Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal-free synthesis of 1,N6-ethenoadenines from N6-propargyl-adenines via NIS mediated radical cascade reaction†
Ruchun Yangab,
Si Dengb,
Xiang-you Dongb,
Xianrong Song b,
Hu Cai
b,
Hu Cai *a,
Jiang Baib and
Qiang Xiao
*a,
Jiang Baib and
Qiang Xiao *b
*b
aInstitute of Chemistry, Nanchang University, Nanchang 330031, Jiangxi Province, China. E-mail: caihu@ncu.edu.cn
bInstitute of Organic Chemistry, Jiangxi Science & Technology Normal University, Key Laboratory of Organic Chemistry, Nanchang 330013, Jiangxi Province, China. E-mail: xiaoqiang@tsinghua.org.cn
First published on 26th November 2019
Abstract
In the present paper, an efficient approach for the construction of 1,N6-ethenoadenines from conveniently prepared N6-propargyl-adenines is developed. This reaction merges N-iodosuccinimide radical initiation and aerobic aminooxygenation in dioxane. This mild, 5-exo-dig, and metal-free cascade reaction could be applied to a wide substrate scope to provide 1,N6-ethenoadenines in moderate to good yields. The reaction mechanism was proposed and tested using radical inhibitor (butylated hydroxytoluene) and isotopic labelling (18O2) experiments.
Introduction
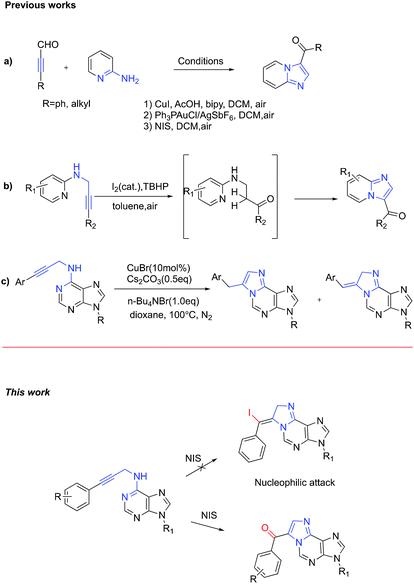
The development of efficient strategies for the synthesis of nitrogen containing heterocycles has attracted tremendous interest from both academic and pharmaceutical companies. In the past decades, various approaches have been developed and great progress has been achieved.1 Among these approaches, the cascade reaction turned out to be the most extensively employed one due to its high efficiency and step economy, without the isolation of possible miscellaneous intermediates.2 However, the corresponding nitrogen or sulphur atoms in the heterocyclic substrates generally possess strong coordinating ability to metal ions, which could lead to catalyst poisoning and side reactions.3 Thus, it is highly desirable to develop a new metal-free cascade reaction particularly for multiple nitrogen-containing heterocycles, such as nucleosides.In our continuous effort to develop fluorogenic nucleosides for nucleic acid analysis,4 1,N6-ethenoadenosines have attracted our attention because of their unique biological activities and conjugated structural skeleton (Fig. 1).5 Specifically, 1,N6-ethenoadenine 1 showed strongly fluorescent emission depending on the surrounding environment, which has been extensively used in analyzing the structure and functions of nucleic acids.6 Furthermore, it has been also recognized as a biomarker for the study of oxidative stress-related diseases and genetic damages associated with cancer.7 In addition, 9-hydroxy-1,N6-benzetheno-2′-deoxyadenosine 2 was firstly identified as DNA adduct with p-benzoquinone formed by peroxidase activation of benzene metabolites, which is well-known to cause acute leukemia in humans and bone marrow toxicity.8 Ethenoadenine 3 is another adducts of adenosine with 4-oxo-2-nonenal, which is a novel product of lipid peroxidation and may play an important role in lipid hydroperoxide-mediated carcinogenesis.9
Despite of the significance of 1,N6-ethenoadenosines mentioned above, literatures survey revealed that the availability of their synthetic approaches was very rare. The typical synthetic route is the reaction of α-halocarbonyl compounds with purine.10 Therefore, the development of novel methodology with high efficiency and structural diversity is in high demand.
In recent years, propargylamine derivatives, bearing electrophilic triple bonds, have emerged as promising cascade synthons for heterocycle synthesis.11 For example, imidazo[1,2-a]pyridines were readily prepared from 3-phenylpropiolaldehyde and 2-aminopyridine by using either copper(I) or gold(I) catalyst along with air as the oxidant (Scheme 1a, (1) and (2)).12 Later on, Das et al. further developed an improved and general metal-free aminooxygenation of alkynes for the rapid construction of 3-aroylimidazo[1,2-a]pyridines (Scheme 1a, (3)).13 According to the proposed mechanism, NIS works as an iodine cation donor, which may coordinate with the alkyne to form iodonium intermediate. Then nucleophilic addition of water followed by elimination of hydrogen iodide afforded the target product. Very recently, Huang et al. reported a metal free synthesis of aroylimidazo[1,2-a]pyridine via intramolecular dehydrogenative aminooxygenation of alkynes, which use I2 as catalyst and TBHP as an oxidant (Scheme 1b).14 But this approach has never been applied to purine substrates. In 2014, Guo et al. developed a novel approach to construct purine-fused 1,N6-ethenoadenine via copper-catalysed intramolecular cyclization of N6-propargyl-adenine at high temperature. However, this approach always affords two regioisomers (Scheme 1c).15
Based on examples mentioned previously in the literature, we envisioned that N6-propargyl-adenine could be activated by NIS to form iodonium intermediate. The subsequent N-1 mediated nucleophilic attacking to the resulting iodonium intermediate would generate 1,N6-ethenoadenines products directly. If it works, this new metal free synthetic route could provide direct access to 1,N6-ethenoadenines in one step cascade reaction from readily available starting material.
Results and discussion
To test our hypothesis, we initially mixed readily prepared N6-propargyl-adenine 1a with 1 eq. NIS in DCM at room temperature (Table 1, entry 1). It is encouraging to find that a florescent product was obtained in moderate yield of 46%. From NMR spectra, there is only one CH2 group present which belong to N9-benzyl group and there is a new carbonyl and a new aromatic CH appeared. In addition, element analysis excluded iodine atom in the target molecule. After extensive characterization analysis, the target structure was determined to be the oxidized cascade cyclization product 2a.| Entry | Halo source | Solvent | Time | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: 0.1 mmol 1a, 1.0 eq. NIS, in 2.0 mL dioxane under air, room temperature.b Isolated yields.c Reaction performed at 50 °C.d Reaction performed at 80 °C.e Dark, 1 atm O2.f Sunlight, argon atmosphere. | ||||
| 1 | NIS | DCM | 12 h | 46 |
| 2 | NIS | CH3OH | 12 h | 34 |
| 3 | NIS | DMSO | 12 h | 36 |
| 4 | NIS | Dioxane | 12 h | 74 |
| 5 | NIS | DMF | 12 h | 23 |
| 6 | NIS | CH3CN | 12 h | 52 |
| 7 | NIS | THF | 12 h | 48 |
| 8 | — | Dioxane | 12 h | None |
| 9 | NIS (0.5 eq.) | Dioxane | 12 h | 33 |
| 10 | NIS (1.2 eq.) | Dioxane | 12 h | 75 |
| 11 | NIS (1.5 eq.) | Dioxane | 12 h | 73 |
| 12c | NIS (1.2 eq.) | Dioxane | 12 h | 65 |
| 13d | NIS (1.2 eq.) | Dioxane | 12 h | 63 |
| 14 | NBS | Dioxane | 12 h | Trace |
| 15 | NCS | Dioxane | 12 h | None |
| 16 | I2 | Dioxane | 12 h | 13 |
| 17e | NIS (1.2 eq.) | Dioxane | 12 h | 46 |
| 18f | NIS (1.2 eq.) | Dioxane | 12 h | Trace |
In order to improve the efficiency, a series of solvents with different polarity, such as DMF, dioxane, CH3CN, CH3OH, and DMSO, were screened (entries 1–7). Dioxane proved to be optimal and gave the best yield of 74% (entry 4). Moreover, 1.2 eq. NIS is sufficient to complete this transformation (entry 10). Increasing the reaction temperature led to slightly lower yields (entries 12–13). Furthermore, the effect of different halo sources (NCS, NBS and iodine) were examined (entries 14–16). Neither NBS nor NCS could promote the cascade reaction and only trace amount of desired product could be detected. Using iodine only provided 13% yield. In addition, the reaction performed under argon gave only trace amount of product, revealing the essential role of oxygen. Furthermore, the reaction became sluggish in absence of light. Thus, we chose dioxane as solvent, 1.2 eq. NIS, room temperature, and opening to air as the reaction conditions for further investigation (Table 2).
| a Reaction conditions: 0.1 mmol 1a–1s, 1.2 eq. NIS, in 2.0 mL dioxane under air; isolated yields. |
|---|
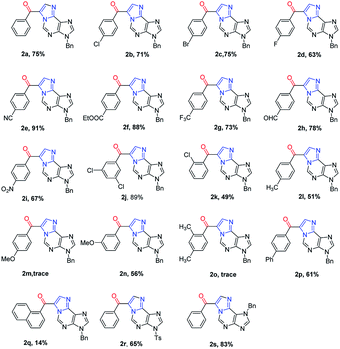 |
To demonstrate the generality of this transformation, various substituted N6-propargyl-adenines were subjected to the optimized conditions. Firstly, the effect of aromatic ring bearing different R substituents attached to alkyne was evaluated. A series of halogens including F, Cl and Br were compatible with this cascade reaction and the desired products were generated in good yields (2b–2d). It was found that substrates bearing electron-withdrawing substituents (CN, NO2, CF3 etc.) afforded product in higher yield than the substrate bearing electron-donating substitutes (Me, OMe, phenyl etc.). However, the strong electron-donating group substituted at the para position of the alkyne remarkably retarded the reaction (2m, trace). The electron-donating group at the meta position also significantly reduced the reaction yield (2n, 56%). Steric hindered substrates proved to be not suitable for the reaction and only low yields were obtained (2k, 2o and 2q). Furthermore, the effect of the substituent attached on purine, including N7-benzyl and N9-Ts substituted substrates also gave the corresponding 1,N6-ethenoadenines in good yields (2r, 65%; 2s, 83%). Fortunately, a single crystal suitable for X-ray crystallography of 2n was obtained and its structure was unambiguously confirmed in Fig. 2, which further verified the proposed structure.
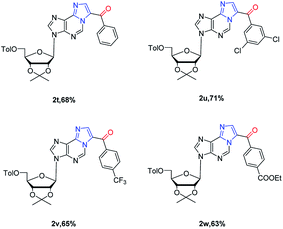
Next, substrate scope was extended to ribose nucleoside substrates 1t–1w under the optimized reaction condition (Table 3). The corresponding 1,N6-ethenoadenosines were also obtained in good yields (2t–2w). Their UV and fluorescent spectroscopies were recorded. From UV spectrum, they showed a unique adsorption at 340 nm (see ESI†). Furthermore, fluorescence spectrum of 2w showed emission wave at 437 nm and excitation at 270 nm, which is very useful for nucleic acid research when incorporated into oligonucleotides. The related work is on-going and will be reported in due course.
| a Reaction conditions: 0.1 mmol 1t–1w, 1.2 eq. NIS, in 2.0 mL dioxane under air; isolated yields. |
|---|
 |
To gain insights into the reaction mechanism, we revisited the optimization process. We observed three clear facts: (1) the oxygen is essential for this transformation. (2) Other NXS except for NIS cannot afford the desired products in good yield. (3) Retarded reaction was observed under dark environment. Considering NIS could generate iodine radical, we assumed that the reaction might be a radical mechanism, which is contrary to our initially proposed mechanism through iodonium intermediate.
In order to test our hypothesis, control experiments were performed as shown in Scheme 2. When the radical inhibitor butylated hydroxytoluene (BHT) was added to the reaction, we observed that the reaction was almost inhibited, which indicated that a radical intermediate maybe involved in this cascade reaction (Scheme 2a). In order to further verify the resource of oxygen atom in oxidation products, isotopic labelling experiment using 18O2 were conducted. The labeled product 2f-O18 can be confirmed by HRMS analysis (Scheme 2b). The result demonstrated that the oxygen atom of oxidation product 2a was utmost originated from O2. To the best of our knowledge, bifunctionalization of alkyne by radical reaction and aerobic aminooxygenation is rarely reported.16 In addition, the cyclization reaction cannot happen if the NH-6 was blocked by methyl group.
Based on these preliminary mechanistic studies and previous reports, a plausible mechanism for this cascade reaction is proposed as shown in Scheme 3. Initially, the reaction of the N6–H of starting material 1 with NIS gave intermediate A, which is easily hemolytic to form radical intermediate B. The tautomerism of radical intermediate B could deliver N-1 radical intermediate C. Then, the N-1 radical attack the triple bond through 5-exo-dig configuration to afford cyclized radical intermediate D in higher yields. After oxidation of D using molecular O2, the resulted intermediate E rearranged to give ethenoadenine 2 after elimination of HOI.
Conclusions
In summary, an efficient approach for the preparation of 1,N6-ethenoadenine from N6-propargyl-adenine was developed. This approach features: (1) easily prepared starting material; (2) NIS mediated radical and metal-free cascade reaction; (3) air used as an oxygen source and as the oxidant. We believe that this methodology provides a complementary synthetic approach to 1,N6-ethenoadenine architectures and the NIS mediated radical cascade reaction of N-propargylamine under air opens up new opportunities for the synthesis of other heterocycles.Experimental
General procedure for the preparation of products
NIS (27.0 mg, 0.12 mmol) was added to a stirred solution of 1a–1w (0.1 mmol) in dioxane (2 mL) under open air atmosphere. The resulting mixture was stirred at room temperature and the progress of the reaction was monitored by thin-layer chromatography. After completion of the reaction, the mixture was quenched by slow addition of saturated sodium thiosulfate and extracted with EtOAc (3 × 5 mL). The combined organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel to give the corresponding product 2a–2w.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the National Natural Science Foundation of China (No. 21676131, No. 21571094, and No. 21861026) and the Bureau of Science & Technology of Jiangxi Province (No. 20143ACB20012) are gratefully acknowledged.Notes and references
- For selected reviews on synthetic progress and pharmaceutical application of heterocycles, see: (a) D. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson and A. Wood, Nat. Chem., 2018, 10, 383 CrossRef CAS PubMed; (b) J. Akhtar, A. A. Khan, Z. Ali, R. Haider and M. S. Yar, Eur. J. Med. Chem., 2017, 125, 143 CrossRef CAS PubMed; (c) A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones and O. Fadeyi, Org. Biomol. Chem., 2016, 14, 6611 RSC; (d) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- For selected reviews on cascade synthesis of heterocycles, see: (a) J. Lei, J.-P. Meng, D.-Y. Tang, B. Frett, Z.-Z. Chen and Z.-G. Xu, Mol. Diversity, 2018, 22, 503 CrossRef CAS PubMed; (b) J. Xuan and A. Studer, Chem. Soc. Rev., 2017, 46, 4329 RSC; (c) B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505 RSC; (d) C. J. Ball and M. C. Willis, Eur. J. Org. Chem., 2013, 425 CrossRef CAS.
- For selected examples about discussing potential poisoning catalysis by heterocycles, see: (a) S. J. Tereniak and S. S. Stahl, J. Am. Chem. Soc., 2017, 139, 14533 CrossRef CAS PubMed; (b) R. P. Downs, A. L. Chin, K. M. Dean and J. D. Carrick, J. Heterocycl. Chem., 2017, 54, 3008 CrossRef CAS; (c) C. Shen, A. Spannenberg and X.-F. Wu, Angew. Chem., Int. Ed., 2016, 55, 5067 CrossRef CAS PubMed; (d) Y.-J. Liu, H. Xu, W.-J. Kong, M. Shang, H.-X. Dai and J.-Q. Yu, Nature, 2014, 515, 389 CrossRef CAS PubMed.
- (a) C. Hu, Z. Ruan, H. Ding, Y. Zhou and Q. Xiao, Molecules, 2017, 22, 643 CrossRef PubMed; (b) H.-X. Ding, M. Wan, R.-C. Yang, W.-H. Xiao and Q. Xiao, Chin. J. Org. Chem., 2008, 28, 330 CAS; (c) Q. Xiao, R. T. Ranasinghe, A. M. P. Tang and T. Brown, Tetrahedron, 2007, 63, 3483 CrossRef CAS.
- (a) Z. Jahnz-Wechmann, G. R. Framski, P. A. Januszczyk and J. Boryski, Front. Chem., 2016, 4, 19 Search PubMed; (b) C.-H. Wu, J. Zhou and C.-B. Chen, Chin. J. Org. Chem., 2006, 26, 1457 CAS.
- (a) P. Virta, T. Holmstrom, T. Munter, T. Nyholm, L. Kronberg and R. Sjoholm, Nucleosides, Nucleotides Nucleic Acids, 2003, 22, 85 CrossRef CAS PubMed; (b) J. Wierzchowski, A. Stachelska-Wierzchowska, B. Wielgus-Kutrowska and A. Bzowska, Curr. Pharm. Des., 2017, 23, 6948 CrossRef CAS PubMed.
- M. T. Leithauser, A. Liem, B. C. Stewart, E. C. Miller and J. A. Miller, Carcinogenesis, 1990, 11, 463 CrossRef CAS PubMed.
- A. Chenna, B. Hang, B. Rydberg, E. Kim, K. Pongracz, W. J. Bodell and B. Singer, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 5890 CrossRef CAS PubMed.
- (a) A. Winczura, A. Czubaty, K. Winczura, K. Maslowska, M. Nalecz, D. A. Dudzinska, M. Saparbaev, K. Staron and B. Tudek, DNA Repair, 2014, 22, 1 CrossRef CAS PubMed; (b) H. Bartsch and J. Nair, Toxicology, 2000, 153, 105 CrossRef CAS PubMed.
- S. Mikkola, N. Koissi, K. Ketomaki, S. Rauvala, K. Neuvonen and H. Lonnberg, Eur. J. Org. Chem., 2000, 2315 CrossRef CAS.
- (a) K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Chem. Rev., 2017, 117, 14091 CrossRef CAS PubMed; (b) V. A. Peshkov, O. P. Pereshivko, A. A. Nechaev, A. A. Peshkov and E. V. Van der Eycken, Chem. Soc. Rev., 2018, 47, 3861 RSC; (c) J. Xie, Z. Guo, Y. Huang, Y. Qu, H. Song, H. Song, Y. Liu and Q. Wang, Adv. Synth. Catal., 2019, 361, 490 CrossRef CAS; (d) E. Vessally, S. Soleimani-Amiri, A. Hosseinian, L. Edjlali and A. Bekhradnia, RSC Adv., 2017, 7, 7079 RSC.
- (a) H. Cao, X. Liu, J. Liao, J. Huang, H. Qiu, Q. Chen and Y. Chen, J. Org. Chem., 2014, 79, 11209 CrossRef CAS PubMed; (b) H. Zhan, L. Zhao, J. Liao, N. Li, Q. Chen, S. Qiu and H. Cao, Adv. Synth. Catal., 2015, 357, 46 CrossRef CAS.
- K. R. Reddy, A. P. Gupta and P. Das, Asian J. Org. Chem., 2016, 5, 900 CrossRef CAS.
- Y. He, W. Yin, J. Wang, J. Huang, X. Pang, C. Gan, F. Yang and C. Huang, Org. Chem. Front., 2018, 5, 1772 RSC.
- R. L. Li, L. Liang, M. S. Xie, G. R. Qu, H. Y. Niu and H. M. Guo, J. Org. Chem., 2014, 79, 3665 CrossRef CAS PubMed.
- For recent examples, see: (a) J. Santhi and B. Baire, Adv. Synth. Catal., 2016, 358, 3817 CrossRef CAS; (b) W. Chen, Y. Zhang, P. Li and L. Wang, Org. Chem. Front., 2018, 5, 855 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1955922. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra09198j |
| This journal is © The Royal Society of Chemistry 2019 |