Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stereoselective organocatalyzed glycosylations – thiouracil, thioureas and monothiophthalimide act as Brønsted acid catalysts at low loadings†
G. A.
Bradshaw‡
,
A. C.
Colgan‡
 ,
N. P.
Allen
,
I.
Pongener
,
N. P.
Allen
,
I.
Pongener
 ,
M. B.
Boland
,
Y.
Ortin
and
E. M.
McGarrigle
,
M. B.
Boland
,
Y.
Ortin
and
E. M.
McGarrigle
 *
*
Centre for Synthesis & Chemical Biology, UCD School of Chemistry, University College Dublin, Belfield, Dublin 4, Ireland. E-mail: eoghan.mcgarrigle@ucd.ie
First published on 22nd October 2018
Abstract
Thiouracil catalyzes stereoselective glycosylations with galactals in loadings as low as 0.1 mol%. It is proposed that in these glycosylations thiouracil, monothiophthalimide, and the previously reported catalyst, Schreiner's thiourea, do not operate via a double H-bonding mechanism but rather by Brønsted acid/base catalysis. In addition to the synthesis of 2-deoxyglycosides and glycoconjugates, we report the first organocatalytic synthesis of 1,1′-linked trehalose-type sugars.
Introduction
Thioureas have found many applications as organocatalysts and it has become generally accepted that they activate their substrates through double-hydrogen bonding.1 Prominent examples include hydrogen bonding to anions and carbonyl groups, thus creating more reactive dienophiles, electrophiles or nucleophiles (Fig. 1). Herein we present evidence that thioureas can activate molecules in at least one other way and describe how this opens the way for new organocatalyst designs, with specific applications in glycosylations detailed.2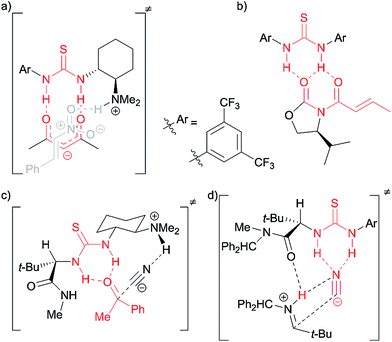 | ||
| Fig. 1 Selected examples of proposed interactions in thiourea catalysis via double H-bonding: (a) enolate binding in Pápai's model for enolate addition to nitroalkenes using Takemoto's catalyst;1d,e (b) proposed complex between Schreiner's catalyst and an N-acyloxazolidinone in Diels–Alder reaction;1f (c) ketone binding in Jacobsen's ketone cyanosilylation;1g (d) cyanide binding in Jacobsen's enantioselective Strecker reaction.1h | ||
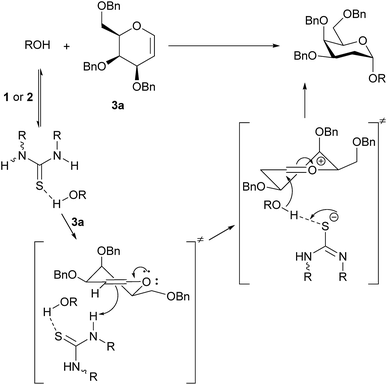
In 2012 Galan, McGarrigle and co-workers reported3 that use of Schreiner's thiourea1f,41 as a catalyst gave high α-selectivity in glycosylations with galactals to form 2-deoxydisaccharides (Scheme 1a).5 Based on experimental evidence, and calculations by Schreiner6 on the related THP-protection of alcohols (Scheme 1b), an ‘oxyanion hole’-type activation was previously proposed whereby the catalyst activated the alcohol by forming two hydrogen bonds to the oxygen, making the alcohol proton more acidic and thus catalysing C–H bond formation to C-2 of the galactal (Scheme 1c). A re-examination of this mechanistic proposal was prompted by recent work on the THP-protection of alcohols by Varga, Pápai and co-workers suggesting a Brønsted acid mode of action (see below),2 challenging the prevailing notion of thiourea catalysts acting as double H-bond donors. We now report the development of a new glycosylation catalyst that is >600-fold cheaper than 1 and is active at loadings as low as 0.1 mol% in the synthesis of disaccharides, glycoconjugates, and trehalose-type sugars. Our results support a Brønsted acid/base mechanism for our new catalysts and for Schreiner's thiourea in these glycosylations.
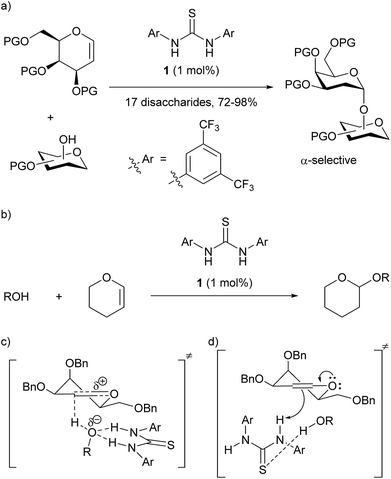 | ||
| Scheme 1 (a) Galan and McGarrigle's thiourea-catalyzed glycosylation reaction;3 (b) Schreiner's THP-protection of alcohols;6 (c) original proposal with catalyst acting as a double hydrogen-bond donor to alcohol;3 (d) analogue of Pápai2 proposal with catalyst acting as a Brønsted acid and accepting a H-bond from the alcohol. | ||
Results and discussion
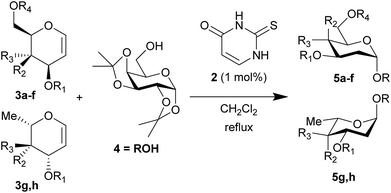
In contrast to the generally accepted mode of action for thiourea catalysts as H-bond donors, calculations by Varga, Pápai and co-workers favoured a Brønsted acid mode of action in the THP-protection of alcohols.2 An analogous transition state for our glycosylation reaction is represented in Scheme 1d. In contrast to Scheme 1c, where the N–H bonds need to be in a parallel orientation so as to enable the archetypal double-H-bonding, the alternative proposed transition state (Scheme 1d) doesn't require this parallel orientation. If such a mode of action were operative in our glycosylations, alternative catalysts designs would be feasible.To test this new hypothesis, we first examined whether 2-thiouracil 2 was capable of catalysing the reaction between selected galactals 37 and diacetone galactose 4 (Table 1). Indeed, using just 1 mol% of thiouracil we found that high yields of disaccharides could be obtained with excellent α-selectivity.7 As 2-thiouracil is >600 times cheaper than Schreiner's catalyst, this is a significant improvement on our previously reported system.8 In fact not all of the thiouracil dissolves at this loading but it wasn't convenient to weigh out smaller amounts of catalyst, as on a ca. 0.5 mmol scale only 0.5 mg catalyst was used; we estimated the amount of dissolved catalyst to be <0.25 mol% under these conditions (see ESI†). In fact on larger scale the catalyst loading could be lowered further (vide infra). Galactals 3a–c worked well, as they had with thiourea 1 as catalyst. Galactal 3d was sluggish but gave a good yield when the reaction was carried out in 2-methyl-THF which allowed a higher reaction temperature.9 Per-acetylated 3e was relatively unreactive, as had been observed with 1. Per-benzylated glucal 3f had been problematic previously, so the yield of up to 46% represented progress.3 We didn't pursue this further due to the formation of Ferrier-rearrangement products which were inseparable from the desired glycoside.10 Rhamnal 3g also gave an inseparable mixture of Ferrier products and desired disaccharide (Entry 10). McGarrigle, Galan and co-workers previously reported a protecting group strategy for accessing α-linked disaccharides from glucal and rhamnal.10a Fucal 3h gave a yield of 52% of α-5h (entry 11) although in this case a small amount of β-5h was also produced. We propose that the greater reactivity of galactals might be due to electrostatic stabilisation of the oxacarbenium ion by the 4-substituent.11 These results are consistent with a common mechanism being operative in thiouracil and thiourea-catalyzed glycosylations.
| Entry | R1 | R2 | R3 | R4 | t (h) | Yield (%)b | α/βc | |
|---|---|---|---|---|---|---|---|---|
| a 1.2 equiv. of the glycal was used. b Yield of Isolated product. c Determined by 1H NMR. d Solvent: 2-methyl-THF (reflux = 83 °C). e Isolated with 10–20% inseparable impurities due to Ferrier rearrangement (see ESI for details). f Isolated with ca. 10% hydration impurity (vide infra). g Ratio prior to chromatography in parentheses. | ||||||||
| 1 | a | Bn | OBn | H | Bn | 18 | 95 | α |
| 2 | b | Allyl | OAllyl | “ | Allyl | 18 | 82 | α |
| 3 | c | Bn | OBn | “ | Ac | 18 | 85 | α |
| 4 | d | TBS | OTBS | “ | TBS | 48 | 11 | α |
| 5 | “ | “ | “ | “ | 18 | 68d | α | |
| 6 | e | Ac | OAc | “ | Ac | 18 | 0 | — |
| 7 | “ | “ | “ | “ | 18 | 7c,d,e | β | |
| 8 | f | Bn | H | OBn | Bn | 18 | 46e | 5/1 |
| 9 | “ | “ | “ | “ | 18 | 39d,e | 4/1 | |
| 10 | g | Bn | “ | OBn | — | 40 | 40e | 4/1 |
| 11 | h | Bn | OBn | H | — | 18 | 52f | α (9/1)g |
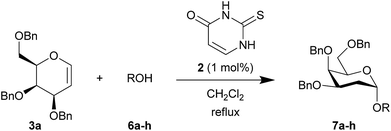
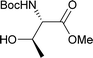
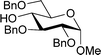
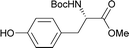
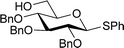
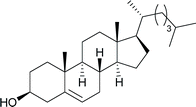
Table 2 shows the scope of the reaction with respect to the acceptor.12 The reaction tolerates primary and secondary alcohols at any position on the ring (entries 1–4). In Schreiner's original report6 on thiourea-catalyzed THP-protection, it was noted that an amine-containing solid support was not compatible with the reaction. Later studies establishing the pKa of 1 as 8.5 (DMSO) suggests that this might be due to deprotonation of the catalyst by amines.13a,b Catalyst 2 has a pKa = 7.75 (H2O).13c Thus we predicted that appropriate protection group choice might enable us to use amino-containing substrates. Indeed, Boc-protected amino acids 6e–g were glycosylated in excellent yields (entries 5–7). Glycoconjugates 7e and 7f thus give access to the 2-deoxy analogues of mucin-type motifs. Entry 7 shows that the reaction also tolerates phenols (although rare, glycosylated tyrosines have been found in nature).14 Finally, cholesterol also proved a viable substrate (entry 8), opening the way to glycoconjugate synthesis beyond amino acids.
| Yieldb (%), α/βc | Yieldb (%), α/βc | ||||
|---|---|---|---|---|---|
| a 1.2 equiv. of the galactal was used and 0.83 M acceptor in CH2Cl2 except where stated. b Yield of Isolated product. c Determined by 1H NMR. d 0.35 M solution w.r.t. acceptor instead of 0.83 M. e 2 equiv. of galactal w.r.t. acceptor was used. f With 17% α,α-9a as an impurity. g Solvent: 2-methyl-THF (reflux = 83 °C). | |||||
| 1 |
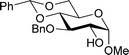
|
a, 84%,d,e α | 5 |
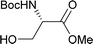
|
e, 94%, α |
| 2 |
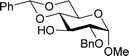
|
b, 89%, α | 6 |
|
f, 91%,g α |
| 3 |
|
c, 55%,f α | 7 |
|
g, 94%, α |
| 4 |
|
d, 82%, α | 8 |
|
h, 98%,d α |
1,1′-Linked sugars and glycosyl sulfonamides
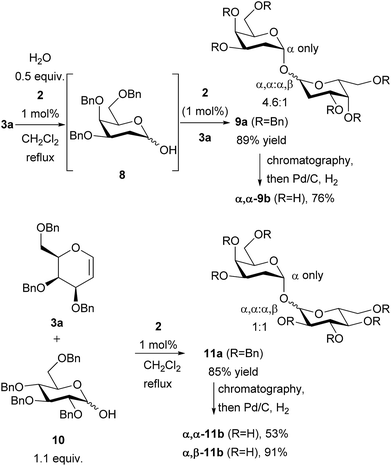
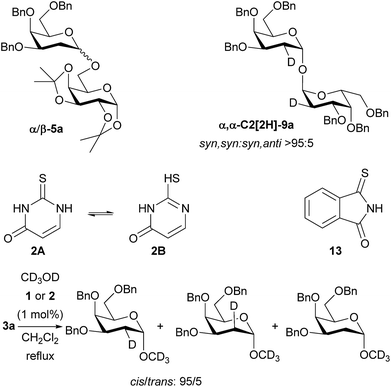
It was noted that the presence of water in the reaction of 3a and 4 was detrimental to yields, and led to the formation of hydrated side-products. This was turned to advantage by replacing the alcohol with water, enabling the synthesis of 1,1′-linked trehalose-like dimer 9a (Scheme 2). Sugars of this type are present in the cell wall of mycobacteria including tuberculosis (TB).15 The dimer 9a was obtained as a mixture of α,α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α,β 4.6
α,β 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. No β,β-dimer was detected. No reaction took place in the absence of catalyst, thus this dimerization is also catalyzed by thiouracil. The stereochemical outcome can be rationalized as follows: following addition of water to galactal 3a the resulting hemiacetal 8 rapidly epimerizes, but addition of 8 to a second galactal occurs with excellent α-selectivity. We suspect, but cannot prove, that the initial addition of water to the galactal is α-selective. The proposed pathway is supported by the stereoselective addition of hemiacetal 10 to galactal 3a giving a mixture of α,α
1. No β,β-dimer was detected. No reaction took place in the absence of catalyst, thus this dimerization is also catalyzed by thiouracil. The stereochemical outcome can be rationalized as follows: following addition of water to galactal 3a the resulting hemiacetal 8 rapidly epimerizes, but addition of 8 to a second galactal occurs with excellent α-selectivity. We suspect, but cannot prove, that the initial addition of water to the galactal is α-selective. The proposed pathway is supported by the stereoselective addition of hemiacetal 10 to galactal 3a giving a mixture of α,α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α,β 1
α,β 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The use of hemiacetals such as 10 thus demonstrates that heterodimeric trehalose-type structures can also be accessed through this methodology. The epimers were separable by chromatography and the disaccharides were deprotected by hydrogenation over Pd/C to give 9b and 11b. Thus the method gives easy access to symmetrical and unsymmetrical 1,1′-linked sugars. Compound 9b is an inhibitor of mycolyltransesterase enzymes Ag85A, Ag85B and Ag85C which serve as essential mediators of cell envelope function and biogenesis in Mycobacterium tuberculosis,15b thus this method may enable access to compounds useful for studying/fighting TB. Further efforts in this regard are ongoing in our laboratory.
1. The use of hemiacetals such as 10 thus demonstrates that heterodimeric trehalose-type structures can also be accessed through this methodology. The epimers were separable by chromatography and the disaccharides were deprotected by hydrogenation over Pd/C to give 9b and 11b. Thus the method gives easy access to symmetrical and unsymmetrical 1,1′-linked sugars. Compound 9b is an inhibitor of mycolyltransesterase enzymes Ag85A, Ag85B and Ag85C which serve as essential mediators of cell envelope function and biogenesis in Mycobacterium tuberculosis,15b thus this method may enable access to compounds useful for studying/fighting TB. Further efforts in this regard are ongoing in our laboratory.
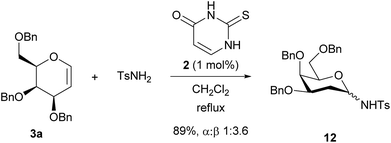
Glycosyl sulfonamides have been shown to inhibit growth of tumour cell lines. Reaction with TsNH2 gave the glycosylated product 12 in 89% yield as a 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of β
1 mixture of β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α anomers (Scheme 3), showing that N-glycosylation is also possible.16 The stereochemical outcome may arise from epimerisation of α-12 to the β-product, indeed the ratio we observe is similar to that reported by Toste.16a,17
α anomers (Scheme 3), showing that N-glycosylation is also possible.16 The stereochemical outcome may arise from epimerisation of α-12 to the β-product, indeed the ratio we observe is similar to that reported by Toste.16a,17
Exploration of mechanism
With regard to the mechanism, we propose that the catalyst is operating via an acid/base-type mechanism, rather than through a double-hydrogen bonding mechanism, as we originally proposed. The reactions to form 2-deoxyglycosides, 1,1′-linked sugars and glycosylated sulfonamides all require catalyst – no reaction is observed in the absence of catalyst. When a mixture of α/β-5a was resubjected to the reaction conditions the α/β ratio remained constant (Scheme 4). Crossover tests also proved negative (see ESI† for details). Thus, the high α-selectivity observed is obtained kinetically, and is not the result of post-reaction anomerisation, except in the case of 1,1-linked disaccharides where prolonged reaction times increased the amount of α,α-dimer 9a. The use of D2O in place of H2O in the formation of α,α-9a showed stereoselective (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) syn-addition of deuterium and oxygen and gives easy access to C2-deuterated α,α-9a. Glycosylation of CD3OD resulted in a cis
5) syn-addition of deuterium and oxygen and gives easy access to C2-deuterated α,α-9a. Glycosylation of CD3OD resulted in a cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio of 95
trans ratio of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for both catalysts 1 and 2 (Scheme 4). This is more cis-selective than related reactions of dihydropyrans studied by Varga and Pápai,2a and Zimmerman and Nagorny,18 but goes against an asynchronous concerted mechanism.6 It might be proposed that 2 could be present in solution as the tautomer 2B, and thus fulfil the role of a double H-bonding catalyst. To counter this, monothiophthalimide 13 was synthesized and shown to be a viable stereoselective catalyst at 1 mol% under our standard glycosylation reaction conditions giving 84% of α-5a (Scheme 4). At the very least, this demonstrates the existence of a catalytic pathway that does not require a double H-bonding catalyst, and given the calculations of Varga and Pápai, we contend that it is most likely that thiourea 1, thiouracil 2, and monothiophthalimide 13 all operate via a common mechanism (we are not aware of any other reports of monothioimides as organocatalysts).
5 for both catalysts 1 and 2 (Scheme 4). This is more cis-selective than related reactions of dihydropyrans studied by Varga and Pápai,2a and Zimmerman and Nagorny,18 but goes against an asynchronous concerted mechanism.6 It might be proposed that 2 could be present in solution as the tautomer 2B, and thus fulfil the role of a double H-bonding catalyst. To counter this, monothiophthalimide 13 was synthesized and shown to be a viable stereoselective catalyst at 1 mol% under our standard glycosylation reaction conditions giving 84% of α-5a (Scheme 4). At the very least, this demonstrates the existence of a catalytic pathway that does not require a double H-bonding catalyst, and given the calculations of Varga and Pápai, we contend that it is most likely that thiourea 1, thiouracil 2, and monothiophthalimide 13 all operate via a common mechanism (we are not aware of any other reports of monothioimides as organocatalysts).
Monothiophthalimide 13 has greater solubility in CH2Cl2 than thiouracil, thus enabling NMR studies. The chemical shift of the NH proton of 13 changed by 0.4 ppm when alcohol 4 (3.7 equiv.) was added to 13, consistent with complex formation (see ESI† for details). In the context of our current proposal we can rationalize these results as follows (Scheme 5): we propose that the catalyst first accepts a H-bond from the alcohol substrate making it a stronger acid. We propose a H-bond to sulfur as uracil does not catalyse the reaction, but several structures are conceivable.19 It is this catalyst–alcohol complex that interacts with the galactal and undergoes proton transfer. Secondly, the anion formed is able to deprotonate the alcohol in the second step of the reaction as C–O bond formation occurs. We note that we had previously discounted3 a ‘simple’ acid-catalyzed mechanism for this reaction as neither Meldrum's acid (pKa = 7.3 (DMSO)) nor Et3N·HCl (pKa = 9.0 (DMSO)) catalyzed the reaction.20 It is clear that the chloride anion that would result from Et3N·HCl would not be sufficiently basic for this role. We suspect that the same is true of the conjugate base of Meldrum's acid if complexation with alcohol made it a strong enough acid for the first step. The α-stereoselectivity and syn-addition of H/D and O–R are consistent with the catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ROH complex favouring approach from the bottom face and the C–O bond forming before the ion pair has time to rearrange.
ROH complex favouring approach from the bottom face and the C–O bond forming before the ion pair has time to rearrange.
Catalyst loading and gram-scale reactions
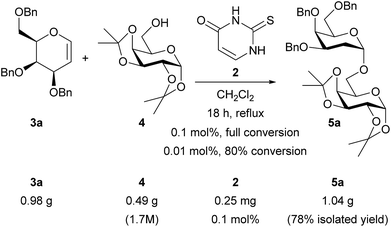
We conducted larger scale experiments in order to explore the catalyst loadings and demonstrate practicality (Scheme 6). Thiouracil 2 was active at loadings down to 0.01 mol% but for reproducible reaction times we chose 0.1 mol% and carried out a 1 g scale reaction.21 The concentration was found to be important on bigger scale (1.7 M w.r.t. acceptor; on smaller scale the initial concentration was 0.8 M but some solvent loss occurred during the experiment so the final concentration was higher – see ESI†). Under these optimized conditions just 0.25 mg of catalyst was required to obtain an isolated yield of 1 g (78%) of 5a.Conclusions
In summary, we have shown that thiouracil can replace Schreiner's thiourea in glycosylation reactions. Thus 2-thiouracil is a simple, cheap and effective catalyst for α-selective glycosylation with galactals of monosaccharides, steroids, and amino acids at loadings as low as 0.1 mol%. The method also enables the synthesis of symmetrical and unsymmetrical 1,1′-linked disaccharides including examples relevant to M. tuberculosis. Further work in this area is underway in our laboratories.Furthermore, in line with Varga, Pápai and co-worker's publication on thiourea-catalyzed THP-protection, we are supporting a mechanism whereby the thiourea or thiouracil engages via general acid/base catalysis rather than acting as a double-hydrogen bond donor. When considering how a thiourea catalyst is activating substrates it is necessary to consider possibilities beyond double H-bonding. These findings should lead to new opportunities to catalyse reactions and to new organocatalyst designs beyond thioureas. We will report in due course on our own efforts in this regard.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
E. M. M. and G. A. B. thank Science Foundation Ireland & the Marie-Curie Action COFUND (11/SIRG/B2154). A. C. C. thanks the Irish Research Council (GOIPG/2013/301), N. P. A. thanks UCD for a Research Demonstratorship. E. M. M. and I. P. thank Science Foundation Ireland (15/CDA/3625). We thank members of EU COST action CM0905 (organocatalysis) and, in particular, Imre Pápai for helpful discussions. We are grateful to Prof Declan Gilheany (UCD) and Dr Kirill Nitikin (UCD) for helpful discussions also.Notes and references
- (a) G. Jakab and P. R. Schreiner in Comprehensive Enantioselective Organocatalysis, ed. P. Dalko, Wiley-VCH, Weinheim, 2013, ch. 12, vol. 2 Search PubMed; (b) K. Hof, K. M. Lippert and P. R. Schreiner in Asymmetric Organocatalysis, ed. K. Maruoka), Thieme, Stuttgart, 2012, ch. 2.2.4, vol. 2 Search PubMed; (c) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713 CrossRef CAS PubMed; (d) A. Hamza, G. Schubert, T. Soós and I. Pápai, J. Am. Chem. Soc., 2006, 128, 13151 CrossRef CAS PubMed; (e) T. Okino, Y. Hoashi and Y. Takemoto, J. Am. Chem. Soc., 2003, 125, 12672 CrossRef CAS PubMed; (f) P. R. Schreiner and A. Wittkopp, Org. Lett., 2002, 4, 217 CrossRef CAS PubMed; (g) S. J. Zuend and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 15872 CrossRef CAS PubMed; (h) S. J. Zuend and E. N. Jacobsen, J. Am. Chem. Soc., 2009, 131, 15358 CrossRef CAS PubMed.
- (a) Á. Madarász, Z. Dósa, S. Varga, T. Soós, A. Csámpai and I. Pápai, ACS Catal., 2016, 6, 4379 CrossRef; (b) Á. Madarász, Z. Dósa, S. Varga, T. Soós, A. Csámpai, and I. Pápai, oral presentation, EU Cost Action CM0905 Meeting, Palermo, May 2014 Search PubMed. A thiourea catalyst with one NH was recently described: (c) P. Jovanovic, M. Petkovic, M. Simic, B. Ivkovic and V. Savic, Org. Biomol. Chem., 2016, 14, 6712 RSC. See also (d) A. Supady, S. Hecht and C. Baldau, Org. Lett., 2017, 19, 4199 CrossRef CAS PubMed. For proposed action of thioureas as Lewis bases see: (e) C. K. Tan, F. Chen and Y.-Y. Yeung, Tetrahedron Lett., 2011, 52, 4892 CrossRef CAS; (f) C. B. Tripathi and S. Mukherjee, J. Org. Chem., 2012, 77, 1592 CrossRef CAS PubMed; (g) S. E. Denmark and M. T. Burk, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20655 CrossRef CAS PubMed; (h) Y. Kobayashi, Y. Nakatsuji, S. Li, S. Tsukuki and Y. Takemoto, Angew. Chem., Int. Ed., 2018, 57, 3646 CrossRef CAS PubMed.
- E. I. Balmond, D. M. Coe, M. C. Galan and E. M. McGarrigle, Angew. Chem., Int. Ed., 2012, 51, 9152 CrossRef CAS PubMed.
- (a) E. Kuehle, L. Eue and O. Bayer, BE 613427, 1962, CA59:8698; (b) E. I. Balmond, M. C. Galan, and E. M. McGarrigle, N,N′-Bis[3,5-bis(trifluoromethyl)phenyl]-thiourea, in Encyclopedia of Reagents for Organic Synthesis, online, ed. L. A. Paquette, D. Crich, P. L. Fuchs, and G. A. Molander, John Wiley & Sons Ltd., 2014, DOI:10.1002/047084289X.rn01642; (c) Z. Zhang, Z. Bao and H. Xing, Org. Biomol. Chem., 2014, 12, 3151 RSC.
- For leading references on organocatalytic glycosylations see: (a) D. J. Cox, M. D. Smith and A. J. Fairbanks, Org. Lett., 2010, 12, 1452 CrossRef CAS PubMed; (b) E. I. Balmond, M. C. Galan and E. M. McGarrigle, Synlett, 2013, 24, 2335 CrossRef CAS; (c) Y. Q. Geng, A. Kumar, H. M. Faidallah, H. A. Albar, I. A. Mhkalid and R. R. Schmidt, Angew. Chem., Int. Ed., 2013, 52, 10089 CrossRef CAS PubMed; (d) S. Das, D. Pekel, J. M. Neudorfl and A. Berkessel, Angew. Chem., Int. Ed., 2015, 54, 12479 CrossRef CAS PubMed; (e) S. Schmalisch and R. Mahrwald, Org. Lett., 2013, 15, 5854 CrossRef CAS PubMed; (f) L. Sun, X. Wu, D.-C. Xiong and X.-S. Ye, Angew. Chem., Int. Ed., 2016, 55, 8041 CrossRef CAS PubMed; (g) M. Tanaka, J. Nashida, D. Takahashi and K. Toshima, Org. Lett., 2016, 18, 2288 CrossRef CAS PubMed; (h) C. Gouliaras, D. Lee, L. Chan and M. S. Taylor, J. Am. Chem. Soc., 2011, 133, 13926 CrossRef CAS PubMed; (i) S. Medina, M. Harper, E. I. Balmond, S. Miranda, G. Crisenza, D. M. Coe, E. M. McGarrigle and M. C. Galan, Org. Lett., 2016, 18, 4222 CrossRef CAS PubMed; (j) Y. Park, K. C. Harper, N. Kuhl, E. E. Kwan, R. Y. Liu and E. N. Jacobsen, Science, 2017, 355, 162 CrossRef CAS PubMed; (k) R. Williams and M. C. Galan, Eur. J. Org. Chem., 2017, 6247 CrossRef CAS; (l) J.-L. Liu, Y.-T. Zhang, H.-F. Liu, L. Zhou and J. Chen, Org. Lett., 2017, 19, 5272 CrossRef CAS PubMed; (m) J. Lee, A. Borovika, Y. Khomutnyk and P. Nagorny, Chem. Commun., 2017, 53, 8976 RSC; (n) C. Palo-Nieto, A. Sau, R. Williams and M. C. Galan, J. Org. Chem., 2017, 82, 407 CrossRef CAS PubMed; (o) T. Kimura, T. Eto, D. Takahashi and K. Toshima, Org. Lett., 2016, 18, 3190 CrossRef CAS PubMed; (p) M. Shaw, Y. Kumar, R. Thakur and A. Kumar, Beilstein J. Org. Chem., 2017, 13, 2385 CrossRef CAS PubMed; (q) Y. Singh, T. Wang, S. A. Geringer, K. J. Stine and A. V. Demchenko, J. Org. Chem., 2018, 83, 374 CrossRef CAS PubMed; (r) J.-H. Tay, A. J. Argüelles, M. D. DeMars, P. M. Zimmerman, D. H. Sherman and P. Nagorny, J. Am. Chem. Soc., 2017, 139, 8570 CrossRef CAS PubMed.
- M. Kotke and P. R. Schreiner, Synthesis, 2007, 779 CAS.
- (a) E. Durantie, C. Bucher and R. Gilmour, Chem.–Eur. J., 2012, 18, 8208 CrossRef CAS PubMed; (b) We note that care must be taken in the synthesis of donor 3a to avoid an impurity that can act as a catalyst poison: A. C. Colgan, H. Müller-Bunz and E. M. McGarrigle, J. Org. Chem., 2016, 81, 11394 CrossRef CAS PubMed.
- Price/mol on www.sigmaaldrich.com and www.tcichemicals.com, accessed 22 June 2018.
- CH2Cl2 was identified as the best solvent from a solvent screen but the reaction also proceeded in toluene, acetonitrile, THF and 2-MeTHF. 2-Methyl-THF is suggested to be more sustainable (bio-based), and less toxic than CH2Cl2: (a) D. F. Aycock, Org. Process Res. Dev., 2007, 11, 156 CrossRef CAS; (b) V. Antonucci, J. Coleman, J. B. Ferry, N. Johnson, M. Mathe, J. P. Scott and J. Xu, Org. Process Res. Dev., 2011, 15, 939 CrossRef CAS; (c) C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879 RSC; (d) V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369 CrossRef CAS PubMed.
- Other methods for accessing 2-deoxyglycosides see ref. 5d, j and n and: (a) E. I. Balmond, D. M. Coe, R. W. Alder, E. M. McGarrigle and M. C. Galan, Angew. Chem., Int. Ed., 2014, 53, 8190 CrossRef CAS PubMed; (b) J. M. Nogueira, M. Bylsma, D. K. Bright and C. S. Bennett, Angew. Chem., Int. Ed., 2016, 55, 10088 CrossRef CAS PubMed; (c) D. Zhu, K. N. Baryal, S. Adhikari and J. Zhu, J. Am. Chem. Soc., 2014, 136, 3172 CrossRef CAS PubMed; (d) A. Borovika and P. Nagorny, J. Carbohydr. Chem., 2012, 31, 255 CrossRef CAS; (e) H. Wang, J. Tao, X. Cai, W. Chen, Y. Zhao, Y. Xu, W. Yao, J. Zeng and Q. Wan, Chem.–Eur. J., 2014, 17319 CrossRef CAS PubMed; (f) V. P. Verma and C.-C. Wang, Chem.–Eur. J., 2013, 19, 846 CrossRef CAS PubMed; (g) G. Zhao and T. Wang, Angew. Chem., Int. Ed., 2018, 57, 6120 CrossRef CAS PubMed; (h) T. M. Beale, P. J. Moon and M. S. Taylor, Org. Lett., 2014, 16, 3604 CrossRef CAS PubMed; (i) C. H. Marzabadi and R. W. Franck, Tetrahedron, 2000, 56, 8385 CrossRef CAS; (j) D. Hou and T. L. Lowary, Carbohydr. Res., 2009, 344, 1911 CrossRef CAS PubMed; (k) C. S. Bennett and M. C. Galan, Chem. Rev., 2018, 118, 7931 CrossRef CAS PubMed; (l) D.-M. Yang, Y. Chen, R. P. Sweeney, T. L. Lowary and X.-Y. Liang, Org. Lett., 2018, 20, 2287 CrossRef CAS PubMed.
- (a) H. H. Jensen and M. Bols, Acc. Chem. Res., 2006, 39, 259 CrossRef CAS PubMed; (b) A. Garcia, J. R. Sanzone and K. A. Woerpel, Angew. Chem., Int. Ed., 2015, 127, 12255 CrossRef; (c) A. Garcia, D. A. L. Otte, W. A. Salamant, J. R. Sanzone and K. A. Woerpel, Angew. Chem., Int. Ed., 2015, 54, 3061 CrossRef CAS PubMed.
- (a) A. Roën, J. I. Padrón and J. T. Vázquez, J. Org. Chem., 2003, 68, 4615 CrossRef PubMed; (b) M. Matwiejuk and J. Thiem, Chem. Commun., 2011, 47, 8379 RSC; (c) P. J. Garegg, T. Iversen and S. Oscarson, Carbohydr. Res., 1976, 50, C12–C14 CrossRef CAS; (d) L. K. Mydock, M. N. Kamat and A. V. Demchenko, Org. Lett., 2011, 13, 2928 CrossRef CAS PubMed; (e) T. Ogawa and T. Kaburagi, Carbohydr. Res., 1982, 103, 53 CrossRef CAS; (f) C.-C. Wang, S. S. Kulkarni, J.-C. Lee, S.-Y. Luo and S.-C. Hung, Nat. Protoc., 2008, 3, 97 CrossRef CAS PubMed; (g) P. J. Garegg, H. Hultberg and S. Wallin, Carbohydr. Res., 1982, 108, 97 CrossRef; (h) Z. Zhang, B. Yua and R. R. Schmidt, Synthesis, 2006, 8, 1301 CrossRef; (i) K. Bao, A. Fan, Y. Dai, L. Zhang, W. Zhang, M. Cheng and X. Yao, Org. Biomol. Chem., 2009, 7, 5084 RSC.
- (a) X. Li, H. Deng, S. Luo and J. P. Cheng, Eur. J. Org. Chem., 2008, 4350 CrossRef CAS; (b) G. Jakab, C. Tancon, Z. Zhang, K. M. Lippert and P. R. Schreiner, Org. Lett., 2012, 14, 1724 CrossRef CAS PubMed; (c) H. N. Christensen, J. Biol. Chem., 1945, 160, 425 CAS.
- (a) J. Peters, S. Rudolf, H. Oschkinat, R. Mengele, M. Sumper, J. Kellermann, F. Lottspeich and W. Baumeister, Biol. Chem. Hoppe-Seyler, 1992, 373, 171 CrossRef CAS PubMed; (b) A. Halim, G. Brinkmalm, U. Ruetschi, A. Westman-Brinkmalm, E. Portelius, H. Zetterberg, K. Blennow, G. Larson and J. Nilsson, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 11848 CrossRef CAS PubMed; (c) C. Steentoft, S. Y Vakhrushev, M. B. Vester-Christensen, K. T.-B. G. Schjoldager, Y. Kong, E. P. Bennett, U. Mandel, H. Wandall, S. B Levery and H. Clausen, Nat. Methods, 2011, 8, 977 CrossRef CAS PubMed; (d) P. O. Larsen, E. Pedersen, H. Sorensen and P. Sorup, Phytochemistry, 1973, 12, 2243 CrossRef CAS; (e) G. Yang, Q. Wang, X. Luo, J. Zhang and J. Tang, Glycoconjugate J., 2012, 29, 453 CrossRef CAS PubMed.
- (a) K. J. Welsh, R. L. Hunter and J. K. Actor, Tuberculosis, 2013, 93, S3–S9 CrossRef CAS PubMed; (b) K. M. Backus, H. l. Boshoff, C. S. Barry, O. Boutureira, M. K. Patel, F. D'Hooge, S. S. Lee, L. E. Via, K. Tahlan, C. E. Barry III and B. G. Davis, Nat. Chem. Biol., 2011, 7, 228 CrossRef CAS PubMed; (c) H. N. Foley, J. A. Stewart, H. W. Kavunja, S. R. Rundell and B. M. Swarts, Angew. Chem., Int. Ed., 2016, 55, 2053 CrossRef CAS PubMed; (d) M. Kamariza, P. Shieh, C. S. Ealand, J. S. Peters, B. Chu, F. P. Rodriguez-Rivera, M. R. Babu Sait, W. V. Treuren, N. Martinson, R. Kalscheuer, B. D. Kana and C. R. Bertozzi, Sci. Transl. Med., 2018, 10, eaam6310 CrossRef PubMed.
- (a) B. D. Sherry, R. N. Loy and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 4510 CrossRef CAS PubMed; (b) P. A. Colinas and R. D. Bravo, Org. Lett., 2003, 5, 4509 CrossRef CAS PubMed.
- We thank a referee for this suggestion. A sample of 12 (α
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β 1
β 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) did not show significant epimerisation under our reaction conditions but we were unable to obtain an α-enriched sample to carry out a similar test on, and to rule out a decomposition pathway for α-12 that would lead to β-enriched product..
9) did not show significant epimerisation under our reaction conditions but we were unable to obtain an α-enriched sample to carry out a similar test on, and to rule out a decomposition pathway for α-12 that would lead to β-enriched product.. - Y. Y. Khomutnyk, A. J. Argüelles, G. A. Winschel, Z. Sun, P. Zimmerman and P. Nagorny, J. Am. Chem. Soc., 2016, 138, 444 CrossRef CAS PubMed.
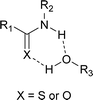
- H-bonds from NH to the alkoxy fragment may also be present. No cross-peak for the NH was found in a HMBC NMR spectrum. We also considered whether a thioglycoside intermediate could arise from the reaction of catalyst with galactal but NMR studies failed to detect such a species even with excess 13 over 3a (see ESI† for details)
 .
. - (a) I. M. Kolthoff, M. K. Chantoon and S. Bhowmik, J. Am. Chem. Soc., 1968, 90, 23 CrossRef CAS; (b) E. M. Arnett, S. G. Maroldo, S. L. Schilling and J. A. Harrelson, J. Am. Chem. Soc., 1984, 106, 6759 CrossRef CAS.
- For other examples of low loading organocatalysts see: ref. 6 and: (a) F. Giacalone, M. Gruttadauria, P. Agrigento and R. Noto, Chem. Soc. Rev., 2012, 41, 2406 RSC; (b) D. Stevanovicć, G. Bertuzzi, A. Mazzanti, M. Fochi and L. Bernardi, Adv. Synth. Catal., 2018, 360, 893 CrossRef; (c) X. Wu, L. Xue, D. Li, S. Jia, J. Ao, J. Deng and H. Yan, Angew. Chem., Int. Ed., 2017, 56, 13722 CrossRef CAS PubMed; (d) D. Bécart, V. Diemer, A. Salaün, M. Oiarbide, Y. R. Nelli, B. Kauffmann, L. Fischer, C. Palomo and G. Guichard, J. Am. Chem. Soc., 2017, 139, 12524 CrossRef PubMed; (e) M. Biedrzycki, A. Kasztelan and P. Kwiatkowski, ChemCatChem, 2017, 9, 2453 CrossRef CAS; (f) X. Companyó and J. Bures, J. Am. Chem. Soc., 2017, 139, 8432 CrossRef PubMed; (g) J.-L. Li, L. Fu, J. Wu, K.-C. Yang, Q.-Z. Li, X.-J. Gou, C. Peng, B. Han and X.-D. Shen, Chem. Commun., 2017, 53, 6875 RSC; (h) M. Yanagi, A. Imayoshi, Y. Ueda, T. Furuta and T. Kawabata, Org. Lett., 2017, 19, 3099 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterisation and copies of NMR spectra. See DOI: 10.1039/c8sc02788a |
| ‡ G. A. B. and A. C. C. contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |