Open Access Article
Open Access ArticleGrowth of ZIF-8 on molecularly ordered 2-methylimidazole/single-walled carbon nanotubes to form highly porous, electrically conductive composites†
James E.
Ellis
a,
Zidao
Zeng
a,
Sean I.
Hwang
a,
Shaobo
Li
 b,
Tian-Yi
Luo
a,
Seth C.
Burkert
a,
David L.
White
a,
Nathaniel L.
Rosi
b,
Tian-Yi
Luo
a,
Seth C.
Burkert
a,
David L.
White
a,
Nathaniel L.
Rosi
 a,
Jeremiah J.
Gassensmith
a,
Jeremiah J.
Gassensmith
 bc and
Alexander
Star
bc and
Alexander
Star
 *a
*a
aDepartment of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA. E-mail: astar@pitt.edu
bDepartment of Chemistry and Biochemistry, University of Texas at Dallas, TX 75080, USA
cDepartment of Bioengineering, University of Texas at Dallas, TX 75080, USA
First published on 25th October 2018
Abstract
The combination of porosity and electrical conductivity in a single nanomaterial is important for a variety of applications. In this work, we demonstrate the growth of ZIF-8 on the surface of single-walled carbon nanotubes (SWCNTs). The growth mechanism was investigated and a molecularly ordered imidazole solvation layer was found to disperse SWCNTs and promote crystal growth on the sidewalls. The resultant ZIF-8/SWCNT composite demonstrates high microporosity and electrical conductivity. The ZIF-8/SWCNT composite displayed semiconducting electrical behavior and an increase in sensor sensitivity toward ethanol vapors versus pristine SWCNTs.
Introduction
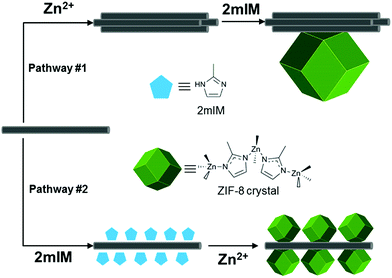
A material that combines the physical properties of periodic porosity and electrical conductivity is an appealing candidate for a variety of applications including sensors, photovoltaics, thermoelectrics, electrocatalysts, and electrical energy storage materials. Electrically conductive metal–organic frameworks (MOFs) contain both of these physical properties;1 however, the mechanism of electrical conductivity found in most conductive MOFs, described as charge hopping, results in a much lower charge mobility than band transport materials. Charge hopping limits MOF electrical conductivity to high electrical fields and short distances, thus limiting their field of application. A potential solution to the low charge mobility inherent to conductive MOFs is to hybridize MOFs with band transport materials like carbon nanotubes (CNTs), thus improving electrical transduction in MOF-based sensors and electrocatalysts.In this study, a zeolitic imidazolate framework (ZIF-8) was chosen as the model MOF because of its water stability, ease of synthesis, and well-documented characterization. ZIF-8 is composed of Zn(II) ions and 2-methylimidazole (2mIM) linkers, and has been studied as both a gas storage material as well as a biomimetic mineralization layer material.2–5 First reports of ZIF-8 employed a solvothermal synthesis in DMF or DEF;6,7 however, room temperature aqueous syntheses that yield highly-crystalline ZIF-8 have recently been investigated.8,9
Several examples of MOF/CNT hybrid composites have been synthesized. Early reports found that the inclusion of CNTs into large MOF crystals enhance gas adsorption compared to the pristine MOF species.10,11 Shim and coworkers were the first to demonstrate a core–shell composite material through homogeneous decoration of small ZIF-8 crystals on PVP-dispersed multi-walled carbon nanotubes (MWCNTs).12 MOF/MWCNT hybrids have been applied toward electrocatalysis and energy storage because they are electrically conductive, high surface area materials.13–16 A pyrolysis step is added to some MOF/MWCNT composites in order to convert the MOF shell into catalytically active metal nanoparticles and heteroatom-doped carbon domains.17–19 A MIL-101/single-walled carbon nanotube (SWCNT) composite has been synthesized by adding SWCNTs to the MOF precursor components.20 An H2 storage improvement was observed for the composite. Unlike MWCNTs, which have metallic electrical characteristics, smaller diameter SWCNTs can have either a metallic or semiconducting electronic structure depending on their chirality.21 For this reason, they are purified based on chirality and applied toward electrical devices such as sensors and field-effect transistors.22–27 SWCNTs have excellent electrical characteristics (charge mobility, charge carrier concentration, etc.) and consist almost entirely of surface atoms, thereby making their electronic structure extremely sensitive to the surrounding chemical environment. To our knowledge, only two MOF/single-walled carbon nanotube (SWCNT) composite has been synthesized;20,28 however, no investigation of MOF growth on the surface of SWCNTs has been reported. Gassensmith and coworkers investigated the growth of ZIF-8 on tobacco mosaic virus,29 a one-dimensional bionanoparticle, and discovered that careful control of the MOF precursor ligand/metal ratio significantly affects the resulting morphology of the composite.30 For this reason we chose to composite ZIF-8 with SWCNTs to fabricate a porous, semiconducting nanowire that could serve as a promising material for chemical sensing.
Results and discussion
In this work, all reactions were performed in ambient and aqueous conditions. Oxidized SWCNTs (ox-SWCNTs) were used in order to obtain well-dispersed carbon nanotubes in aqueous solution. Ox-SWCNTs are approximately 3 atomic% of oxygen in the form of hydroxyl and carboxyl groups on their sidewall and termini. Sonication of ox-SWCNTs in polar solvents promotes dispersive forces, allowing separation of large carbon nanotube agglomerates into smaller bundles and individual ox-SWCNTs. A typical ZIF-8 synthesis involves careful control of zinc salt and 2-methylimidazole (2mIM) concentrations and Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM molar ratio. The addition of ox-SWCNTs as a template adds a complicating factor to the synthesis, wherein the order in which the precursors are added plays an important synthetic role. When ox-SWCNTs are mixed with the zinc salt first or both precursors simultaneously, they immediately re-agglomerate and precipitate out of solution before any ZIF-8 forms. This effect is caused by salt-mediated contraction of the hydration layer around the charged ox-SWCNTs. However, when ox-SWCNTs are first mixed with 2mIM, they remain dispersed in solution for minutes after adding Zn salt (Fig. S1†). It has been previously observed through molecular dynamics simulations that 1-ethyl-3-methylimidazolium tetrafluoroborate (EMI+BF4−) forms an external solvation layer around SWCNTs through π–π interaction between the SWCNTs and EMI+.31 Raman spectroscopy demonstrated that 2mIM-saturated ox-SWCNTs undergo a G peak shape change and red-shift as compared to pristine ox-SWCNTs (Fig. S2†). The ox-SWCNT spectrum has a single G peak which is indicative of SWCNT bundling; in contrast, the G peak of the 2mIM/ox-SWCNT material splits into G− and G+ peaks, which is a signature of single, unbundled SWCNTs.32,33 A redshift in carbon nanotube G peak is an indication of n-doping, which may be the result of charge transfer between 2mIM and ox-SWCNTs.34 Zeta potential measurements indicate that 2mIM does not diminish the negative surface charge of SWCNTs (Table S1†).
2mIM molar ratio. The addition of ox-SWCNTs as a template adds a complicating factor to the synthesis, wherein the order in which the precursors are added plays an important synthetic role. When ox-SWCNTs are mixed with the zinc salt first or both precursors simultaneously, they immediately re-agglomerate and precipitate out of solution before any ZIF-8 forms. This effect is caused by salt-mediated contraction of the hydration layer around the charged ox-SWCNTs. However, when ox-SWCNTs are first mixed with 2mIM, they remain dispersed in solution for minutes after adding Zn salt (Fig. S1†). It has been previously observed through molecular dynamics simulations that 1-ethyl-3-methylimidazolium tetrafluoroborate (EMI+BF4−) forms an external solvation layer around SWCNTs through π–π interaction between the SWCNTs and EMI+.31 Raman spectroscopy demonstrated that 2mIM-saturated ox-SWCNTs undergo a G peak shape change and red-shift as compared to pristine ox-SWCNTs (Fig. S2†). The ox-SWCNT spectrum has a single G peak which is indicative of SWCNT bundling; in contrast, the G peak of the 2mIM/ox-SWCNT material splits into G− and G+ peaks, which is a signature of single, unbundled SWCNTs.32,33 A redshift in carbon nanotube G peak is an indication of n-doping, which may be the result of charge transfer between 2mIM and ox-SWCNTs.34 Zeta potential measurements indicate that 2mIM does not diminish the negative surface charge of SWCNTs (Table S1†).
A synthesis was performed with a large excess of ox-SWCNT to determine whether a similar solvation layer formed between 2mIM and SWCNTs could be coordinated and crystalized by zinc ion addition (Table 1, material 1). If a 2mIM solvation layer forms around ox-SWCNT through π-stacking, then an excess amount of ox-SWCNTs should nucleate all available Zn2+ ions within the solvation layer and prevent the formation of ZIF-8 crystals in solution. The ox-SWCNTs and 2mIM solutions were first combined prior to adding the zinc nitrate hexahydrate solution. The reaction was left unstirred for 4 hours. The reaction suspension was centrifuged to remove the mother liquor, then washed with water and methanol. TEM of material 1 shows dispersed nanotubes with a thick (∼10 nm) diameter (Fig. S3†). Powder X-ray diffraction (PXRD) of this material showed a dominant single peak at 2θ = 17.78° (d-spacing = 4.98 Å) and very small peaks relating to ZIF-8 formation (Fig. S4†). Fukushima et al. have previously reported the molecular ordering of molten salts (in their case, imidazolium-based ionic liquids) by SWCNTs.35 They observed a single XRD peak with d-spacing of 4.60 Å, which they attributed to unimodal long-range molecular ordering of plane-to-plane separated imidazolium ions without polycrystalline character. A similar molecular ordering phenomenon occurs between SWCNTs, 2mIM, and Zn2+. According to XRD, the d-spacing of this material is 4.98 Å, which is large for π-stacked 2mIM. When ox-SWCNTs were mixed with the same amount of 2mIM, but zinc nitrate was not subsequently added, no XRD peak was detected. This observation, in addition to the larger than expected d-spacing, implies that the Zn2+ ions play a role in the long-range ordering of 2mIM around the ox-SWCNTs. Raman spectroscopy of the crystalline 2mIM/ox-SWCNT material 1 demonstrated the same G peak shape change and red-shift as the 2mIM-saturated SWCNTs compared to untreated ox-SWCNTs (Fig. S2†).
| Material | Zinc source | [Zn] (mM) | Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM mol. ratio 2mIM mol. ratio |
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SWCNT (mmol SWCNT (mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mg) mg) |
|---|---|---|---|---|
| 1 | Zn(NO3)2 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
| 2 | Zn(OAc)2 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Zn(NO3)2 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Zn(NO3)2 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | Zn(OAc)2 | 20 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Zn(OAc)2 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Zn(OAc)2 | 6.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 |
Scheme 1 illustrates the effect order of precursor addition has on ox-SWCNT. The agglomeration of ox-SWCNTs caused by addition of zinc salt before 2mIM affects the resulting morphology of the ZIF-8/SWCNT, such that large ZIF-8 crystals grow on islands of agglomerated ox-SWCNTs rather than along the nanotube axis (Fig. S5†). In all later syntheses described in this work, 2mIM-saturated ox-SWCNTs are used as the precursor material in order to prevent agglomeration, to promote nucleation of ZIF-8 crystals according to pathway #2 (Scheme 1), and to prevent 2mIM from further π-stacking on ox-SWCNTs, which would alter the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio.
2mIM ratio.
Factors that are known to influence ZIF growth, size, and morphology in water-based synthesis include zinc precursor source, zinc concentration, zinc to 2mIM ratio, and temperature.9,36 In this work, zinc(II) acetate and zinc(II) nitrate hexahydrate were used as zinc sources. In order to synthesize a ZIF-8/SWCNT composite, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 Zn
40 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio and total zinc concentration of 20 mM was used for each zinc source (materials 2 and 3). The zinc to ox-SWCNT ratio used was 1 mmol Zn
2mIM ratio and total zinc concentration of 20 mM was used for each zinc source (materials 2 and 3). The zinc to ox-SWCNT ratio used was 1 mmol Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mg ox-SWCNT. The reactions were left unstirred at room temperature for 24 hours. The reaction precipitates were collected with centrifugation and washed in water and methanol. The morphology of the resulting products of these syntheses can be described as large ZIF-8 crystals (1–1.5 μm) threaded with SWCNTs (Fig. 1 and S6†). TEM images show crystal growth occurs along a linear path, which is consistent with growth along a CNT axis. XRD of the ZIF-8/SWCNT composites made from both zinc sources contain characteristic ZIF-8 diffraction peaks (Fig. 2). The product composition of each synthesized material determined by PXRD is listed in Table S2.† The peak widths of the ZIF-8/SWCNT composite made with zinc nitrate hexahydrate are ∼3 times broader than the composite made with zinc acetate. The XRD peak broadening of ZIF-8/SWCNT synthesized from zinc nitrate can be attributed to the smaller crystal domain size of the composite. TEM and SEM of the zinc nitrate ZIF-8/SWCNT composite clearly show crystallites that are coalescing into larger crystals (Fig. 1c and d). This observation is consistent with the fast nucleation rate of zinc nitrate with 2mIM to form clusters, which are more likely to form small crystallites compared to zinc acetate.37 A time-dependent experiment was done on material 3 (ZIF-8/SWCNT synthesized from zinc nitrate hexahydrate) to observe the growth mechanism of ZIF-8 on SWCNTs (Fig. S7†). Within 15 minutes, most SWCNTs are covered with small crystals around 50 nm in diameter (Fig. S7,† 15 min). At 30 minutes, the crystals have grown 10-fold in size and smaller crystal domains seem to be agglomerating into large crystals (Fig. S7,† 30 min). At the 2 hour and 20 hour time points, the ZIF-8 crystals have reached their characteristic size (1–1.5 μm), but remain in a linear configuration along SWCNTs (Fig. S7,† 2 h and 20 h).
1 mg ox-SWCNT. The reactions were left unstirred at room temperature for 24 hours. The reaction precipitates were collected with centrifugation and washed in water and methanol. The morphology of the resulting products of these syntheses can be described as large ZIF-8 crystals (1–1.5 μm) threaded with SWCNTs (Fig. 1 and S6†). TEM images show crystal growth occurs along a linear path, which is consistent with growth along a CNT axis. XRD of the ZIF-8/SWCNT composites made from both zinc sources contain characteristic ZIF-8 diffraction peaks (Fig. 2). The product composition of each synthesized material determined by PXRD is listed in Table S2.† The peak widths of the ZIF-8/SWCNT composite made with zinc nitrate hexahydrate are ∼3 times broader than the composite made with zinc acetate. The XRD peak broadening of ZIF-8/SWCNT synthesized from zinc nitrate can be attributed to the smaller crystal domain size of the composite. TEM and SEM of the zinc nitrate ZIF-8/SWCNT composite clearly show crystallites that are coalescing into larger crystals (Fig. 1c and d). This observation is consistent with the fast nucleation rate of zinc nitrate with 2mIM to form clusters, which are more likely to form small crystallites compared to zinc acetate.37 A time-dependent experiment was done on material 3 (ZIF-8/SWCNT synthesized from zinc nitrate hexahydrate) to observe the growth mechanism of ZIF-8 on SWCNTs (Fig. S7†). Within 15 minutes, most SWCNTs are covered with small crystals around 50 nm in diameter (Fig. S7,† 15 min). At 30 minutes, the crystals have grown 10-fold in size and smaller crystal domains seem to be agglomerating into large crystals (Fig. S7,† 30 min). At the 2 hour and 20 hour time points, the ZIF-8 crystals have reached their characteristic size (1–1.5 μm), but remain in a linear configuration along SWCNTs (Fig. S7,† 2 h and 20 h).
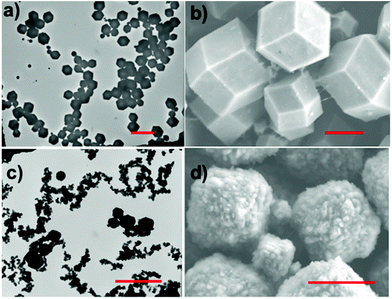 | ||
| Fig. 1 TEM and SEM of ZIF-8/SWCNT. (a and b) Material 2 and (c and d) material 3. Scale bars are (a and c) 5 μm and (b and d) 1 μm. | ||
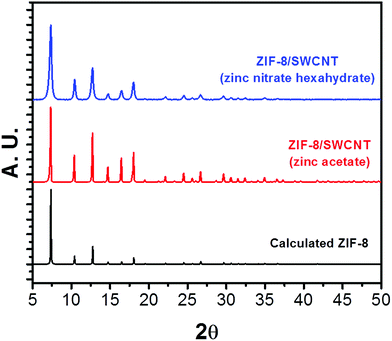 | ||
| Fig. 2 Calculated PXRD pattern of ZIF-8 (bottom); experimental PXRD pattern of ZIF-8/SWCNT synthesized from zinc acetate (middle) and zinc nitrate hexahydrate (top). | ||
The zinc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio controls what type of polymorph/pseudopolymorph is produced in water-based syntheses. ZIF-L and dia(Zn) are polymorphs comprised of Zn(II) and 2mIM, but with different crystal topologies than ZIF-8.36,38,39 Control of Zn(II)
2mIM ratio controls what type of polymorph/pseudopolymorph is produced in water-based syntheses. ZIF-L and dia(Zn) are polymorphs comprised of Zn(II) and 2mIM, but with different crystal topologies than ZIF-8.36,38,39 Control of Zn(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio and temperature allows one polymorph to be favored over the other two during aqueous synthesis. The crystal morphology of ZIF-L resembles a thin, almost two-dimensional leaf-like shape, while ZIF-8's crystal morphology is rhombic dodecahedral. Unlike ZIF-8, ZIF-L and dia(Zn) are nonporous at 77 K; however, at 298 K, ZIF-L outperforms ZIF-8 in CO2 uptake and selectivity over N2 and CH4.38 High ratios (1
2mIM ratio and temperature allows one polymorph to be favored over the other two during aqueous synthesis. The crystal morphology of ZIF-L resembles a thin, almost two-dimensional leaf-like shape, while ZIF-8's crystal morphology is rhombic dodecahedral. Unlike ZIF-8, ZIF-L and dia(Zn) are nonporous at 77 K; however, at 298 K, ZIF-L outperforms ZIF-8 in CO2 uptake and selectivity over N2 and CH4.38 High ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 and above) produce ZIF-8, while lower ratios produce ZIF-L, dia(Zn), or a mixture of one with ZIF-8. A low zinc
35 and above) produce ZIF-8, while lower ratios produce ZIF-L, dia(Zn), or a mixture of one with ZIF-8. A low zinc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio (1
2mIM ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) using zinc acetate produces pure dia(Zn) while zinc nitrate produces pure ZIF-L.9 A low ratio synthesis was performed with 2mIM-saturated ox-SWCNTs (material 4). XRD of this material yields a mixture of ZIF-8 and ZIF-L peak features (Fig. S8†); moreover, TEM shows large (>3 μm) crystals (Fig. S9†) with a morphology unlike ZIF-8 (rhombic dodecahedron) or ZIF-L (thin, leaf-shaped) (Fig. S14B†). This product can be interpreted as the result of two different reaction environments, solution and ox-SWCNT surface, which contain different precursor ratios. 2mIM is expected to concentrate at the surface of ox-SWCNTs through π-stacking, which will promote ZIF-8 crystal growth. In contrast, the solution zinc
8) using zinc acetate produces pure dia(Zn) while zinc nitrate produces pure ZIF-L.9 A low ratio synthesis was performed with 2mIM-saturated ox-SWCNTs (material 4). XRD of this material yields a mixture of ZIF-8 and ZIF-L peak features (Fig. S8†); moreover, TEM shows large (>3 μm) crystals (Fig. S9†) with a morphology unlike ZIF-8 (rhombic dodecahedron) or ZIF-L (thin, leaf-shaped) (Fig. S14B†). This product can be interpreted as the result of two different reaction environments, solution and ox-SWCNT surface, which contain different precursor ratios. 2mIM is expected to concentrate at the surface of ox-SWCNTs through π-stacking, which will promote ZIF-8 crystal growth. In contrast, the solution zinc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio remains 1
2mIM ratio remains 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, leading to ZIF-L crystal growth. It has been previously reported that when separately synthesized ZIF-8 and ZIF-L crystals are combined into a single solution, they will coalesce into a new core–shell composite.40 The addition of ox-SWCNTs into low zinc
8, leading to ZIF-L crystal growth. It has been previously reported that when separately synthesized ZIF-8 and ZIF-L crystals are combined into a single solution, they will coalesce into a new core–shell composite.40 The addition of ox-SWCNTs into low zinc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio solutions produces similar ZIF-L/ZIF-8/ox-SWCNT composites in a one-pot synthesis. When the 1
2mIM ratio solutions produces similar ZIF-L/ZIF-8/ox-SWCNT composites in a one-pot synthesis. When the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 Zn
8 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM synthesis was done with zinc acetate as precursor (material 5), the morphology was pure dia(Zn). Zinc acetate has a slower rate of cluster nucleation, which may explain why only a single morphology was observed for material 5.37
2mIM synthesis was done with zinc acetate as precursor (material 5), the morphology was pure dia(Zn). Zinc acetate has a slower rate of cluster nucleation, which may explain why only a single morphology was observed for material 5.37
Decreasing the overall precursor concentration slows the kinetics of the reaction and achieves smaller ZIF-8 coverage on the surface of ox-SWCNTs. 10 mM and 6.6 mM zinc acetate reactions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 zinc
40 zinc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratio) were done with 2mIM-saturated ox-SWCNTs and collected after 24 hours (materials 6 and 7, respectively). Both syntheses produced a smaller ZIF-8 crystal shell growth (approximately 90 nm and 50 nm, respectively) on the ox-SWCNTs (Fig. S10 and S11†). However, the decrease in precursor concentration also lowers the pH, such that pseudopolymorph dia(Zn) (Fig. S14A†) forms in solution if the reaction is left for more than 24 hours (Fig. S12, S13, and S15†).
2mIM ratio) were done with 2mIM-saturated ox-SWCNTs and collected after 24 hours (materials 6 and 7, respectively). Both syntheses produced a smaller ZIF-8 crystal shell growth (approximately 90 nm and 50 nm, respectively) on the ox-SWCNTs (Fig. S10 and S11†). However, the decrease in precursor concentration also lowers the pH, such that pseudopolymorph dia(Zn) (Fig. S14A†) forms in solution if the reaction is left for more than 24 hours (Fig. S12, S13, and S15†).
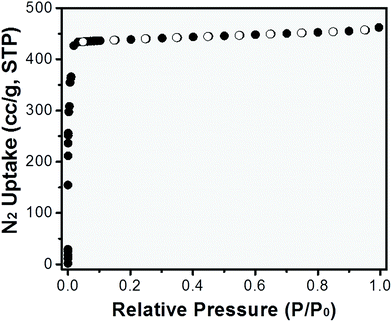
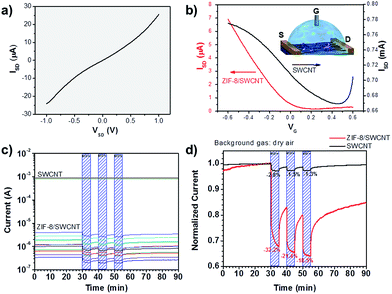
The gas adsorption performance of ZIF-8/SWCNT (shown in Fig. 1a and b) was tested (Fig. 3). ZIF-8/SWCNT displayed a type-I isotherm and the BET surface area of ZIF-8/SWCNT was calculated to be 1792 m2 g−1, which is near the reported BET surface area of pure ZIF-8 (1851 m2 g−1).41 The CO2vs. N2 uptake at 298 K was tested and showed the same preference for CO2 uptake as pure ZIF-8 (Fig. S16†), indicating that these composite materials may exhibit selectivity for specific gases. In order to test I–V performance, a 3 μL droplet of ZIF-8/SWCNT solution was dropcast on a device of two interdigitated gold electrodes (Fig. S17†). The ZIF-8/SWCNT current–voltage curve displays a conductivity of 0.056 S cm−1 (Fig. 4a). Conductivity (σ) was calculated from the following equation:
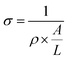 | (1) |
This conductivity is on the higher end for conductive 3-D MOFs, which range from 1 × 10−7 to 1.8 S cm−1.42,43 Among the conductive MOF/carbon composites, this work demonstrated the highest BET surface area and achieved conductivity with the least amount of carbon loading (0.40 ± 0.09%; see Table S3†).44,45 Carbon nanotube loading was calculated by dividing the initial amount of carbon nanotube by the total mass of the ZIF-8/SWCNT product, filtered and dried. These experiments demonstrate both the highly microporous and electrically conductive nature of the reported ZIF-8/SWCNT composite.
Liquid FET measurements of ZIF-8/SWCNT (material 2) demonstrate p-type semiconductor behavior (Fig. 4b). Semiconducting nanowires are ideal for gas sensing because of their large surface area to volume ratio, their sensitivity toward doping interactions, and their sensitivity toward changes in inter-nanowire distance.46 Both the SWCNT and ZIF-8/SWCNT devices showed a decrease in conductance when exposed to 3 cycles of air saturated with ethanol vapor (7.73 vol%) (Fig. 4c and d). The sensitivity to ethanol vapor was quantified by calculating the relative sensor response (S):
 | (2) |
Conclusions
In conclusion, this work demonstrates the molecular ordering of 2-methylimidazole (2mIM) as a solvation layer on the surface of oxidized single-walled carbon nanotubes (ox-SWCNTs). This 2mIM solvation layer gives rise to ZIF-8 growth on ox-SWCNT sidewalls. The high concentration of 2mIM on ox-SWCNTs produces ZIF-8 at the surface of the nanotube even at low zinc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2mIM ratios that typically produce ZIF-L. The size of ZIF-8 growth on ox-SWCNTs can be controlled by precursor concentration, wherein decreasing the precursor concentration decreases the size of ZIF-8. The ZIF-8/SWCNT composite was shown to combine the porous nature of ZIF-8 with the electrically conductive nature of carbon nanotubes. Finally, the ZIF-8/SWCNT material was incorporated into a chemiresistor device and demonstrated a higher sensitivity toward saturated ethanol vapor versus pristine SWCNT. Such a highly porous, electrically conductive nanomaterial will be of interest for a variety of applications such as sensing, electrocatalysis, and energy storage.
2mIM ratios that typically produce ZIF-L. The size of ZIF-8 growth on ox-SWCNTs can be controlled by precursor concentration, wherein decreasing the precursor concentration decreases the size of ZIF-8. The ZIF-8/SWCNT composite was shown to combine the porous nature of ZIF-8 with the electrically conductive nature of carbon nanotubes. Finally, the ZIF-8/SWCNT material was incorporated into a chemiresistor device and demonstrated a higher sensitivity toward saturated ethanol vapor versus pristine SWCNT. Such a highly porous, electrically conductive nanomaterial will be of interest for a variety of applications such as sensing, electrocatalysis, and energy storage.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Raman characterization was completed through the assistance of a ONR (N000141410765) grant. We thank the Department of Biological Sciences and the Nanoscale Fabrication and Characterization Facility (NFCF) at the University of Pittsburgh for access to the TEM, SEM, and XRD instrumentation. We especially thank Dr Susheng Tan for the help he provided in collecting HRTEM images of material 6. JJG and SL acknowledge UTD for provided start-up funds.References
- L. Sun, M. G. Campbell and M. Dinca, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS PubMed.
- L. Mu, B. Liu, H. Liu, Y. Yang, C. Sun and G. Chen, J. Mater. Chem., 2012, 22, 12246 RSC.
- K. Liang, C. J. Coghlan, S. G. Bell, C. Doonan and P. Falcaro, Chem. Commun., 2016, 52, 473–476 RSC.
- H. He, H. Han, H. Shi, Y. Tian, F. Sun, Y. Song, Q. Li and G. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 24517–24524 CrossRef CAS PubMed.
- N. K. Maddigan, A. Tarzia, D. M. Huang, C. J. Sumby, S. G. Bell, P. Falcaro and C. J. Doonan, Chem. Sci., 2018, 9, 4217–4223 RSC.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- Y. Pan, Y. Liu, G. Zeng, L. Zhao and Z. Lai, Chem. Commun., 2011, 47, 2071–2073 RSC.
- M. Jian, B. Liu, R. Liu, J. Qu, H. Wang and X. Zhang, RSC Adv., 2015, 5, 48433–48441 RSC.
- S. J. Yang, J. Y. Choi, H. K. Chae, J. H. Cho, K. S. Nahm and C. R. Park, Chem. Mater., 2009, 21, 1893–1897 CrossRef CAS.
- Z. Xiang, X. Peng, X. Cheng, X. Li and D. Cao, J. Phys. Chem. C, 2011, 115, 19864–19871 CrossRef CAS.
- J. Yoo, S. Lee, C. K. Lee, C. Kim, T. Fujigaya, H. J. Park, N. Nakashima and J. K. Shim, RSC Adv., 2014, 4, 49614–49619 RSC.
- S. Sohrabi, S. Dehghanpour and M. Ghalkhani, J. Mater. Sci., 2017, 53, 3624–3639 CrossRef.
- Q. Wang, Q. Wang, B. Xu, F. Gao, F. Gao and C. Zhao, Electrochim. Acta, 2018, 281, 69–77 CrossRef CAS.
- H. Zhang, W. Zhao, M. Zou, Y. Wang, Y. Chen, L. Xu, H. Wu and A. Cao, Adv. Energy Mater., 2018, 8, 1800013 CrossRef.
- Y. Pu, W. Wu, J. Liu, T. Liu, F. Ding, J. Zhang and Z. Tang, RSC Adv., 2018, 8, 18604–18612 RSC.
- L. Ge, Y. Yang, L. Wang, W. Zhou, R. De Marco, Z. Chen, J. Zou and Z. Zhu, Carbon, 2015, 82, 417–424 CrossRef CAS.
- Y. Pan, K. Sun, S. Liu, X. Cao, K. Wu, W. C. Cheong, Z. Chen, Y. Wang, Y. Li, Y. Liu, D. Wang, Q. Peng, C. Chen and Y. Li, J. Am. Chem. Soc., 2018, 140, 2610–2618 CrossRef CAS PubMed.
- H. Zhang, Y. Wang, W. Zhao, M. Zou, Y. Chen, L. Yang, L. Xu, H. Wu and A. Cao, ACS Appl. Mater. Interfaces, 2017, 9, 37813–37822 CrossRef CAS PubMed.
- K. P. Prasanth, P. Rallapalli, M. C. Raj, H. C. Bajaj and R. V. Jasra, Int. J. Hydrogen Energy, 2011, 36, 7594–7601 CrossRef CAS.
- S. N. Kim, J. F. Rusling and F. Papadimitrakopoulos, Adv. Mater., 2007, 19, 3214–3228 CrossRef CAS PubMed.
- H. R. Byon and H. C. Choi, J. Am. Chem. Soc., 2006, 128, 2188–2189 CrossRef CAS PubMed.
- S. Boussaad, B. A. Diner and J. Fan, J. Am. Chem. Soc., 2008, 130, 3780–3787 CrossRef CAS PubMed.
- J. E. Ellis, U. Green, D. C. Sorescu, Y. Zhao and A. Star, J. Phys. Chem. Lett., 2015, 6, 712–717 CrossRef CAS PubMed.
- G. Peng, S. Wu, J. E. Ellis, X. Xu, G. Xu, C. Yu and A. Star, J. Mater. Chem. C, 2016, 4, 6575–6580 RSC.
- S. Ishihara, C. J. O'Kelly, T. Tanaka, H. Kataura, J. Labuta, Y. Shingaya, T. Nakayama, T. Ohsawa, T. Nakanishi and T. M. Swager, ACS Appl. Mater. Interfaces, 2017, 9, 38062–38067 CrossRef CAS PubMed.
- C. Paoletti, M. He, P. Salvo, B. Melai, N. Calisi, M. Mannini, B. Cortigiani, F. G. Bellagambi, T. M. Swager, F. Di Francesco and A. Pucci, RSC Adv., 2018, 8, 5578–5585 RSC.
- Z. Ghiamaty, A. Ghaffarinejad, M. Faryadras, A. Abdolmaleki and H. Kazemi, J. Nanostruct. Chem., 2016, 6, 299–308 CrossRef CAS.
- S. Li, M. Dharmarwardana, R. P. Welch, Y. Ren, C. M. Thompson, R. A. Smaldone and J. J. Gassensmith, Angew. Chem., Int. Ed., 2016, 55, 10691–10696 CrossRef CAS PubMed.
- S. Li, M. Dharmarwardana, R. P. Welch, C. E. Benjamin, A. M. Shamir, S. O. Nielsen and J. J. Gassensmith, ACS Appl. Mater. Interfaces, 2018, 10, 18161–18169 CrossRef CAS PubMed.
- Y. Shim and H. J. Kim, ACS Nano, 2009, 3, 1693–1702 CrossRef CAS PubMed.
- U. D. Venkateswaran, A. M. Rao, E. Richter, M. Menon, A. Rinzler, R. E. Smalley and P. C. Eklund, Phys. Rev. B, 1999, 59, 10928–10934 CrossRef CAS.
- S. B. Cronin, A. K. Swan, M. S. Ünlü, B. B. Goldberg, M. S. Dresselhaus and M. Tinkham, Phys. Rev. B, 2005, 72 Search PubMed.
- R. Voggu, C. S. Rout, A. D. Franklin, T. S. Fisher and C. N. R. Rao, J. Phys. Chem. C, 2008, 112, 13053–13056 CrossRef CAS.
- T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300, 2072–2074 CrossRef CAS PubMed.
- Y. Lo, C. H. Lam, C.-W. Chang, A.-C. Yang and D.-Y. Kang, RSC Adv., 2016, 6, 89148–89156 RSC.
- S. Tanaka, T. Shimada, K. Fujita, Y. Miyake, K. Kida, K. Yogo, J. F. M. Denayer, M. Sugita and T. Takewaki, J. Membr. Sci., 2014, 472, 29–38 CrossRef CAS.
- R. Chen, J. Yao, Q. Gu, S. Smeets, C. Baerlocher, H. Gu, D. Zhu, W. Morris, O. M. Yaghi and H. Wang, Chem. Commun., 2013, 49, 9500–9502 RSC.
- Q. Shi, Z. Chen, Z. Song, J. Li and J. Dong, Angew. Chem., Int. Ed., 2011, 50, 672–675 CrossRef CAS PubMed.
- W. C. Lee, H. T. Chien, Y. Lo, H. C. Chiu, T. P. Wang and D. Y. Kang, ACS Appl. Mater. Interfaces, 2015, 7, 18353–18361 CrossRef CAS PubMed.
- F. M. Hinterholzinger, A. Ranft, J. M. Feckl, B. Rühle, T. Bein and B. V. Lotsch, J. Mater. Chem., 2012, 22, 10356 RSC.
- S. K. Bhardwaj, N. Bhardwaj, R. Kaur, J. Mehta, A. L. Sharma, K.-H. Kim and A. Deep, J. Mater. Chem. A, 2018, 6, 14992–15009 RSC.
- L. S. Xie, L. Sun, R. Wan, S. S. Park, J. A. DeGayner, C. H. Hendon and M. Dinca, J. Am. Chem. Soc., 2018, 140, 7411–7414 CrossRef CAS PubMed.
- O. Fleker, A. Borenstein, R. Lavi, L. Benisvy, S. Ruthstein and D. Aurbach, Langmuir, 2016, 32, 4935–4944 CrossRef CAS PubMed.
- D. Kim, D. W. Kim, W. G. Hong and A. Coskun, J. Mater. Chem. A, 2016, 4, 7710–7717 RSC.
- J. F. Fennell Jr, S. F. Liu, J. M. Azzarelli, J. G. Weis, S. Rochat, K. A. Mirica, J. B. Ravnsbaek and T. M. Swager, Angew. Chem., Int. Ed., 2016, 55, 1266–1281 CrossRef PubMed.
- K. Zhang, R. P. Lively, M. E. Dose, A. J. Brown, C. Zhang, J. Chung, S. Nair, W. J. Koros and R. R. Chance, Chem. Commun., 2013, 49, 3245–3247 RSC.
- A. Nalaparaju, X. S. Zhao and J. W. Jiang, J. Phys. Chem. C, 2010, 114, 11542–11550 CrossRef CAS.
- G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed.
- C. Li, E. T. Thostenson and T.-W. Chou, Appl. Phys. Lett., 2007, 91, 223114 CrossRef.
- H. C. Wang, Y. Li and M. J. Yang, Sens. Actuators, B, 2007, 124, 360–367 CrossRef CAS.
- S. F. Liu, L. C. H. Moh and T. M. Swager, Chem. Mater., 2015, 27, 3560–3563 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional TEM, PXRD, Raman spectroscopy, and zeta potential measurement of synthesized materials; comparison table of this work with other conductive MOFs. See DOI: 10.1039/c8sc03987a |
| This journal is © The Royal Society of Chemistry 2019 |