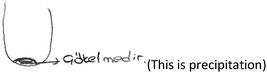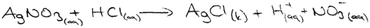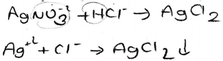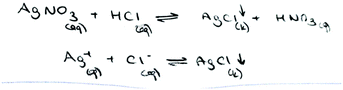Exploring prospective chemistry teachers’ perceptions of precipitation, conception of precipitation reactions and visualization of the sub-microscopic level of precipitation reactions
Canan
Nakiboğlu
 *a and
Nuri
Nakiboğlu
*a and
Nuri
Nakiboğlu
 b
b
aBalıkesir University, Necatibey Education Faculty, Chemistry Education Division, Balıkesir, Turkey. E-mail: canan@balikesir.edu.tr
bBalıkesir University, Science and Art Faculty, Chemistry Department, Balıkesir, Turkey. E-mail: nnuri@balikesir.edu.tr
First published on 14th August 2019
Abstract
In this study, how prospective chemistry teachers (PCTs) perceive precipitation and conceptualize precipitation reactions at the symbolic level was explored. Further, it was of interest to identify PCTs’ visualization of the sub-microscopic level of precipitation reactions. The sample was drawn from the Chemistry Education Department at the Education Faculty of a Turkish Public University. A total of 46 PCTs (10 in the 4th semester, 15 in the 6th semester, and 21 in the 8th semester) participated in the study. The data were collected using an instrument with three open-ended questions developed by the authors and with interviews. It was revealed that the PCTs thought about precipitation in qualitatively different ways depending on their practices of phenomena. The PCTs’ perceptions of precipitation were grouped into three issues coded as (1) reacting two salt solutions, (2) undissolved solid and (3) residue. It was found that half of the PCTs (24 of the 46 PCTs) did not use state symbols when writing the precipitation equations and more than half of them included the molecular dissolution features in their drawings. It was suggested that instruction should be to address incorporating a relation between the sub-microscopic, symbolic and macroscopic levels by using the animations.
Introduction
The term “precipitation” can be used to explain the formation of a solid substance, either by reacting two salts or by adding an excess amount of solute to a saturated solution. The second situation is also a crystallization process. Students’ perceptions of the concept of precipitates/precipitation can change depending on their experience with the phenomena. The difference between precipitation and crystallization lies fundamentally in whether the emphasis is placed on the process by which the solubility is decreased or on that by which the structure of the solid becomes arranged. The terminology can seem a bit confusing and can lead to students’ misconceptions or learning difficulties in learning precipitation reactions, solubility equilibria, and solubility products.The reaction where the precipitate is formed by reacting the ions of salt solutions is called the precipitation reaction. The precipitation reactions taught at the secondary level are again encountered by chemistry students in both general chemistry and analytical chemistry courses. For the reasons above and to provide meaningful learning concerning precipitation reactions, it is essential to explore how the students perceive and conceptualize the concept of precipitation at the beginning of the general chemistry and analytical chemistry courses. Additionally, in order for the precipitation reactions to be understood, it is crucial that the students are provided opportunities to distinguish these two events at the beginning of the teaching period of this phenomenon.
Another problem associated with the learning of precipitation and especially precipitation reactions is related to the triplet nature of this phenomenon. Precipitation reactions are a process that can be observed at the macroscopic level, but the explanations of the process are at the sub-microscopic level based on the interactions between ions. Besides, the process is represented by using a chemical equation at the symbolic level. Macroscopic and sub-microscopic levels have been emphasized as distinct and different perspectives for the precipitation phenomena. Another research study has shown that teachers tend to teach chemistry far more at the symbolic level than at the macroscopic and sub-microscopic levels (Johnstone, 1991). For this reason, representations play a critical role in understanding the phenomena at the sub-microscopic level where the precipitation reaction occurs as a result of the interaction between the ions of two salt solutions.
Another thing that causes difficulties with the learning of the phenomena is the fact that the precipitate is an ionic solid in a solution and still contains ions at the end of the reaction. This requires that students understand what is happening in the solution and the transfer between different representations in solution, and also master the ionic bonding concept. Student understanding of solution chemistry and ionic bonding at the sub-microscopic level are the primary problems for chemistry students at all levels as seen in many research studies (Butts and Smith, 1987; Taber, 1994; Taber et al., 2012). Taber (2002) designed a probe to elicit students’ ideas about precipitation reactions. It was found that many 14–16 year-old students have genuine problems making any real sense of the precipitation process. Most of the students did not explain the structure and bonding of the precipitate, silver chloride. He found that the main problem was related to students’ difficulties with the concept of the ionic bond. Students thought that the reason for the formation of the ionic bond in the reaction mixture was electron transfer. In another study, Smith and Metz (1996) evaluated student understanding of solution chemistry through sub-microscopic representation, and they found that many graduate students had failed to dissociate the ionic species in the precipitation reactions.
Another problem involves the chemical equations which are used to show the reactions in progress. The precipitation reaction can be written as a molecular equation, an ionic equation, and a net ionic equation. In a molecular equation, the formulas of the compounds are written as though all species existed as molecules or whole units (Chang and Goldsby, 2013). When ionic compounds dissolve in water, they break apart into their component cations and anions. For the reaction to show the dissociation of dissolved ionic compounds into ions, the molecular equation is turned into the ionic equation, which shows dissolved species as free ions. At the end of a precipitation reaction, an insoluble compound is formed and separate ions, which are called spectator ions, remain in solution. These ions are not involved in the overall reaction, and because they appear on both sides of an equation, they can be eliminated from the ionic equation. The final equation is called the net ionic equation, which shows only the species that actually take part in the reaction. All three reactions are symbolized by an arrow. A saturated solution of a salt is an equilibrium between the solid salt and its dissolved ions. The solubility equilibrium of a reaction can be represented by an equation which contains double arrows and is the reverse of the net ionic equation.
Students’ conceptions of precipitation reactions
A precipitate can form either when a compound is added to a solvent or when two solutions are mixed. This depends on the solubility of the solute. For this reason, solubility is a crucial concept for understanding the process of precipitation. There are a lot of research studies regarding students’ conception of solubility (Fensham and Fensham, 1987; Ebenezer and Gaskell, 1995; Ebenezer and Erickson, 1996; Ebenezer and Fraser, 2001; Raviolo, 2001; Kelly and Jones, 2007, 2008; Chandrasegaran et al., 2008, 2009; Adadan and Savasci, 2012; Naah and Sanger, 2012, 2013; Adadan, 2014; Şen and Yılmaz, 2017). Although studies that focus on only precipitation or precipitation reactions were very few (Raviolo, 2001; Kelly et al., 2010; Naah and Sanger, 2012; Nakiboğlu and Nakiboğlu, 2017), it was seen that a number of researchers included precipitation reactions in their research focusing on reactions in aqueous solutions (Butts and Smith, 1987; Fensham and Fensham, 1987; Smith and Metz, 1996; Chandrasegaran et al., 2008, 2009; Kelly and Jones, 2008).Since the dissolution and sub-microscopic processes of the dissolution of ionic substances in water are prerequisites for learning precipitation reactions meaningfully, it is essential to examine studies about students’ understanding concerning the nature of the dissolution process of ionic solids. When these studies are examined, it is seen that the problems can be grouped into categories. The first one is about the particles in the solution, i.e., the formation of ions (Butts and Smith, 1987; Kelly and Jones, 2007; Tien et al., 2007; Devetak et al., 2009; Kelly et al., 2010; Nyachwaya et al., 2011). Devetak et al. (2009) found that the most frequent misconception of secondary school students regarding solutions of ionic substances was that the molecules were drawn in the solution instead of separate ions, and the size and/or the amount ratio between anions and cations were incorrect. Butts and Smith (1987) showed that high school students depicted NaCl units as molecules in a solution of sodium chloride instead of separate sodium and chloride ions. Similar findings were also obtained from studies with college and university level students. These studies indicated that students think an ionic compound dissolves as neutral atoms or molecules in the water (Tien et al., 2007; Nyachwaya et al., 2011; Naah and Sanger, 2012).
The second issue about the dissolution process of ionic solids involves the representation of the molecules of water/particles of the solvent. Boo and Watson (2001) reported that one of the misconceptions of high school students about solutions was that “water (solvent) does not play any part in the dissolution process”. Regarding this issue, Butts and Smith (1987) showed that the role of the polar water molecule in this process was poorly understood by high school students. Kelly and Jones (2007) described incorrect features displayed in students’ drawings of a sodium chloride and water mixture. One of them was that water molecules break apart into separate atoms.
The findings of students’ conceptions related to precipitation and precipitation reactions can also be grouped into several areas of difficulty. The first difficulty involves why a precipitate forms in a reaction between certain ions in solution. Butts and Smith (1987) indicated that it was difficult for students to explain the formation of a precipitate from a mixture of ionic compounds in solution. They found that high school students thought that all ionic compounds are soluble. They based this misconception on the students’ difficulty in explaining why some ions unite to form an insoluble solid when mixed in the same aqueous solution. Butts and Smith (1987) also found that very few students could associate the formation of a silver chloride precipitate with its low solubility. Some of the students reasoned that silver chloride could not be an ionic compound since it does not dissolve in water. Similarly, Tan et al. (2009) determined that students cannot relate the formation of a precipitate in a double displacement reaction to the low solubility of the salt. They explained the reason for this problem, which is as follows: “The students have seldom been introduced to the sub-microscopic level representations of the formation of precipitate-ions attracting each other and aggregating together to form larger masses which became visible at the macroscopic level” (Tan et al., 2009, p. 143).
Another difficulty concerns interpreting the symbolic language used in the precipitation equations. Naah and Sanger (2012) investigated college students’ misconceptions and difficulties in writing symbolic-level balanced equations for dissolving ionic compounds in water. They identified several misconceptions; one of them was confusion regarding the appropriate use of subscripts and coefficients. These findings agree with several previous studies. For example, Nyachwaya et al. (2011) investigated college students’ ability to balance chemical equations and draw appropriate particulate representations of those reactions. They explored that students have problems with interpreting the symbolic language used in the precipitation equations. They cited that “the conceptual misunderstandings stem from students’ inabilities to translate between the symbols used in a chemical reaction to the particulate level” (p. 127). A similar finding was obtained in the study of Kelly, Barrera, and Mohamed (2010). Raviolo (2001) also investigated students’ conceptual understandings of solubility equilibrium in his study, and he found that the students have difficulty in relating the micro and symbolic levels while writing a chemical equation of a system in equilibrium between AgCl and its ions.
As well as the students’ conceptions mentioned above related to the precipitation reactions, another important issue in understanding students’ conceptualizations of all types of chemical reactions is to determine the students’ model-based reasoning. Cheng (2018) investigated Grade 10–12 students’ model-based reasoning for the reaction of magnesium with oxygen at the sub-microscopic level. He said that it had been proposed that chemical reactions can be conceptualized using two models: “(i) the particle model, in which a reaction is regarded as the simple combination and rearrangement of reactant particles and does not involve any change in the identity of the reactants, and (ii) the atomic model, wherein a reaction involves the transformation of one chemical species into another” (p. 227). He aimed to determine whether students’ understanding of chemical reactions could be made sense of using these two models and whether the particle model of reactions can support students’ understanding of some advanced chemical concepts. He found that both were useful in assessing the students’ understanding of the reaction and showing the different ways that the students reasoned the chemical reaction.
Theoretical framework
Constructivism was used as the theoretical framework for this study in which learning is a process of knowledge construction in the mind of the learner (von Glasersfeld, 1989, 1990). Constructivism is one of the most appreciated guidelines for science teaching and learning as well as for research in these fields in the last forty years. von Glasersfeld (1990) asserted that “knowledge is not passively received either through the senses or by way of communication, and knowledge is actively built up by the cognising subject”.The constructivist considers that learners can construct models of the world as they try to make sense of their experiences. For this reason, these constructions can have a significant epistemological status for the learner, and may not easily be changed (Taber, 2001a, 2001b). Besides, what the learners already know is of significant status in the construction process. Bodner (1986) stated that constructivism is based on preexisting cognitive structures or schemes and provides a theoretical basis for Ausubel's distinction between meaningful and rote learning. Meaningful learning is a constructive process in which students try to produce links among the existing concepts, knowledge, and observations of science to acquire understanding (Ausubel, 1968, cited in Vachliotis et al., 2013). A more critical point in meaningful learning is what has previously been learned. The existing knowledge can also act as an impediment to subsequent learning (Taber, 2001b) and so students’ understanding may not always be scientifically correct. This situation may lead to conceptual or propositional knowledge that does not correspond to the currently held scientific theory. Taber (2000) defined an alternative conception “as a conception which does not match the accepted scientific version” (p. 8). Taber (2001a) also stated that much of the literature about learners’ ideas in chemistry refers to misconceptions, but he said that “this term is considered (by some authors) to imply a minor misunderstanding of the teacher's words that is readily put right, whereas many learners’ ideas have been found to be tenacious and stable over long periods” (p. 43). Although Taber (2000) demonstrated the difference between misconceptions and alternative conceptions when the literature about learners’ ideas in chemistry is examined, it was seen that the terms misconceptions and alternative conceptions might be used interchangeably by researchers. Besides, the difference between them seems not to be precise in many research studies. Stefani and Tsaparlis (2009) indicated that alternative conceptions and misconceptions are the standard terms that describe such knowledge that is different from or inconsistent with the accepted scientific definition.
Reading a chemical equation requires an understanding of several concepts, and the relationships between and among these concepts (Keig and Rubba, 1993, cited in Nyachwaya et al., 2011). If the concepts which are prerequisite knowledge for the learning of the chemical reaction are not understood meaningfully, this can prevent students from learning the topic. To learn chemical equations meaningfully, individuals must relate new knowledge to relevant concepts and propositions they already know (Bodner, 1986). It is beneficial to identify the misconceptions held by students at the beginning of the topic, so appropriate strategies may be employed that will challenge students’ understandings in order to help students develop more scientifically acceptable conceptions.
Phenomenography was used as an analytical framework for part of the study related to PCTs’ perceptions of precipitation. From the findings of our pilot studies, it was decided that phenomenography would be very effective in generating the type of categories of students’ perceptions of precipitation. In phenomenography, the categories point to possible ways in which the individuals can perceive the phenomenon. Phenomenography is an area of research which focuses on identifying and describing the qualitatively different ways in which people understand phenomena in the world around them. The research which aims at the description, analysis and understanding of experiences was labelled as phenomenography by Marton (1981). Tóth and Ludányi (2007) cited that although individuals would have different experiences and conceptualisations of a phenomenon in a given context, the number of qualitatively different conceptualisations was limited.
For this reason, they asserted that these different conceptualizations were the focus of a phenomenographic study rather than each individual learner's conceptualisations and individuals could accurately express their experiences and conceptualisations. It takes some discovery to find the qualitatively different ways in which people experience or conceptualize a specific phenomenon. There are no algorithms for such discoveries (Marton, 2005).
Marton advocated that “phenomenology is concerned with the relations that exist between human beings and the world around them” (Marton, 2005, p. 143). Additionally, he said that the point of departure in phenomenography was always relational and phenomenography provided descriptions that were relational, experiential, content-oriented, and qualitative. However, he cited that what was focused on by researchers must be a function of both the problem and the subject of the study. So, the researcher looked for the most essential and distinctive structural aspects of the relation between the individual and the phenomenon, and it is essential to discover the structural framework within which various categories of understandings exist (Marton, 2005, p. 146).
The rationale and research questions
Precipitation reactions have essential functions in industry and daily life. They are used for purification, removing or recovering salts, for making pigments, and to identify substances in qualitative analysis. The synthesis of many industrial chemicals is based on these reactions. They are also a vital part of analytical chemistry classes and typical experiments carried out by students in analytical chemistry laboratories. The ability to predict precipitation reactions has practical value in industrial and chemistry laboratory preparations. On the other hand, this knowledge is tested with mathematically based questions. The correct resolution of these questions by the students does not always indicate that they understand the chemical concept meaningfully. The students often can solve problems successfully using memorized algorithms (Nurrenbern and Pickering, 1987; Smith and Metz, 1996; Raviolo, 2001; Stamovlasis et al., 2005; Nyachwaya et al., 2014).It can be seen from the previous discussion of the phenomena that teaching of the precipitation reactions requires students to transfer between macro (observable, concrete), sub-microscopic (invisible, abstract), and symbolic levels of reaction (Johnstone, 1991). The students have difficulties visualizing particles’ behaviour and arrangement in a chemical reaction microscopically as well as in relating chemical equations as a symbolic representation of chemical reactions at the microscopic level (Lee, 1999). All of these explanations made us think that meaningful understanding of this phenomenon by prospective chemistry teachers is crucial for their future students and so it is essential to understand how prospective chemistry teachers (PCTs) perceive and conceptualize this phenomenon.
For this reason, how PCTs perceive precipitation and conceptualize precipitation reactions at the symbolic level was explored. Further, it was of interest to identify PCTs’ visualization of the sub-microscopic level of the precipitation reaction. The research questions this study attempted to answer are the following:
(1) What are the PCTs’ perceptions of precipitation?
(2) What are the PCTs’ conceptualizations of the precipitation reactions at the symbolic level?
(3) Concerning the particle model and the atomic model, how do the PCTs represent the precipitation reaction at the sub-microscopic level?
(4) What is the PCTs’ visualizations of the reactants and products of precipitation reactions at the sub-microscopic level?
(5) What types of misconceptions are uncovered by PCTs’ drawings of the sub-microscopic representations of precipitation reactions?
Methods
The sample and context of the study
A qualitative survey methodology and a purposeful convenience sampling method (Patton, 2002) were used in this study. The sample was drawn from the Chemistry Education Department at the Education Faculty of a Turkish Public University. In Turkey, any student who completes eight years of primary education begins secondary education which includes the four years of high school (called Lycée). The high school graduates need to pass a nationwide Higher Education Institutions Exam (YKS) to enter a university. The Higher Education Council (YOK) governs the University system in Turkey. Each university consists of different faculties, institutes, and four-year schools. One of the faculties is the Education Faculty which contains different departments. These departments have 4 year pre-service teacher training programs and train the undergraduates to teach at pre-school, primary school, high school, and many other branches’ education programmes. One of the programs of education faculties is the chemistry teacher training program, and its purpose is to educate teacher candidates for chemistry teaching in high schools.A total of 46 undergraduate chemistry (10 in the 4th semester, 15 in the 6th semester, and 21 in the 8th semester) participated in the study. The subjects ranged in ages between 20 and 25 years. All of the participants studied General Chemistry courses 1 and 2 in their first year at university. All of them took an Analytical Chemistry 1 course and Analytical Chemistry Laboratory 1 course in the first semester of their second year. Ten of the participants who were in the 4th semester were taking the Analytical Chemistry 2 and Analytical Chemistry Laboratory 2 courses while the study was being undertaken. The other participants had taken these courses in the second semester of their second year. All the participants took analytical chemistry courses from the second author of this study. The study was conducted in the first author's department. At the beginning of the study, the authors informed the participants about the study's purpose and the researchers guaranteed their anonymity when results were to be presented. All the participating students were volunteers and provided informed consent.
The precipitation and dissolution processes are regular parts of the Turkish high school chemistry curriculum; the precipitation reactions are first taught to students in high school chemistry lessons. The same topic is taught in the General Chemistry courses in the PCTs’ first year briefly. Detailed teaching of the precipitation-reactions is carried out in the Analytical Chemistry 2 and Analytical Chemistry Laboratory 2 courses.
Data collection
The data were collected as written responses to an instrument with subsequent interviews by the researchers. The simultaneous collection of data eliminates the risk that details, presented in early interviews, might influence the pattern or details of subsequent interviews. Gathering data with written responses allows for the collection of data from more than one student and enables the interpretation of results. For this reason, the written responses were chosen as a primary data source. The interviews were used as a secondary data source in order to provide an in-depth investigation of the ways that the PCTs’ ideas reflected in their drawings.The instrument with three open-ended questions was developed by the authors. The first author of the study who has a PhD degree in Inorganic Chemistry is a chemistry educator. The second author who has a PhD degree in Analytical Chemistry is a professor with 25 years of teaching experience. All the participants were taught Analytical Chemistry courses by the second author of the study. The questions contain two precipitation reactions (between silver nitrate and hydrochloric acid, and between silver nitrate and potassium chromate) which can be representative of the possible questions about precipitation reactions. The content validity of the instrument was provided by the second author's expert judgment. The three questions on the instrument were linked with the objectives of the study logically which establishes the face validity (Kumar, 1999, p. 138).
The development of the instrument was based on the V diagrams used for precipitation titrations in analytical chemistry laboratories by the authors for three years. In the first year, the students were asked about the precipitation, precipitation reaction and related concepts about precipitation titrations in the conceptual part of the V-diagram and the students were asked to write the equations of precipitation reactions in the experiment in the methodical part of the V-diagram. The data of the V diagram showed that students experienced difficulties with the concept of precipitation and precipitation reactions. It was noted that the students’ explanations were not based on the sub-microscopic dimension. For this reason, a new part was added where the students were asked to draw a precipitation reaction at the sub-microscopic level. In this drawing, we provided the ions for the solutions in the first and second containers, and the PCTs were asked to sketch the particles only for the third container which contains the reactants. It was decided to change this question after the analysis of the student responses in the second year. Finally, a new instrument was developed. If an instrument is consistent and stable, and hence, predictable and accurate, it is said to be reliable (Kumar, 1999, p. 140). The instrument of the study was applied three times. Although some additions were made to improve the instrument, the consistent findings of the main points were produced under the same conditions.
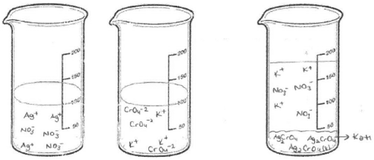
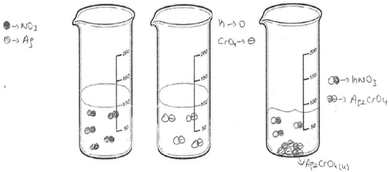
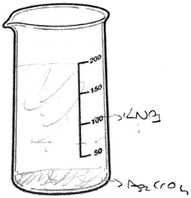
The first question of the instrument asks the PCTs to give a definition of precipitation and the second question asks them to write the equation of a precipitation reaction between hydrochloric acid and silver nitrate solutions. The third question is “100 ml of the solution of AgNO3 in the first beaker glass is mixed with the same concentration of 100 ml of K2CrO4 solution in the second beaker glass. The reaction is 2AgNO3(aq) + K2CrO4(aq) → Ag2CrO4(s) + 2KNO3(aq). Draw the microscopic size (particle size-ion, molecule, etc.) of this chemical reaction for each beaker glasses in below.”
After the analysis of the PCTs’ responses, it was decided to conduct interviews with the PCTs to investigate some situations detected in their answers to the second and third questions. Interviews were conducted with 18 PCTs selected according to their responses. Each interviewee was asked questions specific to his/her situation. The interviews were tape recorded and transcribed.
Analysis of the questions
An analysis similar to those used in the studies of Cheng (2018) and Cheng and Gilbert (2017) was followed. Since this analysis focused on model use rather than students’ misconceptions, the PCTs’ representations about precipitation reactions were categorised based on the defining features of the particle model and the atomic model. The findings of this analysis are presented under the subheading entitled Particle and atomic models used in representing the chemical reaction.
Reliability and validity of the analysis
The reliability of the content analysis includes three criteria, one of which is stability. Stability is the tendency for coders to consistently re-code the same data in the same way over time. To obtain the inter-rater reliability of the analysis, the data were analysed by the authors independently at first (the inter-rater reliability was 90%), and all interpretation and coded data were checked by the authors together. Additionally, after validating the coding scheme, the first author independently coded all of the interviews at two different times, and the intra-judge reliability was provided by the first author (Gay and Airasion, 2000, p. 175).Three criteria comprise the validity of the content analysis. One of them is the closeness of categories. The closeness of categories can be achieved by utilizing multiple classifiers to arrive at an agreed upon definition of each specific category. Using multiple classifiers, a concept category that may be an explicit variable can be broadened to include synonyms or implicit variables. Additionally, validity was gained through triangulation of findings from two data sources including written questions and interviews.
Findings
| List of issues | Frequency | |||
|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | |
| Reacting two salt solutions | 10 | 2 | 1 | 13 |
| Undissolved solid | 10 | 11 | 8 | 29 |
| Residue | 1 | 2 | — | 3 |
| Total | 21 | 15 | 9 | 45 |
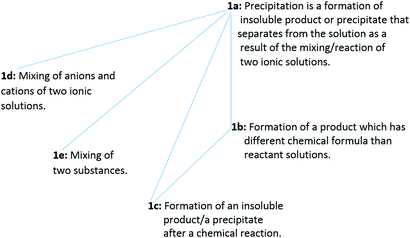
Regarding issue 1, 13 of the 46 PCTs viewed the precipitation as an event that was happening between two salt solutions. For this reason, issue 1 was coded as “reacting two salt solutions”. For issue 1, five ways of understanding the phenomenon were found. The sequencing in decreasing levels with structured coding for issue 1 and the frequency distribution of levels are presented in Table 2. These levels are hierarchically related and associated with the depth of information (level 1a implies levels 1b, 1c, 1d, and 1e; level 1b implies level 1c) and constitute together the outcome space (presented in Fig. 1).
| List of levels | Frequency | ||||
|---|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | ||
| 1a | Precipitation is a formation of an insoluble product or a precipitate that separates from the solution as a result of the mixing/reaction of two ionic solutions | 1 | — | — | 1 |
| 1b | The formation of a product which has different chemical formula from those of reactant solutions | — | 1 | — | 1 |
| 1c | The formation of an insoluble product/a precipitate after a chemical reaction | 6 | 1 | — | 7 |
| 1d | Mixing of anions and cations of two ionic solutions | 1 | — | — | 1 |
| 1e | Mixing of two substances | 2 | — | 1 | 3 |
| Total | 10 | 2 | 1 | 13 | |
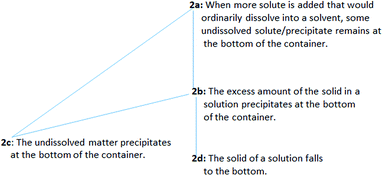
Regarding issue 2, more than half of the PCTs (29 of the 46 PCTs) viewed precipitation as a process in which dissolved solute comes out of solution, and the undissolved solute/precipitate remains at the bottom of the container (presented in Table 1). Issue 2 was coded as “undissolved solid”, and four ways of understanding the phenomenon were found. The sequencing in decreasing levels with structured coding for issue 2 and the frequency distribution of levels are presented in Table 3. These levels are hierarchically related (level 2a implies both levels 2b and 2c, and level 2b implies level 2d) and constitute together the outcome space (presented in Fig. 2).
| List of levels | Frequency | ||||
|---|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | ||
| 2a | When more solute is added that would ordinarily dissolve into a solvent, some undissolved solute/precipitate remains at the bottom of the container. | 2 | 2 | 3 | 7 |
| 2b | The excess amount of the solid in a solution precipitates at the bottom of the container. | 1 | 1 | — | 2 |
| 2c | The undissolved matter precipitates at the bottom of the container. | 3 | 4 | — | 7 |
| 2d | The solid of a solution falls to the bottom. | 4 | 4 | 5 | 13 |
| Total | 10 | 11 | 8 | 29 | |
Three of the 46 PCTs perceived precipitation as an event where an insoluble residue is observed at the bottom of the solution after a reaction has taken place. One of them states that “after a chemical reaction occurs, some substance remains at the bottom of the solution.” Issue 3 was coded as “residue”; the three ways of understanding the phenomenon were found. The sequencing in decreasing levels with structured coding for issue 3 and the frequency distribution of levels are presented in Table 4. These levels are hierarchically related (level 3a implies level 3b implies level 3c) and constitute together the outcome space (presented in Fig. 3).
| List of levels | Frequency | ||||
|---|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | ||
| 3a | After a reaction has taken place, a certain amount of substance remains at the bottom | 1 | — | — | 1 |
| 3b | Residue after a reaction | — | 1 | — | 1 |
| 3c | Residue | — | 1 | — | 1 |
| Total | 1 | 2 | — | 3 | |
As seen in Tables 2–4, the PCTs thought precipitation in qualitatively different ways depending on their experiences of phenomena. It is important to know what the PCTs perceive when the teacher talks about precipitation related to reacting two salt solutions while teaching precipitation reactions. If the PCTs consider precipitation as the crystallization process instead of the insoluble product formation while working with precipitation reactions, this would cause problems in understanding the precipitation reactions.
In order to understand the relationship between the prospective teachers’ definitions of precipitation and their experiences, 4 PCTs were interviewed. It was understood that the pre-service teachers’ experiences influenced their explanations. It was found that these experiences are “the most common practices in the laboratory”, “the representation used in the lectures” and “the daily life experiences”. Below are excerpts from a few of the interviews in which PCT28 explains why she wrote such an explanation to define precipitation (R stands for the researcher and PCT# identifies the PCT by number).
R: You wrote, “When more solute is added that would ordinarily dissolve into a solvent, some precipitate remains at the bottom of the container”. Why did you write this definition?
PCT28: I explained the application I made.
R: Look at the second question, is this also a precipitation event?
PCT28: Yes.
R: What is the difference between them?
PCT28: There is not any difference between them. The precipitation occurs in both. However, they are different events. I recognized that I explained the saturation here.
R: Why did you think saturation first?
PCT28: When I wrote this, a practice came to my mind, and I wanted to explain it.
Similarly, PCT30 says:
“I have written this definition based on our experiments.”
We have also found that the experience of the prospective teachers concerning their daily life influences their perceptions.
Another reason is concerning the drawings which were used in their lessons. An excerpt that was taken from the interview conducted with PCT26 gave the reason.
R: You wrote, “If the excess amount of the solid in a solution is added to the solution, precipitation occurs.” When we look at the second question, this is also a precipitation event, isn’t it? What do you think?
PCT26: This is also precipitation.
R: What is the difference between them?
PCT26: Could it be related to visual or invisible?
R: In both cases, you can see the precipitate, right?
PCT26: Yes. There is no difference.
R: Why did you think the first event firstly?
PCT26: This event is more visual for me. I’ve seen a drawing like this before in my lessons. That's why the representation came to my mind directly. Our teacher has drawn on the board this way.
(PCT26 is only one PCT who draws an image when explaining the first question. Her drawing is given below). [Although PCT26 indicated the precipitate with an arrow, she labelled it as a precipitation process.]
| Thematic category | Example | Frequency | |||
|---|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | ||
| All state symbols used and all were true | AgNO3(aq) + HCl(aq) → AgCl(s) + HNP3(aq) | 7 | 3 | — | 10 |
| No state symbol used | AgNO3 + HCl → AgCl + HNO3 | 6 | 8 | 10 | 24 |
| Confusion about the aqueous state symbol (aq) with the liquid state symbol (l) | AgNO3(I) + HCl → AgCl(s) + HNO3(l) | 7 | 4 | — | 11 |
| To use the aqueous state symbol (aq) for AgCl | AgNO3(aq) + HCl(aq) → AgCl(aq) + HNO3(aq) | 1 | — | — | 1 |
| Total | 21 | 15 | 10 | 46 | |
When Table 5 is examined, we see that while half of the PCTs (24 of the 46 PCTs) did not use state symbols when writing the precipitation equation, only 10 out of the 46 PCTs (most of them are 4th-year students) wrote the precipitation equation using correct state symbols. 11 students confused the aqueous state symbol (aq) with the liquid state symbol (l). Below are excerpts from a few of the interviews in which a PCT explains why she did not use any state symbol in her equation. The first excerpt was taken from the interview conducted with PCT6, who used the liquid state symbol (l) for all aqueous solutions in the equation.
R: Why did you use the liquid state symbol (l) in the equation?
PCT6: Because they’re liquids.
R: Well, in the question, it is written that, for example, AgNO3 is an aqueous solution. In this case, do you think this representation is correct?
PCT6: It's not correct; I should have written it as (aq).
R: What is (aq)?
PCT6: It means aqua. I should have written it as (aq) because they were solutions.
R: Ok, do you want to rewrite the equation?
PCT6: Yes. (He writes all state symbols correctly.)
R: So, what happens if we do not write state symbols? Why do we write state symbols in the equations?
PCT6: To specify the physical state of the substance.
Another excerpt was taken from the interview conducted with PCT15, who used the liquid state symbol (l) for all aqueous solutions in the equation.
R: What does (l) mean in this equation?
PCT15: In the liquid, that means that it was dissolved.
So, we just told him.
R: Does not the (l) represent a pure liquid?
PCT15: Actually, it's true.
R: You called it a solution. Then why did you use (l)?
PCT15: There are ions. I tried to tell this.
R: Is not AgNO3 solid?
PCT15: Yes.
R: Then, does the liquid state of this solution indicate that it is a pure liquid?
PCT15: I used (l) to show that it was in the water.
R: Ok, You know this is a solution, but you show it with (l).
PCT15: Yeah.
From the interview above, the PCT thinks of the homogeneous solution as a liquid. This conception has been labelled as a misconception in this study. The third excerpt was taken from the interview conducted with PCT4, who did not use any state symbol in the equation.
R: Okay, You haven’t written any state symbols in your equation. Why are the state symbols written in a chemical equation?
PCT4: In fact, the state symbols should be written. The state symbols are written to show the physical states of the products after the reaction.
R: Is it only for products?
PCT4: No, for reactants also.
R: Why didn’t you write them?
PCT4: It's because of not paying attention. (She was asked to write the state symbols of reactants and products, and she wrote all of them correctly.)
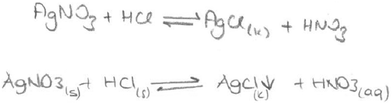
Chemical equations are used to show the reactions in progress. The precipitation reaction can be written as a molecular equation, an ionic equation, and a net ionic equation. All three reactions are symbolized with an arrow. A saturated solution of a salt is in contact with its solid. An equilibrium exists between a solid salt and its dissolved ions in a saturated solution. The solubility equilibrium of a reaction can be represented by an equation which contains a double-headed arrow symbol and the reverse of the net ionic equation. In our previous study, we found that the students did not know the meaning of a double-headed arrow symbol. Additionally, the findings of the first question analysis indicated that the PCTs viewed precipitation either as an event of reaction between two salt solutions or as a process in which dissolved solute comes out of solution. For this reason, the PCTs’ equations were analysed according to which equations (molecular, ionic or net ionic) are preferred in their writing and whether the PCTs write the equation as an equilibrium equation. After analysing the PCTs’ equations, their representations of precipitation equations were grouped into eight categories. The findings about the presentations of precipitation equations written by the PCTs are given in Table 6.
| Categories of equations | Frequency | |||
|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | |
| Molecular equations (correctly written) | 14 | 7 | 3 | 24 |
| Molecular equations (contains significant errors) | 4 | — | 4 | 8 |
| Molecular equations (written as equilibrium equations) | — | 7 | — | 7 |
| Molecular equations (contains missing formulas or ions) | 2 | — | 1 | 3 |
| Molecular equations (contains both formulas and ions) | — | 1 | — | 1 |
| Net ionic equations | — | — | 1 | 1 |
| Both molecular and net ionic equations (written as equilibrium equations) | 1 | — | — | 1 |
| Both molecular and net ionic equations (contains significant errors) | — | — | 1 | 1 |
| Total | 21 | 15 | 10 | 46 |
As can be seen from Table 6, more than half of the PCTs preferred to write the molecular equation for representing a precipitation reaction, and the details of the PCTs’ molecular equations showed a great deal of variety. A representative example of the molecular equation which contains both formulas and ions is given below. (The state symbol (k) means solid in Turkish.)
While no PCTs wrote an ionic equation, it was determined that only one PCT (PCT26) wrote a net ionic equation. When PCT26's equation is examined, which is given below, we see that she did not write the oxidation state of silver and the subscript for AgCl correctly.
It was found that 8 of the 46 PCTs wrote the molecular equation using a double-headed arrow for this equation. Two typical examples of this type of response are given below. (The state symbol (s) means liquid in Turkish.)
This situation also occurred in the interview of one of the prospective chemistry teachers (PCT3) who wrote the equation for the second question as if it was a solubility equilibrium (given below).
Excerpts taken from the interview with PCT3 are presented below:
R: You used a double-headed arrow symbol in this equation. Why did you use this kind of arrow?
PCT3: It is in equilibrium. Isn’t it?
R: Is this reaction that shows the equilibrium?
PCT3: Yeah.
R: Why did you think it was an equilibrium?
PCT3: I don’t know why, but I’m trying to figure out what I’m writing for. When the AgCl solid is dissolved in water, is there an equilibrium to ionization? (She thinks the precipitation reaction acts as a solubility equilibrium of AgCl).
R: Is it a solubility equilibrium equation or a precipitation reaction?
PCT3: This is the precipitation reaction.
R: In which case is a double-headed arrow symbol used?
PCT3: For a solubility equilibrium equation. As a matter of fact, I thought about it that way.
R: Why did you think so?
PCT3: The solid is dissolved in the water and dissociates into ions, and the ions are combined to form solids.
R: So, how did this precipitation reaction occur? Is there an insoluble compound formed by decreasing the solubility or is there a precipitate formed by mixing two salt solutions?
PCT3: Two solutions are mixed in this equation; I should use an arrow symbol in this case.
Particle and atomic models used in representing the chemical reaction
The frequency distribution of PCTs’ drawings that used different models in representing the precipitation reaction is presented in Table 7.| Model | Frequency | |||
|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | |
| Particle model | 12 | 10 | 5 | 27 |
| Atomic model | 9 | 5 | 3 | 17 |
| No drawing | — | — | 2 | 2 |
| Total | 21 | 15 | 10 | 46 |
When Table 7 is examined, it is seen that most of the PCTs prefer the particle model in their sub-micro representation concerning precipitation reactions. A representative example of this model is presented in Fig. 4.
 | ||
| Fig. 4 A sample taken from PCTs’ sub-microscopic representations of the reaction between silver nitrate and potassium dichromate based on the particle model. | ||
As seen from Fig. 4, the particles of reactants and products simply rearrange, and they do not involve any subatomic particles. There are no changes in the identities of the particles of the reactants and products.
As seen from Table 7, 17 out of the 46 PCTs visualize the precipitation reaction by using the atomic model. A typical example of PCTs’ drawings for the atomic model is presented in Fig. 5.
When Fig. 5 is examined, it is seen that the particles of reactants and products are more precisely defined; that is, the reactant solutions contain the ions. There are changes in the identity of the reacting particles, i.e., from silver and chromate ions to the ionic compound, silver chromate.
Dissolution models of ionic compounds
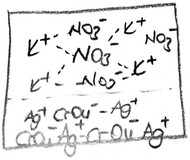
The findings of PCTs’ drawings according to the dissolution models of ionic compounds are presented in Table 8. According to this analysis, the drawings of the PCTs were collected into three different groups. The first one is called as the ionic dissolution model, which is a scientific model (presented in Fig. 5). According to this model, the reactant solutions of AgNO3 and K2CrO4 and the aqueous product involve their separate ions. As seen from Table 8, only 13 PCTs thought that the solutions of reactants and the aqueous product consisted of separate ions. When Fig. 5 is examined, PCT8 drew both the reactant solutions and product solution as separate ions. The second model is called the molecular dissolution model, which is accepted as a misconception. When Table 8 is examined, it is seen that more than half of the PCTs included the molecular dissolution features in their drawings. A sample of the molecular dissolution models of ionic compounds is presented in Fig. 6.| Dissolution model | Frequency | |||
|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | |
| Molecular dissolution model | 10 | 9 | 5 | 24 |
| Ionic dissolution model | 7 | 4 | 2 | 13 |
| Particle distribution model | 4 | 2 | 1 | 7 |
| No drawing | — | — | 2 | 2 |
| Total | 21 | 15 | 10 | 46 |
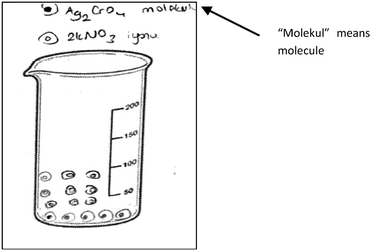
In the interview with PCT10 whose drawing was seen in Fig. 6, we investigated whether she drew these molecules consciously. It was found that she thought that when AgNO3 and HCl dissolved in the water, molecular pairs formed. She said that the solid ionic product, Ag2CrO4, formed after mixing the AgNO3 and HCl solutions, and this product involves molecules. She also thought that the aqueous product involves molecular pairs. Regarding the idea that the Ag2CrO4 consists of molecules, another PCT wrote “molecule” for Ag2CrO4 on his drawing as seen in Fig. 7.
In addition to these two dissolution models of ionic salts, the PCTs used different representations to show the particles of ionic salts in their drawings, and this model is called the particle distribution model. This model includes separate particles that were neither ions nor molecules (presented in Fig. 8).
Representations of the ionic product
After analysing the PCTs’ drawings, the PCTs’ representations concerning the ionic product of the precipitation reaction were grouped into five categories: the ionic product is depicted as (a) molecules which were collected at the bottom; (b) molecules/ions/separate particles distributed in the beaker; (c) a solid which is drawn at the bottom; (d) molecules/ions distributed in the beaker glass; and (e) particles which are drawn at the bottom (sub-micro) and labelled ‘solid’. The frequency distribution of these categories and sample representations are presented in Table 9.| Categories concerning the representation of the ionic product | Frequency | |||
|---|---|---|---|---|
| 4th year | 3rd year | 2nd year | Total | |
| The ionic product is depicted as molecules which were collected at the bottom | 5 | 5 | 2 | 12 |
| The ionic product is depicted as molecules/ions/separate particles distributed in the beaker glass | 6 | 3 | 2 | 11 |
| The ionic product is depicted as a macroscopic solid at the bottom | 6 | 2 | 1 | 9 |
| The ionic product is depicted as spherical particles which were aggregated at the bottom | 5 | 2 | 1 | 8 |
| The ionic product is depicted as an ionic crystal structure which was collected at the bottom | 1 | 1 | — | 2 |
| The ionic product is depicted as a formula | — | — | 1 | 1 |
| No drawing | — | — | 3 | 3 |
| Total | 22 | 15 | 10 | 46 |
It was found that 12 PCTs depicted the solid ionic product as molecules which were collected at the bottom. A sample drawing is given in Fig. 7 in the previous sub-section. In his drawing, PCT24 drew the spherical particles at the bottom of the container and wrote that these particles represent the Ag2CrO4 molecules on the top of his drawing.
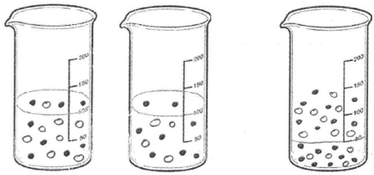
11 of the 46 PCTs depicted the solid ionic product as molecules/ions/separate particles distributed in the beaker randomly. Three sample drawings are shown in Fig. 9.
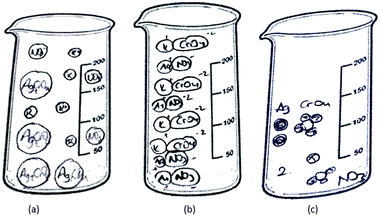 | ||
| Fig. 9 The solid ionic product is depicted as molecules (a)/ions (b)/separate particles (c) distributed in the container. | ||
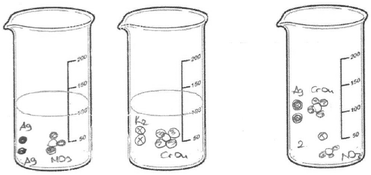
As seen in Fig. 9a, PCT41, who is a third-year student, drew separate Ag2CrO4 molecules, and potassium and nitrate ions randomly distributed in the container without taking into account the stoichiometric ratio. While PCT38, who is a third year PCT, sketched separate silver ions, chromate ions and spectator ions randomly distributed in the container (shown in Fig. 9b), PCT19, who is a senior, sketched separate Ag, CrO4 and NO3 without any ionic charge. She also put number 2 near the particles which represent the K and NO3 in order to depict 2 moles of KNO3 (shown in Fig. 9c).
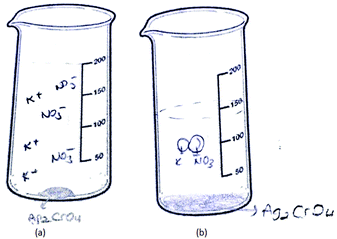
9 of the 46 PCTs depicted the solid ionic product as a macroscopic solid at the bottom of the container. It was seen that the student drawings in this category were divided into two groups. In the drawings in the first group, while PCTs depicted the solid ionic product as a macroscopic solid, they represented the spectator ions at the sub-microscopic level. That is, the PCTs used both the macroscopic and sub-microscopic levels together in their drawings. Two sample drawings which belong to senior PCTs placed in the first group are shown in Fig. 10. They sketched the spectator ions by using their symbol or the spherical particles on top of the ionic solid at the sub-microscopic level.
It was found that only PCT31's drawing was placed in the second group. PCT31, who is a second year student, depicted both the ionic solid and the solution, which contains the spectator ions, at the macroscopic level (shown in Fig. 11).
8 of the 46 PCTs depicted the solid ionic product as spherical particles which were aggregated at the bottom of the container. A representative drawing is shown in Fig. 12.
Two PCTs depicted the ionic product as an ionic crystal structure. A simple drawing is given in Fig. 13. (PCT32 preferred to sketch her drawing in the space under the beaker instead of in the given empty beaker. For this reason, the shape of this representation is different from the others.)
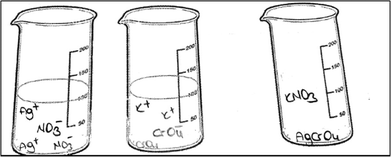
Only PCT28 depicted the ionic product as an AgCrO4 formula and she also wrote a KNO3 formula without drawing any particles. When examined, her drawing showed the ions of the ionic reactant solutions in beakers 1 and 2 (presented in Fig. 14).
The excerpt taken from the interview conducted with PCT28 is presented below.
R: Why did you show the solutions of reactants as the separate ions?
PCT28: I wanted to indicate that they were dissolved into their ions in water.
R: Ok, please look at the third beaker. You wrote KCrO4. What does it mean?
PCT28: Since KCrO4 precipitated and later ions of KNO3, I wrote it down.
R: Why is KNO3 written this way? Does it dissolve as molecules?
PCT28: I should have written the ions of KNO3, which dissolves into their ions, like others.
Additionally, all PCTs’ drawings were analysed to determine whether the volume of the third beaker glass after mixing the solutions was taken into account, We have put a volume line on all empty beakers in our study. We drew a line at 100 ml on the two beakers where only the reactant solutions are to be illustrated. It was stated that the solutions had a volume of 100 ml and equal concentrations before mixing. Only one student took into account the volumes of solutions and drew a line at 200 ml on the beaker containing the product solution.
Conclusions
At the end of this study, several important issues were revealed regarding the perceptions of precipitation, the conception of precipitation reactions and the visualization of the sub-microscopic level of a precipitation reaction.Regarding the PCTs’ perceptions when describing precipitation, it was seen that PCTs thought about the precipitation in qualitatively three different ways depending on their experiences with the phenomena. They are called “reacting two salt solutions”, “undissolved solid”, and “residue”. While “reacting two salt solutions” and “undissolved solid” are the major thinking patterns concerning the perception of precipitation, the last one is minor. Both issue 1 and issue 2 are related to the solubility of the ionic compounds in water. However, regarding issue 1, it was seen that PCTs thought that precipitation occurs in aqueous solution when cations and anions of two soluble salts react in solution. This thinking is related to the conceptions of precipitation reactions, solubility equilibria, and solubility products. For issue 2, it was revealed that PCTs thought that precipitation occurs when an excess amount of solute is added to a saturated solution. This thinking is actually related to the supersaturated solution and also the crystallization process. It is important to know whether the learners think of precipitation as in issue 1, when the teacher talks about precipitation related to reacting two salt solutions while teaching precipitation reactions. In case the learners think of precipitation as in issue 1, they can grasp the precipitation reactions easily. Conversely, if the learners think of it as in issue 2, problems may arise in grasping the precipitation reactions.
The findings indicated that the experience of the PCTs about precipitation had an impact on their perceptions of precipitation. The PCTs’ experiences seem mostly to be related to the chemistry laboratory work and their observation during experiments. While the PCTs practise in the laboratory, they can observe the formation of a precipitate by mixing two different salt solutions, as well as observe the formation of a precipitate in the process of preparing an oversaturated solution or in crystallization. As revealed by the findings of this study, one of these processes comes to their mind first with the effect of these experiences when the PCTs were asked to define precipitation. Besides, it was found that the representations used during their lessons have considerable effects on the PCTs’ perceptions.
Regarding PCTs’ writings of precipitation equations, it was concluded that many PCTs did not use state symbols of reactants and products in a chemical equation or they did not know what state symbols meant exactly. The studies found that college students (Kelly et al., 2010; Nyachwaya et al., 2011) and Grade 9 students (Chandrasegaran et al., 2008) fail to recognize the significance of the symbols and formulas that are used to represent precipitation reactions, and the findings of this study indicated that the PCTs have also similar problems about interpreting the symbolic language used in the precipitation equations. Kelly et al. (2010) found that the some general chemistry students stated that (aq) stood for aqueous, while the other students did not specifically state what the abbreviation meant. After analysing, it appeared that students tried to describe some of the properties of aqueous solutions when they attempted a definition. They found that several students focused on the macroscopic properties and referred to aqueous as being the liquid state of a traditional solution and some thought that aqueous was a transitional state between a solid and a liquid. From our interview, it is understood that there are two reasons why PCTs used the subscript (l) instead of subscript (aq). The first one is that PCTs are not very careful when using subscripts. The latter is a much more serious problem, and the PCTs are confused about the difference between a liquid and an aqueous solution. They think of the homogeneous solution as a liquid and use these terms interchangeably. This thinking pattern found in this study has been labelled as a misconception – “the homogeneous solution is a pure liquid”.
Accordingly, the equation of the precipitation reaction can be confused with the equation of the solubility equilibrium. This has been labelled as a misconception – “The equation of the solubility equilibrium is an equation of the precipitation reaction”. The confusion and different thinking patterns of the learners prior to the teaching of precipitation reactions may inhibit the meaningful understanding of precipitation and precipitation reactions.
Several studies have found that graduate and undergraduate students had problems with writing and balancing ionic equations (Smith and Metz, 1996; Naah and Sanger, 2012). Smith and Metz (1996) found that some of the undergraduate and graduate students incorrectly associated the coefficients in the balanced equations in a precipitation reaction. This study revealed that PCTs had similar problems. A problem concerning PCTs’ writings of precipitation equations was that they could use different species (i.e., ions, formulas) together in the same precipitation equation. While some of the PCTs wrote the reactants as the formulas (whole units), they wrote the products as ions or vice versa. This problem can stem from different equation writing styles for the same precipitation reaction. This reaction can be written as a molecular equation, an ionic equation, and a net ionic equation. Regarding this issue, Kelly and Jones (2008) suggested that the net ionic equation could also lead students to the wrong assumption that the ions come together to form a molecule. Specifically, ions change in a solution at the microscopic level, with a macroscopic observable colour change of the solution and precipitation, and students need to write a net ionic equation, which is at the symbolic level.
Another issue was associated with arrow symbol use. Several PCTs used a double-headed arrow for the equation of precipitation reaction. Although this situation could be interpreted as the equation of the precipitation reaction mixed with the equation of the solubility equilibrium, another assumption was that PCTs did not know the meaning of these arrows clearly. Regarding arrow symbol use in chemistry, Taber (2009) indicated that “the symbolism used in introductory courses sets up a false dichotomy between reversible (signified↔) and irreversible (signified→) reactions. While this may seem to be good pedagogy moving from a simple model to a more complex one, it can also act as [a] pedagogic learning impediment” (p. 97).
Regarding PCTs’ thinking patterns in the visualization of precipitation equations, it was concluded that many PCTs had several problems about the dissolution of ionic salts and representation of the ionic solid product. The dissolution of salts in water is one of the essential phenomena for precipitation reactions, and many researchers found that college level and high school students have difficulties and misconceptions (Devetak et al., 2009; Kelly et al., 2010; Naah and Sanger, 2012) about this matter. Naah and Sanger (2012) demonstrated that students illustrated the dissolution of ionic salts as neutral atoms or molecules in water. Kelly et al. (2010) showed that students depicted aqueous reactants as molecular pairs prior to mixing and only two to three students thought that the aqueous product was present as separate ions in solution, while the precipitate remained as a molecular pair. Similarly, this study found that more than half of the PCTs included the molecular dissolution features for ionic dissolution in their drawings. There are many reasons for these students’ difficulties with the process. First, dissolution is an abstract process since it happens at the sub-microscopic-level. Many researchers have revealed that students have difficulty in relating the micro and symbolic levels during the interpretation of the dissolution process (Blanco and Prieto, 1997; Raviolo, 2001). In addition, the students have to know ionic lattices, ionic bonds, and polarity. Butts and Smith (1987) reported that the actual process of aqueous dissolution, and the role of the polar water molecule in this process, seemed to be poorly understood by year 12 students.
Regarding PCTs’ thinking patterns in the visualization of the products of precipitation reactions and water, the findings revealed that some of the PCTs used the macroscopic representation of the precipitate with the sub-microscopic symbols together. Besides, many of them use macroscopic representations about water. Kelly et al. (2010) found that most of their college students omitted water molecules and some of them provided an ionic, macroscopic representation of water at the surface level or marked wavy lines in the solution. In our study, we saw this similar macroscopic representation of water and almost all the PCTs omitted the water molecules.
The final discussion is about use of students’ particle and atomic models in representing the chemical reaction. Most of the PCTs preferred the particle model to visualize the precipitation reaction. Cheng (2018) reported that, although the particle model looked simpler than the atomic model, it could help in supporting the learning of some advanced chemical concepts such as energetics and collision theory. Therefore, it is postulated that students who reason using the particle model can demonstrate some advanced ideas about chemical reactions. The conceptualization of reactions in terms of the atomic model and the particle model allows a flexible understanding of students’ learning.
Finally, the results of this study showed that it is extremely important to explore the students’ conceptions and visualization concerning previous learning experiences prior to the lecture when the goal is to provide meaningful learning. From a constructivist viewpoint, critical steps in promoting meaningful learning involve identifying students’ perceptions, thinking patterns and misconceptions in order to build toward scientifically accepted views. One of the two obstacles to the meaningful learning of the precipitation reaction phenomenon is related to its abstract nature. While the formation of a precipitate from reaction of two salt solutions is observed as a macroscopic process, this process is explained at the sub-microscopic level. Symbolic equations are also used to connect these two sides together. This requires that students understand these three-representations and associate them. For this reason, high school chemistry teachers should integrate the three representations in their teaching and move between them with an emphasis on their interconnection. Secondly, precipitation reactions require an understanding of several concepts (such as solubility, dissolving processes, writing chemical equations, balanced chemical equations, the mole concept, ionic bonding and bonding in the ionic lattice and bonding energy) and the relationship between and among them. Besides, when talking about solubility, it is important to clearly distinguish whether the solubility of a soluble solid or the solubility of a precipitate formed after precipitation reaction is mentioned.
These concepts are prerequisite knowledge for the precipitation reaction and lack of this knowledge hinders students’ learning about precipitation reactions. For this reason, instruction should be designed according to teaching strategies based on the constructivist approach where the students’ conceptions are viewed. This instruction should be used to address incorporating a relation between the sub-microscopic, symbolic and macroscopic levels by using the animations. Kelly and Jones (2008) cited that chemistry students understand particulate phenomena when they receive instruction that includes animations of these phenomena. On the other hand, Kelly (2017) indicated that “repairing inaccuracies in understanding by showing or telling students the correct answers have had limited success” (p. 181). Regarding this dilemma, researchers have indicated that when students were only shown animations and then attempted to draw or explain their new understandings, alternative perceptions persisted (Kelly and Akaygun, 2016; Kelly, 2017).
Final remarks
In conclusion, although this study shows several similar results to previous studies on the visualization of precipitation reactions, the finding of the phenomenographic analysis of PCTs’ perceptions about precipitation are different from the previous studies. It is expected that these findings can be helpful for researchers and can provide a different perspective to researchers. From our findings, it can be said that before the precipitation reactions are taught, to understand the students’ perceptions regarding the prerequisite knowledge of the topic would be helpful for teaching. Also, it is seen that the use of different analysis methods for student drawings together can provide the opportunity to interpret students’ thinking patterns from different points of view. Therefore, it can be suggested that different methods of analysis be used together for student drawings.So many studies indicated that high school and college level students have problems with understanding and interpreting the three levels of representing chemistry. This study also revealed that PCTs had similar issues. Gabel (1999) said that the primary barrier to understanding chemistry was not the existence of the three levels of representing matter, but the problem was about the chemistry instruction based predominantly on the most abstract level, the symbolic level. Gabel (1999) also indicated that even after making presentations to high school teachers about integrating the three levels, many teachers have not considered it in their own thinking. It is understood from this critical conclusion that it is not easy to provide high school chemistry teachers to integrate the three representations in their teaching. For this reason, this obstacle should be solved by taking this situation into consideration during the training of PCTs. Furthermore, this study found that PCTs had many problems with their own understanding of the triplet nature of chemistry and misconceptions about precipitation reactions. For this reason, if the misconceptions of PCTs are not corrected and their knowledge about this matter is not improved, they can convey these problems to their students in their future classes.
Conflicts of interest
There are no conflicts to declare.References
- Adadan E., (2014), Investigating the influence of pre-service chemistry teachers’ understanding of the particle nature of matter on their conceptual understanding of solution chemistry, Chem. Educ. Res. Pract., 15(2), 219–238.
- Adadan E. and Savasci F., (2012). An analysis of 16–17 year-old students’ understanding of solution chemistry concepts using a two-tier diagnostic instrument, Int. J. Sci. Educ., 34(4), 513–544.
- Alsop G. and Tompsett C., (2004) Being Pragmatic: Experiencing Phenomenography in the Context of Activity Theory, in ALT-C 2004: Blue skies and pragmatism – learning technologies for the next decade; 14–16 Sept 2004, Devon, UK: University of Exeter, (International Conference of the Association for Learning Technology (ALT), no. 11) ISBN 9780954587017. Retrieved from https://www.researchgate.net/publication/42531706_Being_Pragmatic_Experiencing_Phenomenography_in_the_context_of_Activity_Theory.
- Blanco A. and Prieto T., (1997), Pupils’ views on how stirring and temperature affect the dissolution of a solid in a liquid: a cross-age study (12 to 18), Int. J. Sci. Educ., 19(3), 303–315.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63, 873–878.
- Boo H.-K. and Watson J. R., (2001), Progression in high school students’ (aged 16–18) conceptualizations about chemical reactions in solution, Sci. Educ., 85(5), 568–585.
- Butts B. and Smith R., (1987), HSC chemistry students’ understanding of the structure and properties of molecular and ionic compounds, Res. Sci. Educ., 17, 192–201.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M. (2008), An Evaluation of a Teaching Intervention to Promote Students’ Ability to Use Multiple Levels of Representation When Describing and Explaining Chemical Reactions, Res. Sci. Educ., 38, 237–248.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2009), Emphasizing Multiple Levels of Representation to Enhance Students’ Understandings of the Changes Occurring during Chemical Reactions, J. Chem. Educ., 6(12), 1433–1436.
- Chang R. and Goldsby K. A., (2013), Chemistry: Eleventh edition, New York: McGraw-Hill Companies, Inc.
- Cheng M. M. W., (2018), Students’ visualisation of chemical reactions – insights into the particle model and the atomic model, Chem. Educ. Res. Pract., 19, 227–239.
- Cheng M. M. W. and Gilbert J. K., (2017), Modelling students’ visualisation of chemical reaction, Int. J. Sci. Educ., 39(9), 1173–1193.
- Cohen L., Manion L. and Morrison K., (2007), Research methods in education, New York: Routledge.
- Devetak I., Vogrinc J. and Glažar S. A., (2009), Assessing 16 year-old students’ understanding of aqueous solution at the submicroscopic level, Res. Sci. Educ., 39(2), 157–179.
- Ebenezer J. and Erickson G., (1996), Chemistry students’ conceptions of solubility: a phenomenography. Sci. Educ., 80(2), 181–201.
- Ebenezer J. and Fraser D., (2001), First Year Chemical Engineering Students’ Conceptions of Energy in Solution Processes: Phenomenographic Categories for Common Knowledge Construction, Sci. Educ.85, 509–535.
- Ebenezer J. and Gaskell P. J., (1995), Relational Conceptual Change in Solution Chemistry, Sci. Educ., 79(1), 1–17.
- Fensham N. and Fensham P., (1987), Descriptions and Frameworks of Solutions and Reactions in Solutions, Res. Sci. Educ., 17(1), 139–148.
- Gabel D., (1999), Improving Teaching and Learning through Chemistry Education Research: A Look to the Future, Chem. Educ., 76(4), 548–554.
- Gay L. R. and Airasion P., (2000), Educational research: Competencies for analysis and application, New Jersey: Prentice-Hall.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assist. Learn., 7, 75–83.
- Kelly R. M., (2017), Learning from contrasting molecular animations with a metacognitive monitor activity, Educ. Quím., 28, 181–194.
- Kelly R. M. and Akaygun S., (2016), Insights into how students learn the difference between a weak acid and a strong acid from cartoon tutorials employing visualizations, J. Chem. Educ., 93(6), 1010–1019.
- Kelly R. M. and Jones L. L., (2007), Exploring How Different Features of Animations of Sodium Chloride Dissolution Affect Students’ Explanations, J. Sci. Educ. Technol., 16, 413–429.
- Kelly R. M. and Jones L. L., (2008), Investigating students’ ability to transfer ideas learned from molecular animations of the dissolution process, J. Chem. Educ., 85(2), 303.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kumar R., (1999), Research methodology: a step-by-step guide for beginners, Sage Publications, 2nd edn.
- Lee K. W. L., (1999), Particulate Representation of a Chemical Reaction Mechanism, Res. Sci. Educ., 29(3), 401–415.
- Marton F., (1975), On non-verbatim learning I: level of processing and level of outcome, Scand. J. Psychol., 16, 273–279.
- Marton F., (1981), Phenomenography – describing conceptions of the world around us, Instruct. Sci., 10, 177–200.
- Marton F., (2005), Phenomenography: A Research Approach to Investigating Different Understandings of Reality, in Sherman R. R. and Webb R. B. (ed.), Qualitative Research in Education: Focus and Methods, London and New York.
- Naah B. M. and Sanger M. J., (2012), Student misconceptions in writing balanced equations for dissolving ionic compounds in water, Chem. Educ. Res. Pract., 13(3), 186–194.
- Naah B. M. and Sanger M. J., (2013), Investigating students’ understanding of the dissolving process, J. Sci. Educ. Technol., 22(2), 103–112.
- Nakiboğlu C. and Nakiboğlu N., (2017), Kimya öğrencilerinin “çökelme kavramini” anlama düzeyleri ve tanecik boyutunda gösterimlerinin incelenmesi, Paper presented in the 2nd International Contemporary Educational Research Congress, October, 2017, Muğla.
- Nurrenbern S. C. and Pickering M., (1987), Concept Learning versus Problem Solving: Is There a Difference? J. Chem. Educ., 64(6), 508–510.
- Nyachwaya J. M., Mohamed A.-R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students’ understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12, 121–132.
- Nyachwaya J. M., Warfa A. R. M., Roehrig G. H. and Schneider J. L., (2014), College chemistry students’ use of memorized algorithms in chemical reactions, Chem. Educ. Res. Pract., 15(1), 81–93.
- Patton M. Q., (2002), Qualitative Research and Evaluation Methods, 3rd edn, Thousand Oaks, CA: Sage.
- Raviolo A., (2001), Assessing students’ conceptual understanding of solubility equilibrium, J. Chem. Educ., 78(5), 629–631.
- Şen S., and Yılmaz A., (2017), Investigation of Preservice Teachers’ Conceptual Understanding of Dissolution through CHAID Analysis (In Turkish), Bartin University Journal of Faculty of Education, 6(3), 932–954.
- Smith K. J. and Metz P. A., (1996), Evaluating Student Understanding of Solution Chemistry through Microscopic Representations, J. Chem. Educ., 73(3), 233–235.
- Stamovlasis D., Tsaparlis G., Kamilatos C., Papaoikonomou D. and Zarotiadou E., (2005), Conceptual understanding versus algorithmic problem solving: further evidence from a national chemistry examination, Chem. Educ. Res. Pract., 6(2), 104–118.
- Stefani C. and Tsaparlis G., (2009), Students’ levels of explanations, models, and misconceptions in basic quantum chemistry: a phenomenographic study, J Res. Sci. Teach., 46(5), 520–536.
- Taber K. S., (1994), Misunderstanding the ionic bond, Educ. Chem., 31(4), 100–103.
- Taber K. S., (2000), Molar and molecular conceptions of research into learning chemistry: towards a synthesis, in The Chemical Education Research Group Lecture 2000, Overton T. (ed.) Variety in Chemistry Teaching 2000 Proceedings, Royal Society of Chemistry Tertiary Education Group, pp. 8–14.
- Taber K. S., (2001a), Constructing chemical concepts in the classroom? Using research to inform practice. Chem. Educ. Res. Pract. Eur., 2(1), 43–51.
- Taber K. S., (2001b), The mismatch between assumed prior knowledge and the learner's conceptions: a typology of learning impediments, Educ. Stud., 27(2), 159–171.
- Taber K. S., (2002), Chemical misconceptions – prevention, diagnosis and cure: classroom resources, London: Royal Society of Chemistry, vol. 2.
- Taber K. S., (2009), Learning at the Symbolic Level, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education. Models and Modeling in Science Education, Dordrecht: Springer, vol 4.
- Taber K. S., Tsaparlis C., and Nakiboğlu C., (2012), Student Conceptions of Ionic Bonding: Patterns of thinking across three European contexts, Int. J. Sci. Educ., 34(18), 2843–2873.
- Tan K. C. D., Goh N. K., Chia L. S. and Treagust D. F., (2009), Linking the Macroscopic, Sub-microscopic and Symbolic Levels: The Case of Inorganic Qualitative Analysis, in Gilbert J. K. and Treagust D. (ed.), Multiple Representations in Chemical Education. Models and Modeling in Science Education, Dordrecht: Springer, vol. 4.
- Tien L. T., Teichert M. A., and Rickey D., (2007), Effectiveness of a MORE laboratory module in prompting students to revise their molecular-level ideas about solutions, J. Chem. Educ., 87, 113–118.
- Tóth Z. and Ludányi L., (2007), Combination of Phenomenography with Knowledge Space Theory to study students’ thinking patterns in describing an atom, Chem. Educ. Res. Pract., 8 (3), 327–336.
- Vachliotis T., Salta K. and Tzougraki C., (2013), Meaningful Understanding and Systems Thinking in Organic Chemistry: Validating Measurement and Exploring Relationships, Res. Sci. Educ., 44(2), 239–266.
- von Glasersfeld E. (1989), Cognition, construction of knowledge, and teaching, Synthese, 80,121–140.
- von Glasersfeld E., (1990), An exposition of constructivism: why some like it radical, in Davis R. B., Maher C. A. and Noddings N. (ed.), Constructivist views on the teaching and learning of mathematics, Reston, Virginia: National Council of Teachers of Mathematics, pp. 19–29.
| This journal is © The Royal Society of Chemistry 2019 |