Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unusual fcc-structured Ag10 kernels trapped in Ag70 nanoclusters†
Yan-Min
Su‡
a,
Zhi
Wang‡
a,
Gui-Lin
Zhuang
 b,
Quan-Qin
Zhao
a,
Xing-Po
Wang
a,
Chen-Ho
Tung
a and
Di
Sun
b,
Quan-Qin
Zhao
a,
Xing-Po
Wang
a,
Chen-Ho
Tung
a and
Di
Sun
 *a
*a
aKey Lab for Colloid and Interface Chemistry of Education Ministry, School of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, People's Republic of China. E-mail: dsun@sdu.edu.cn
bCollege of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou, 310032, People's Republic of China
First published on 18th October 2018
Abstract
Controlled trapping atom-precise ultrasmall silver nanoparticles into silver nanoclusters is challenging; thus only limited progress has been made in this area. We are therefore inspired to isolate two novel silver nanoclusters, Ag10@Ag70 (SD/Ag80a and SD/Ag80b; SD = SunDi), where a novel fcc-structured Ag10 kernel built from two single-edge opened Ag6 octahedra by sharing one edge is trapped. The bioctahedral Ag10 kernel is locked by a pair of Mo7O2610− anions to form an inner Ag10@(Mo7O26)2 core which is further encapsulated by an outer Ag70 shell to form three-shell Ag10@(Mo7O26)2@Ag70 nanoclusters. Notably, the bioctahedral Ag10 kernel has not been observed in silver nanoclusters ever before, thus representing a new embryo state of silver nanoparticles. SD/Ag80a emits in the near infrared (NIR) region (λem = 730 nm) at low temperature. This work will deepen our understanding on the atomic-level growth of silver nanoparticles and complicated three-shell self-assembly involving polyoxometalate (POM) and two different silver nanoclusters.
Introduction
Ultrasmall silver nanoparticles (e.g., few-atom clusters, <1 nm) represent the embryo states of larger silver nanoparticles (typically >2 nm) to some extent, which have defined molecular structures and compositions and thus can deepen the understanding on the size evolution of silver nanoparticles.1 Given this, X-ray single crystal structures become a prerequisite to get atomic-level information including the surface ligands, inorganic–organic interfaces and silver atoms packed in silver nanoparticles.2 While chasing large silver nanoclusters such as Ag14, Ag21, Ag23, Ag44, Ag50, Ag62, Ag67, Ag74, and Ag141 and even the largest known Ag374,3 chemists almost neglect the significance of the embryo states of silver nanoparticles that however are quite difficult to be captured due to their typical kinetics-controlled growth course.4 Therefore, controlling the reductive transformation from Ag(I) to Ag0 and then trapping the transient Ag aggregates into the thermodynamically stable crystalline product during the self-assembly is an urgent need and thus a major challenge.Learning from the solvent-controlled synthesis of multiple-twin decahedral and icosahedral silver nanoparticles with special favourable [111] facets,5 we found that DMF (N,N-dimethylformamide), compared to widely used NaBH4, is a much more mild reductive agent which facilitates the formation of Ag6 octahedral kernels during the slow reduction process as seen in Ag34 and Ag62 nanoclusters.6 Such Ag6 octahedra can be seen as the smallest fragment cut from the unit cell of face-centered cubic (fcc) bulk silver metal, whereas other silver nanoclusters smaller than the most common icosahedral Ag13 are still not directly observed in any reported silver nanoclusters.7 Thus, the species in the early evolution from discrete Ag atoms to the metallic state are still largely vague and the exploration of a suitable synthesis strategy to trap them is scientifically desired.
With these considerations in mind, we used a DMF-containing mixed solvent system to isolate two novel silver nanoclusters [Ag10@(Mo7O26)2@Ag70(MoO4)2(CyhS)36(CF3SO3)16(DMF)6]·2DMF·4nPrOH (SD/Ag80a; SD = SunDi; CyhSH = cyclohexanethiol) and [Ag10@(Mo7O26)2@Ag70(MoO4)2(iPrS)36(CF3SO3)16(DMF)6] (SD/Ag80b). Two silver nanoclusters have the same metallic core but different organic coatings. In the innermost of cluster, an unusual fcc-structured Ag10 nanocluster constructed from two single-edge opened Ag6 octahedra by sharing one edge is locked by a pair of Mo7O2610− anions to form an inner Ag10@(Mo7O26)2 core which acts as a template to support the outer Ag70 nanocluster to form a final three-shell Ag10@(Mo7O26)2@Ag70 nanocluster. This unprecedented bioctahedral Ag10 nanocrystal can be deemed as a new nanofragment cut from fcc silver metal and represents a possible transient species in the growth of large silver nanoparticles.
Results and discussion
X-ray structures of SD/Ag80a and SD/Ag80b
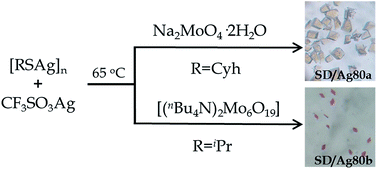
SD/Ag80a and SD/Ag80b were synthesized through a facile one-pot solvothermal reaction of silver-thiolate polymeric precursors, CF3SO3Ag and molybdates in different DMF-containing mixed solvent systems (Scheme 1). In spite of several attempts, we still couldn't isolate SD/Ag80a and SD/Ag80b using the same Mo sources. Their samples were collected as brown-yellow and red crystals, respectively, after evaporation of solvents at room temperature for 1–2 weeks. Several synthetic parameters were optimized and are listed in Tables S1 and S2 (ESI)† for details. Details of the synthesis and some basic characterization are shown in the ESI.†The molecular structures of SD/Ag80a and SD/Ag80b were revealed by single-crystal X-ray diffraction (SCXRD) analysis. They crystallize in monoclinic P21/n and triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space groups, respectively. In each asymmetric unit only half of the corresponding clusters were resolved. Due to the structural similarities, only that of SD/Ag80a is described in detail here. The structural diagrams of SD/Ag80b are shown in Fig. S1.† Selected details of the data collection and structure refinements are listed in Table S3.†
space groups, respectively. In each asymmetric unit only half of the corresponding clusters were resolved. Due to the structural similarities, only that of SD/Ag80a is described in detail here. The structural diagrams of SD/Ag80b are shown in Fig. S1.† Selected details of the data collection and structure refinements are listed in Table S3.†
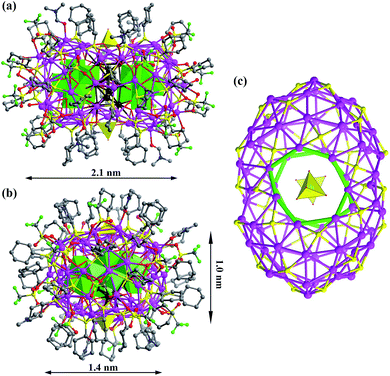
SD/Ag80a is an elongated spheroid (1.0 × 1.4 × 2.1 nm) that sits on the crystallographic inversion center (i). The Ag80 nanocluster is composed of a Ag70 shell and a Ag10 kernel. The Ag70 shell is capped by 36 CyhS−, 16 CF3SO3−, 2 MoO42− and 6 DMF (Fig. 1a and b). All cyclohexyl groups of 36 CyhS− ligands show a unified chair configuration. Two different coordination modes (μ3 and μ4) are found in 36 CyhS− ligands capped on the silver trigons or tetragons (Ag–S distances: 2.389(5)–2.722(5) Å). The 16 CF3SO3− anions exhibit three different coordination fashions including μ3-η1:η2:η0, μ3-η1:η1:η1, and μ2-η1:η1:η0. Two MoO42− anions (yellow tetrahedra in Fig. 1) adopt a μ8-η2:η3:η3 mode to bind in the equatorial region of the Ag70 shell. Six DMF molecules as terminal ligands finished the organic ligand coverage on the surface of the Ag70 shell. Three different O donor ligands (CF3SO3−, MoO42−, and DMF) interact with Ag atoms with the bonding distances in the ranges of 2.406(15)–2.789(17), 2.251(11)–2.568(11) and 2.390(13)–2.458(14) Å, respectively. The Ag70 shell was further consolidated by the argentophilic interaction8 ranging from 2.833(2) to 3.4394(16) Å. The surface of the Ag70 shell consists silver trigons, tetragons, pentagons and heptagons (Fig. 1c). The silver trigons, tetragons, and pentagons are capped by CyhS− or CF3SO3−, whereas MoO42− shapes the large silver heptagons (green rings in Fig. 1c).
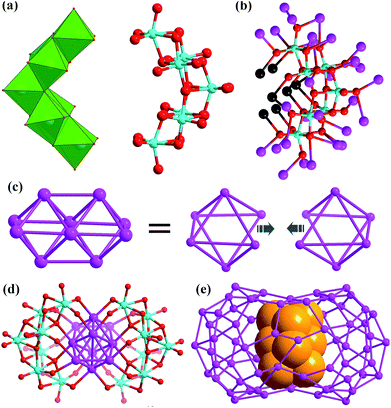
There are two crescent-like Mo7O2610− anions under the Ag70 shell (Fig. 2a). During the synthesis of SD/Ag80a and SD/Ag80b, although different Mo sources, Na2MoO4·2H2O and [(nBu4N)2Mo6O19], were used, respectively, the same Mo7O2610− anion was trapped as the template in the final silver nanoclusters. Thus, the novel Mo7O2610− anions should be in situ transformed from Na2MoO4·2H2O or [(nBu4N)2(Mo6O19)] in different solvent environments. We used Bond-Valence Sum (BVS) calculations for seven Mo atoms of Mo7O2610−, which confirmed that all of them are in the +6 oxidation state (Table S5†).9 The Mo7O2610− is constructed from seven edge-shared MoO6 octahedra. The total 26 O atoms are divided into four kinds based on their binding fashion to Ag atoms, 2 μ0, 4 μ1, 16 μ2, and 4 μ3. Such highly negative-charged Mo7O2610− totally binds 35 silver atoms. Among them, 7 are from the inner Ag10 kernel and the remaining 28 are from the Ag70 shell (Fig. 2b). Notably, this novel molybdate has neither been observed in classic POM chemistry nor in silver nanoclusters. More importantly, this molybdate carries the second highest negative charges10 which effectively enhanced its template effect by binding more Ag atoms (Table S6†).
The most interesting feature in SD/Ag80a is the unusual Ag10 kernel underlying the equatorial region of the Ag70 shell which is built from two single-edge opened Ag6 octahedra by sharing one edge (Fig. 2c). The shared edge is the longest Ag⋯Ag edge (Ag38⋯Ag38i = 3.457(1) Å, symmetry code i: −x + 1, −y + 1, −z + 1) within the Ag10 kernel, which is out of the normal Ag⋯Ag interaction range. All other eleven Ag⋯Ag edges are distributed in the range of 2.659(2)–2.980(1) Å (Fig. S2†) and the average Ag⋯Ag distance is 2.814 Å, which is 2.5% shorter than the Ag⋯Ag distance in metallic silver (2.886 Å),11 indicating strong argentophilic interactions as in bulk silver metal. All exposed trigons of the Ag10 bioctahedral kernel are [111] facets which are capped by Mo7O2610− anions through Ag–O bonding (Ag–O distances: 2.284(10)–2.433(10) Å; Fig. 2d). As such, the Ag10 bioctahedron is doubly clamped by a pair of Mo7O2610− anions to form an inner Ag10@(Mo7O26)2 core, which was enwrapped by an outer Ag70 shell to form a three-shell Ag10@(Mo7O26)2@Ag70 nanocluster. The two polar sites of the Ag10 bioctahedron are also linked with the outer Ag70 shell through argentophilic interactions (Ag⋯Ag: 2.8523(19)–3.3621(18) Å; Fig. S3†).
Although the single Ag6 octahedron has been observed in several inorganic compounds12 and a few silver nanoclusters,13 its dimer, Ag10 bioctahedron, has never been observed before in silver nanoclusters. Such a Ag10 bioctahedron can be seen as a bigger nanofragment than a Ag6 octahedron. An important driven force for its formation should be the suitable reducibility of DMF.6 The oxidation product of DMF in the assembly process is Me2NCOOH14 which can be recognized from the 13C NMR (nuclear magnetic resonance) of HCl digested reaction mother solution (Fig. S4†). In the chemical shift scale corresponding to aldehydes and carboxylates (δ = 150–200 ppm), two peaks appeared at δ = 164.64 and 162.92 ppm, which are assigned to the carbon resonances of DMF and Me2NCOOH, respectively. We didn't observe any peaks in 13C NMR corresponding to the oxidation product of nPrOH, which clearly excluded the possible reductive effect of nPrOH in this assembly system. These results clearly evidenced the redox reaction between Ag(I) and DMF occurred during the self-assembly process. The emergence of a fcc-structured Ag10 nanocluster, on the other hand, answered an important question, which is how the common observed smaller Ag6 kernel grew up to larger structures. Based on the above structural information, we can tentatively assign a new edge-fusion mode to its growth mechanism, although several other growth modes for noble metal nanoparticles have been proposed such as face-fusion, interpenetration, shell-by-shell, layer-by-layer, and tetrahedron-based vertex-sharing growth modes.2 Based on the formulae and charge neutrality considerations, we can determine that the valence of the Ag10 kernel is +6, which means such a kernel carries four free electrons, belonging to a 4e superatom network. We also performed DFT calculations at the B3LYP/SDD theoretical level to study the free electron distributions on the frontier orbitals of the Ag10 kernel (see details in the ESI†). According to the results identified experimentally, the inner Ag10 kernel features Ci symmetry with +6 valence and four free electrons. Thus, frontier molecular orbital analysis (Fig. S5†) reveals that four free electrons occupy two Au-symmetry HOMO-1 and HOMO. HOMO-1 and HOMO exhibit different components. HOMO involves in the 5s orbitals of two ends of Ag10, while HOMO-1 concentrates on the 4d orbitals of two ends of Ag10. Moreover, HOMO-2 features Ag symmetry with similar components to HOMO-1, and LUMO consists of 5s orbitals in the centre of Ag10.
Combining the structural analysis and DMF-involved reductive process, we tentatively proposed a total shell-by-shell formation mechanism for such new silver nanoclusters. Weakly reductive DMF firstly induced the formation of an inner Ag10 kernel (1st shell), which exposes highly active [111] facets that are quickly passivated by the formation of Ag–O interaction with Mo7O2610− (2nd shell). The inner [Ag10@(Mo7O26)2] core acts as the authentic template to support an outer Ag70 shell, forming the final core–shell type silver nanoclusters. Such a formation route resembled the mechanism revealed in the [Ag6@(MoO4)7@Ag56] family by electrospray ionization mass spectrometry.6b
We also noted that the Au21(S-Adm)15 nanocluster has been reported by the Zhu group,15 who firstly found the bioctahedral Au10 kernel formed by edge-sharing of two single-edge opened Au6 octahedra. However, the shared edge is not the longest one (opened edge) and the overall Au6 octahedral framework is severely disordered. Anyhow, as a counterpart of this Au10 kernel, the bioctahedral Ag10 kernel has not been reported before in silver nanoclusters.
The optical properties of SD/Ag80a
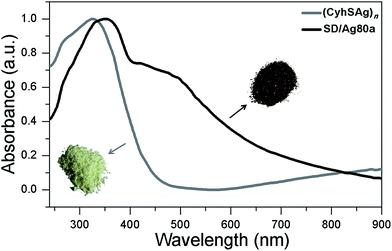
The UV/Vis spectrum of SD/Ag80a was measured in the solid state using diffuse reflectance mode. As shown in Fig. 3, SD/Ag80a showed an absorption maximum at 344 nm and a shoulder peak in the visible region (∼490 nm), which should be ascribed to ligand-based absorption and the charge transfer transition from the S 3p to Ag 5s orbitals, respectively. Similar assignments were also made in a hypothetical silver sulfide monomer and molecular [Ag62S13(SBut)32]4+ cluster.16 Based on the Kubelka–Munk function (Fig. S6†), the band gap of SD/Ag80a was estimated to be ∼1.06 eV, which indicates that SD/Ag80a is a potential narrow-band-gap semiconductor. In comparison, the optical energy gap of the precursor (CyhSAg)n is ∼2.09 eV.The luminescence properties of SD/Ag80a were studied in the solid state. As shown in the insets of Fig. 4, we can observe that SD/Ag80a isn't emissive under the UV light irradiation (λex = 365 nm) at room temperature; however, it emits red luminescence at 77 K. The varied-temperature emission spectra of SD/Ag80a in the solid state were recorded from 293 to 83 K with 30 K as an interval, showing luminescence thermochromic behavior. When gradually cooled to 83 K, the intensity of emission shows an 18-fold enhancement, which should be assigned to the low-temperature induced increase of radiative decay. The emission maximum was blue-shifted from 754 to 730 nm (λex = 469 nm) in the temperature range of 173–83 K (Fig. 4), which may be related to the enhanced molecular rigidity at lower temperature.17 This near-infrared (NIR) emission should be assigned to ligand-to-metal-charge-transfer (LMCT) transition from S 3p to Ag 5s orbitals.18 The emission lifetime of SD/Ag80a, falling on the microsecond scale at 83 K (Fig. S7†), suggests the triplet phosphorescence origin.
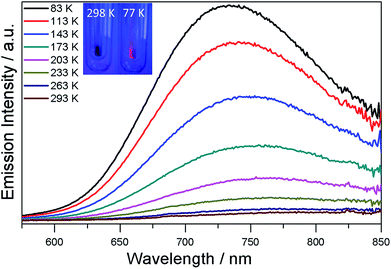 | ||
| Fig. 4 Varied-temperature luminescence spectra of SD/Ag80a from 293–83 K in the solid state. Insets show the photographs of the sample SD/Ag80a under a hand-held UV lamp (365 nm) at 298 and 77 K. | ||
Conclusions
In conclusion, we developed a DMF-controlled strategy to successfully capture an atom-precise ultrasmall Ag10 kernel into a gigantic silver nanocluster. The DMF with mild reductive ability plays a key role in the formation of such a novel cluster-in-cluster silver nanocluster. The fcc-structured Ag10 kernel is built from two single-edge opened Ag6 octahedra by sharing one edge and further locked by a pair of Mo7O2610− anions to form an inner Ag10@(Mo7O26)2 core which is finally encapsulated by an outer Ag70 shell to form three-shell Ag10@(Mo7O26)2@Ag70 nanoclusters. Notably, both the bioctahedral Ag10 kernel and crescent-like Mo7O2610− have not been observed in silver nanocluster and POM chemistry ever before, respectively. The bioctahedral Ag10 core can be deemed as a brand-new embryo state of silver nanoparticles; moreover, it also provides a new edge-fusion growth route for silver nanoparticles from the smallest Ag6 nanofragment of metallic silver. We hope that this work can popularize the new controllable synthetic method to expand the scope of silver nanoclusters with higher complexity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21822107, 21571115, and 21671172), the Natural Science Foundation of Shandong Province (No. JQ201803 and ZR2017MB061), the Qilu Youth Scholar Funding of Shandong University and the Fundamental Research Funds of Shandong University (104.205.2.5).References
- (a) T. U. B. Rao and T. Pradeep, Angew. Chem., Int. Ed., 2010, 49, 3925–3929 CrossRef CAS PubMed; (b) T. U. B. Rao, B. Nataraju and T. Pradeep, J. Am. Chem. Soc., 2010, 132, 16304–16307 CrossRef CAS PubMed; (c) H. Xiang, S.-H. Wei and X. Gong, J. Am. Chem. Soc., 2010, 132, 7355–7360 CrossRef CAS PubMed.
- (a) R. C. Jin, C. J. Zeng, M. Zhou and Y. X. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed; (b) S. L. Zhuang, L. W. Liao, Y. Zhao, J. Y. Yuan, C. H. Yao, X. Liu, J. Li, H. T. Deng, J. L. Yang and Z. K. Wu, Chem. Sci., 2018, 9, 2437–2442 RSC.
- (a) H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Chem. Commun., 2013, 49, 300–302 RSC; (b) R. S. Dhayal, J.-H. Liao, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 3702–3706 CrossRef CAS PubMed; (c) C. Liu, T. Li, H. Abroshan, Z. M. Li, C. Zhang, H. J. Kim, G. Li and R. C. Jin, Nat. Commun., 2018, 9, 744 CrossRef PubMed; (d) A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed; (e) W. J. Du, S. Jin, L. Xiong, M. Chen, J. Zhang, X. J. Zou, Y. Pei, S. X. Wang and M. Z. Zhu, J. Am. Chem. Soc., 2017, 139, 1618–1624 CrossRef CAS PubMed; (f) S. Jin, S. X. Wang, Y. B. Song, M. Zhou, J. Zhong, J. Zhang, A. D. Xia, Y. Pei, M. Chen, P. Li and M. Z. Zhu, J. Am. Chem. Soc., 2014, 136, 15559–15565 CrossRef CAS PubMed; (g) M. J. Alhilaly, M. S. Bootharaju, C. P. Joshi, T. M. Besong, A.-H. Emwas, R. Juarez-Mosqueda, S. Kaappa, S. Malola, K. Adil, A. Shkurenko, H. Hakkinen, M. Eddaoudi and O. M. Bakr, J. Am. Chem. Soc., 2016, 138, 14727–14732 CrossRef CAS PubMed; (h) M. Qu, H. Li, L. H. Xie, S. T. Yan, J. R. Li, J. H. Wang, C. Y. Wei, Y. W. Wu and X. M. Zhang, J. Am. Chem. Soc., 2017, 139, 12346–12349 CrossRef CAS PubMed; (i) L. Ren, P. Yuan, H. Su, S. Malola, S. Lin, Z. Tang, B. K. Teo, H. Hakkinen, L. Zheng and N. Zheng, J. Am. Chem. Soc., 2017, 139, 13288–13291 CrossRef CAS PubMed; (j) H. Y. Yang, Y. Wang, X. Chen, X. J. Zhao, L. Gu, H. Q. Huang, J. Z. Yan, C. F. Xu, G. Li, J. C. Wu, A. J. Edwards, B. Dittrich, Z. C. Tang, D. D. Wang, L. Lehtovaara, H. Hakkinen and N. F. Zheng, Nat. Commun., 2016, 7, 12809 CrossRef CAS PubMed.
- (a) Y. Wang, Y. Q. Zheng, C. Z. Huang and Y. N. Xia, J. Am. Chem. Soc., 2013, 135, 1941–1951 CrossRef CAS PubMed; (b) M. Zhu, P. Wang, N. Yan, X. Q. Chai, L. Z. He, Y. Zhao, N. Xia, C. H. Yao, J. Li, H. T. Deng, Y. Zhu, Y. Pei and Z. K. Wu, Angew. Chem., Int. Ed., 2018, 57, 4500–4504 CrossRef CAS PubMed; (c) L. Z. He, J. Y. Yuan, N. Xia, L. W. Liao, X. Liu, Z. B. Gan, C. M. Wang, J. L. Yang and Z. K. Wu, J. Am. Chem. Soc., 2018, 140, 3487–3490 CrossRef CAS PubMed.
- (a) A. Tao, P. Sinsermsuksakul and P. D. Yang, Angew. Chem., Int. Ed., 2006, 45, 4597–4601 CrossRef CAS PubMed; (b) M. Tsuji, Y. Maeda, S. Hikino, H. Kumagae, M. Matsunaga, X. L. Tang, R. Matsuo, M. Ogino and P. Jiang, Cryst. Growth Des., 2009, 9, 4700–4705 CrossRef CAS; (c) M. Tsuji, M. Ogino, R. Matsuo, H. Kumagae, S. Hikino, T. Kim and S. H. Yoon, Cryst. Growth Des., 2010, 10, 296–301 CrossRef CAS; (d) M. Tsuji, X. L. Tang, M. Matsunaga, Y. Maeda and M. Watanabe, Cryst. Growth Des., 2010, 10, 5238–5243 CrossRef CAS.
- (a) D. Sun, G.-G. Luo, N. Zhang, R.-B. Huang and L.-S. Zheng, Chem. Commun., 2011, 47, 1461–1463 RSC; (b) Z. Wang, H. F. Su, M. Kurmoo, C. H. Tung, D. Sun and L.-S. Zheng, Nat. Commun., 2018, 9, 2094 CrossRef PubMed.
- R. S. Dhayal, Y.-R. Lin, J.-H. Liao, Y.-J. Chen, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Chem.–Eur. J., 2016, 22, 9943–9947 CrossRef CAS PubMed.
- (a) H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS PubMed; (b) H. Liu, C.-Y. Song, R.-W. Huang, Y. Zhang, H. Xu, M.-J. Li, S.-Q. Zang and G.-G. Gao, Angew. Chem., Int. Ed., 2016, 55, 3699–3703 CrossRef CAS PubMed; (c) S. Li, X.-S. Du, B. Li, J.-Y. Wang, G.-P. Li, G.-G. Gao and S.-Q. Zang, J. Am. Chem. Soc., 2018, 140, 594–597 CrossRef CAS PubMed; (d) R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- W. Liu and H. H. Thorp, Inorg. Chem., 1992, 31, 1585–1588 CrossRef.
- R.-W. Huang, Q.-Q. Xu, H.-L. Lu, X.-K. Guo, S.-Q. Zang, G.-G. Gao, M.-S. Tang and T. C. W. Mak, Nanoscale, 2015, 7, 7151–7154 RSC.
- P. Pyykkö, Chem. Rev., 1997, 97, 597–636 CrossRef.
- (a) H. G. V. Schnering and K. G. Hausler, Rev. Chim. Miner., 1976, 13, 71–81 Search PubMed; (b) W. Beesk, P. G. Jones, H. Rumpel, E. Schwarzmann and G. M. Sheldrick, J. Chem. Soc., Chem. Commun., 1981, 664–665 RSC; (c) C. Linke and M. Jansen, Inorg. Chem., 1994, 33, 2614–2616 CrossRef CAS; (d) M. Jansen and C. Linke, Angew. Chem., Int. Ed. Engl., 1992, 31, 653–654 CrossRef.
- (a) Y. Kikukawa, Y. Kuroda, K. Suzuki, M. Hibino, K. Yamaguchi and N. Mizuno, Chem. Commun., 2013, 49, 376–378 RSC; (b) Z.-Y. Wang, M.-Q. Wang, Y.-L. Li, P. Luo, T.-T. Jia, R.-W. Huang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076 CrossRef CAS PubMed.
- I. Pastoriza-Santos and L. M. Liz-Marzan, Pure Appl. Chem., 2000, 72, 83–90 CAS.
- S. Chen, L. Xiong, S. X. Wang, Z. Y. Ma, S. Jin, H. T. Sheng, Y. Pei and M. Z. Zhu, J. Am. Chem. Soc., 2016, 136, 10754–10757 CrossRef PubMed.
- G. Li, Z. Lei and Q. M. Wang, J. Am. Chem. Soc., 2010, 132, 17678–17679 CrossRef CAS PubMed.
- (a) C. Yang, O. Elbjeirami, C. S. P. Gamage, H. V. R. Dias and M. A. Omary, Chem. Commun., 2011, 47, 7434–7436 RSC; (b) C.-M. Che, M.-C. Tse, M. C. W. Chan, K.-K. Cheung, D. L. Phillips and K.-H. Leung, J. Am. Chem. Soc., 2000, 122, 2464–2468 CrossRef CAS; (c) D. Sun, L. L. Zhang, H. F. Lu, S. Y. Feng and D. F. Sun, Dalton Trans., 2013, 42, 3528–3532 RSC; (d) J. Jin, W.-Y. Wang, Y.-H. Liu, H.-W. Hou and Y.-T. Fan, Chem. Commun., 2011, 47, 7461–7463 RSC.
- V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589–7728 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: IR, 13C NMR, CV, UV, EDS, PXRD and luminescence decay curve, and details of the data collection and structure refinements, and crystal data. CCDC 1850394 and 1850395 for SD/Ag80a and SD/Ag80b. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc03396j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |