Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An electrochemical study of pH influences on corrosion and passivation for a Q235 carbon steel in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions
Xuan Lia,
Pei Zhangb,
Huiju Huanga,
Xiaochen Hua,
Yong Zhou *a and
Fuan Yana
*a and
Fuan Yana
aKey Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan 430205, China. E-mail: zhouyong@wit.edu.cn
bCollege of Chemistry and Food Science, Yulin Normal University, Yulin 537000, China
First published on 28th November 2019
Abstract
In this study, the influences of different pH values on the corrosion and passivation behaviors of a Q235 carbon steel in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions were studied by electrochemical methods. The manifestations of the electrochemical characteristics were revealed and the variations in the electrochemical parameters were clarified. Moreover, for the Q235 steel in the three solutions with different pH values, the decrease in the corrosion current density (icorr) and the increase in the charge transfer resistance (Rct) in each solution, indicated a decrease in the corrosion rate. The decrease in the critical passivation current density (icrit) and increase in the passive film resistance (Rf) suggested the reinforcement of passivation capability. On the other hand, in the three solutions at the same pH value, the corrosion rate increased and the passivation capability weakened in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions. Simultaneously, the related electrochemical mechanisms of corrosion and passivation for Q235 carbon steel in acidic solutions containing nitrite anions (NO2−) were also discussed.
1. Introduction
Carbon steel materials are widely used in the construction of large engineering structures.1–3 However, in electrolytic environments, the corrosion of structural steel products is unavoidable,4 especially in strong electrolytes.5 The addition of corrosion inhibitors, mainly including oxidation and/or adsorption species, into electrolytic environments can inhibit the corrosion process of metals and alloys effectively.6As an oxidizing type inhibitor, the addition of nitrite anions (NO2−) into alkaline and neutral electrolytes significantly decreases the corrosion rate for carbon steels, which is attributed to the function of NO2− on the repassivation of the steel surface,7–12 and the crucial electrochemical mechanism of NO2− is as follows:
| 2Fe2+ + 2OH− + 2NO2− → 2NO + γ-Fe2O3 + H2O | (1) |
At present, in alkaline and neutral electrolytes containing NO2−, many studies concerning the corrosion and passivation behaviors of carbon steels have been reported.7–12
In contrast, there are relatively few related studies in acidic electrolytes containing NO2−.13–17 Zhou et al.13 studied the corrosion and passivation behaviors of Q235 carbon steel in CO2 saturated solutions containing NO2−. The authors reported that the electrochemical characteristics of the Q235 steel transferred from the active dissolution in the single CO2 solution free of NO2− to the anodic passivation in the corresponding solutions containing NO2−, which was due to the formation of the Fe2O3 passive film under the FeCO3 layer. Zuo et al.14 studied the pitting and passivation behaviors of the X70 carbon steel in acidic NaCl solutions containing NO2− and thioureido imidazoline (TAI). The authors reported that although the interactive and superimposed mechanism between NO2− and TAI was present, NO2− (rather than TAI) was responsible for the passivation of the X70 steel. However, in the studies of Zhou et al.13 and Zuo et al.,14 the pH value was kept at pH 3.7 and pH 5.5, respectively, which is too limited in scope and not systematic. In contrast, Garces et al.16 studied the corrosion and passivation behaviors of a corrugated steel bar in simulated pit solutions containing NO2− from pH 1.46 to pH 6.38. For the corrugated steel bar in the simulated solutions, the authors reported that the addition of NO2− promoted the surface passivation and restrained the pitting corrosion, but the general corrosion rate also increased. Zhou et al.17 studied the corrosion and passivation behaviors of Q235 carbon steel in HCl solutions containing NO2− from pH 1 to pH 6. The authors reported that uniform corrosion, intergranular corrosion and pitting corrosion occurred on the surface of the Q235 carbon steel in sequence with the increasing pH value, and NO2− played a critical role in the above three corrosion stages. Nevertheless, in the studies of Garces et al.16 and Zhou et al.,17 the influences of the pH value and NO2− presence on the variations in the electrochemical parameters were not discussed in detail.
In a previous study,18 we carried out potentiodynamic polarization tests on the L80, N80, X65 and Q235 steels in HNO3–NaNO2, HCl–NaNO2, HAc–NaNO2 and CO2–NaNO2 solutions and reported the relationship between the activation–passivation transition and the grain boundary dissolution for both carbon steels and alloy steels in acidic solutions containing NO2−. However, in the above study, the detailed pH influences on the corrosion and passivation behaviors, particularly on the manifestations of electrochemical characteristics and the variations of electrochemical parameters, were not discussed. Therefore, in this study, the influence of different pH values on the corrosion and passivation behaviors of Q235 carbon steel in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions are studied by electrochemical methods, and the related electrochemical mechanisms are also discussed in detail.
2. Experimental
The studied material was Q235 carbon steel with the following chemical composition (weight percent): C, 0.160; Mn, 0.530; Si, 0.300; S, 0.045; P, 0.015, and Fe, balance. Samples were manually abraded up to 1000 grit with SiC abrasive papers, rinsed with de-ionized water and degreased in alcohol.The studied solutions were HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions. The diluted HNO3, HAc and HCl solutions were each introduced into a 0.01 mol L−1 NaNO2 solution to adjust the pH value and obtain the three final solutions.
The electrochemical tests of potentiodynamic polarization, cyclic voltammetry, electrochemical impedance spectroscopy (EIS) and potential step were performed at ambient temperature by a CS310 electrochemical workstation (China). A typical three-electrode system was used for all electrochemical tests. The system was composed of a saturated calomel electrode (SCE) or a silver–silver chloride electrode (Ag/AgCl) as the reference electrode, a platinum sheet as the counter electrode and a Q235 sample as the working electrode. The SCE electrode was applied in the electrochemical tests of potentiodynamic polarization, EIS and potential step; the Ag/AgCl electrode was applied in the cyclic voltammetry tests. Before each electrochemical test, the working electrode was immersed in the corresponding studied solution for a certain period of time until the open circuit potential (OCP) was stable. In the potentiodynamic polarization tests, the potential scanning rate was 0.5 mV s−1, and the potential scanning range was from −0.5 VOCP to the potential value corresponding to the objective electrochemical characteristic. In the cyclic voltammetry tests, the potential scanning rate was 30 mV s−1, and the potential scanning range was from −2.0 VAg/AgCl to 2.0 VAg/AgCl. In the EIS tests, a perturbation potential of 10 mV amplitude was used in the frequency range from 105 to 10−2 Hz. In the potential step tests, an applied potential was suddenly added on the working electrode, and the recording frequency of the current density was 5 Hz.
3. Results and discussion
3.1 Electrochemical characteristic
Fig. 1 shows the polarization curves of the Q235 samples in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions. From Fig. 1, the influences of the pH values on the electrochemical characteristics of the Q235 carbon steel in the three solutions are very prominent. In the HNO3–NaNO2 solutions of pH 1–4 and in the HAc–NaNO2 solutions of pH 3–6, the Q235 samples show a similar electrochemical characteristic: the anodic current density first increased gradually with the positive shift of applied potential, but then decreased suddenly when the applied potential reached the activation–passivation transition potential (Etrans) value corresponding to the critical passivation current density (icrit); after that, the anodic current density remained at the maintaining passivation current density (imain) until the occurrence of transpassivation. The above electrochemical characteristic is called “activation–passivation–transpassivation (A–P–T)” in this work. The anodic current density also first increased gradually with the positive shift of applied potential in the HNO3–NaNO2 solutions at pH 5 and pH 6, but directly reached the imain value without the activation–passivation transition, which is called “self-passivation–transpassivation (sp-T)”. In the HCl–NaNO2 solutions at pH 1 and pH 2, the anodic current density increased continuously with the positive shift of applied potential. This is called “activation (A)” in this work. In the HCl–NaNO2 solutions at pH 3 to pH 6, the initial evolution of the anodic current density in these solutions was very similar to that in the HNO3–NaNO2 solutions at pH 1 to pH 4. However, due to the occurrence of pitting corrosion induced by Cl−,19 the anodic current density increased suddenly when the applied potential reached the pitting potential (Epit). This electrochemical characteristic is called “activation-passivation-pitting (A–P–P)”.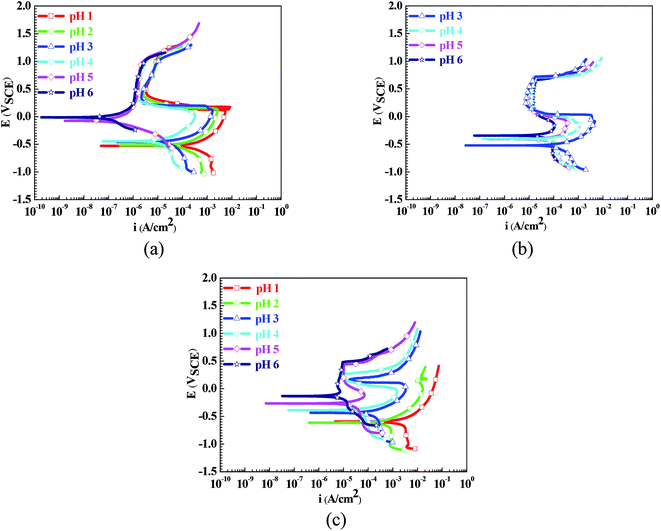 | ||
| Fig. 1 Polarization curves of the Q235 samples in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions: (a) HNO3–NaNO2 solutions, (b) HAc–NaNO2 solutions and (c) HCl–NaNO2 solutions. | ||
When the scanning of the applied potential is close to the corrosion potential (Ecorr) for carbon steels in acidic electrolytes, the main anodic reaction is the Fe oxidation with a standard potential (Est) of −0.441 VSHE,20 which is given as follows:
| Fe → Fe2+ + 2e (Est = −0.441 VSHE) | (2) |
At the same time, the main cathodic reactions depend on the pH value of the acidic electrolytes. The H+ reduction and the O2 reduction occur at relatively low and high pH values,15 respectively, which are given as follows:
| 2H+ + 2e → H2 (Est = 0 VSHE) | (3) |
| O2 + 2H2O + 4e → 4OH− (Est = 0.401 VSHE) | (4) |
It is generally accepted that carbon steels usually show an electrochemical characteristic of active dissolution in acidic electrolytes.21 The manifestation on the polarization curve is that the anodic current density keeps increasing with the positive shift of the applied potential.22 In this work, similar results were also observed on the polarization curves when the scanning of the applied potential slightly exceeded Ecorr, as shown in Fig. 1.
However, except in the pH 1 and pH 2 HCl–NaNO2 solutions, a passivation occurrence was present on the polarization curves with a positive shift of the applied potential, as shown in Fig. 1. One is the anodic passivation like the polarization curves in the HNO3–NaNO2 solutions at pH 1 to pH 4, in the HAc–NaNO2 solutions at pH 3 to pH 6 and in the HCl–NaNO2 solutions at pH 3 to pH 6: the anodic current density decreased suddenly when the applied potential reached the Etrans value. The other is the spontaneous passivation like the polarization curves in the HNO3–NaNO2 solutions at pH 5 and pH 6: the anodic current density remained at the imain value before the applied potential reached the transpassivation potential. A large number of studies have reported that the oxidation from Fe2+ to Fe3+ plays a critical role in the surface passivation of steel materials.23–25 The anodic reaction of Fe2+ oxidation is given as follows:
| Fe2+ → Fe3+ + e (Est = 0.771 VSHE) | (5) |
In order to confirm the oxidation from Fe2+ to Fe3+ on the surface of the Q235 carbon steel in the three solutions, the electrochemical tests of cyclic voltammetry were carried out. Fig. 2 shows the cyclic voltammograms of the Q235 samples in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions at pH 4. The reason why the pH 4 solutions are chosen is that the cathodic reactions involving NO2− reduction and their equilibrium potential at pH 4 have been reported by Li et al.26 From Fig. 2, for the Q235 steel in the pH 4 solutions, the CV peaks corresponding to the oxidation from Fe2+ to Fe3+27–29 are observed on the cyclic voltammograms. In pure acidic electrolytes free of oxidants, the anodic reaction of Fe2+ oxidation is not available, which is attributed to the Est value of Fe2+ oxidation (0.771 VSHE) being greater than that of H+ reduction (0 VSHE) or O2 reduction (0.401 VSHE). However, in this work, due to the presence of NO2− in the three solutions, the cathodic reaction of NO2− reduction made the anodic reaction of Fe2+ oxidation possible.13,15,18 It is noteworthy from Fig. 2 that the CV peaks corresponding to the reduction of NO2− were absent, and similar results were also obtained by Li et al.26 and Valcarce et al.11 Regarding the cathodic reduction of NO2−, Zhou et al.13 reported the following electrode reaction in a CO2-saturated solution:
| NO2− + e → NO + O2− (Est = 0.715 VSCE or 0.959 VSHE) | (6) |
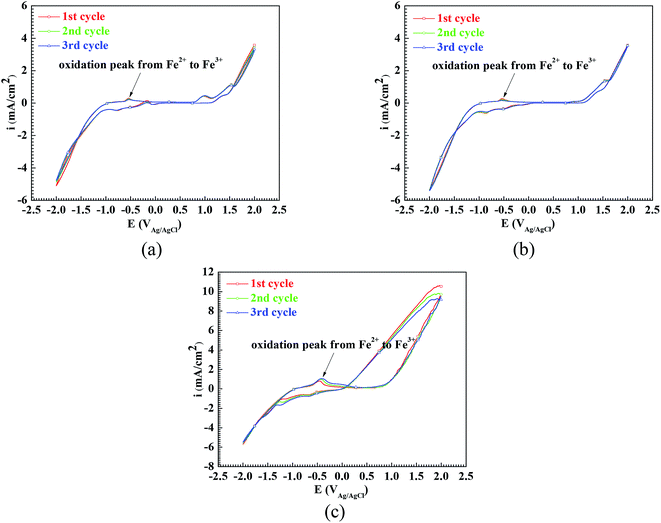 | ||
| Fig. 2 Cyclic voltammograms of the Q235 samples in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions at pH 4: (a) HNO3–NaNO2 solution, (b) HAc–NaNO2 solution and (c) HCl–NaNO2 solution. | ||
In addition, Li et al.26 reported the following electrode reactions:
| 2NO2− + 8H+ + 6e → N2 + 4H2O (Est = 0.944 VSCE or 1.188 VSHE) | (7) |
| 2NO2− + 6H+ + 4e → N2O + 3H2O (Est = 0.771 VSCE or 1.015 VSHE) | (8) |
Because there was a cathodic reaction whose Est was more positive than the Est of Fe2+ oxidation, the anodic reaction of Fe2+ oxidation was possible, resulting in the occurrence of passivation on the surface of the Q235 carbon steel.
From the above discussion, for the Q235 carbon steel in the three solutions, the corrosion occurred when the applied potential was close to Ecorr, followed by the occurrence of passivation with the gradual positive shift of the applied potential. The influences of pH values on the electrochemical parameters of corrosion and passivation are significant, which will be discussed as follows. In this work, in order to obtain the electrochemical parameters, the polarization curves were fitted by the CVIEW software according to the Tafel interpretation, and the EIS were fitted by the ZVIEW software according to the equivalent electrical circuit (EEC) interpretation.
3.2 Corrosion behavior (corrosion rate)
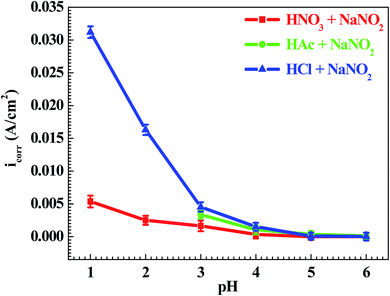
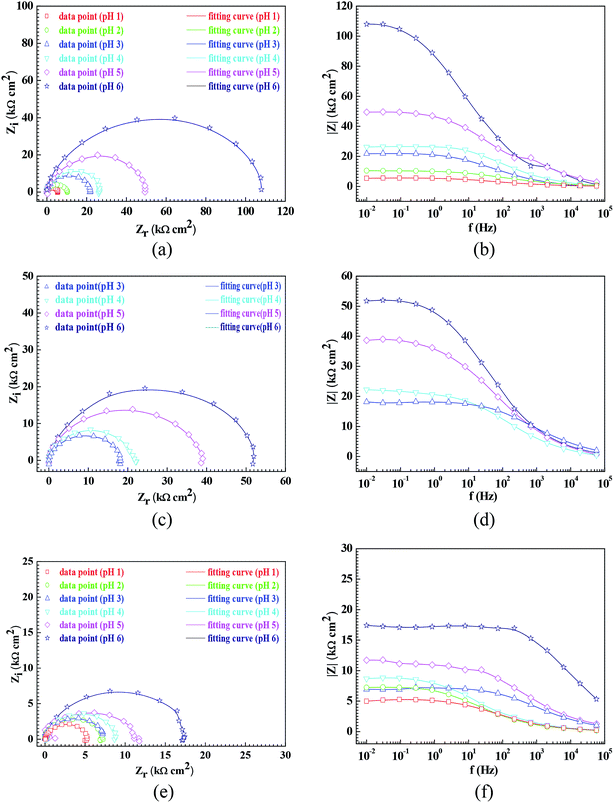
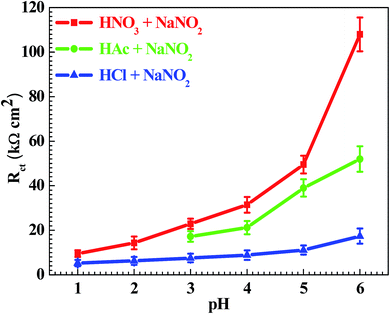
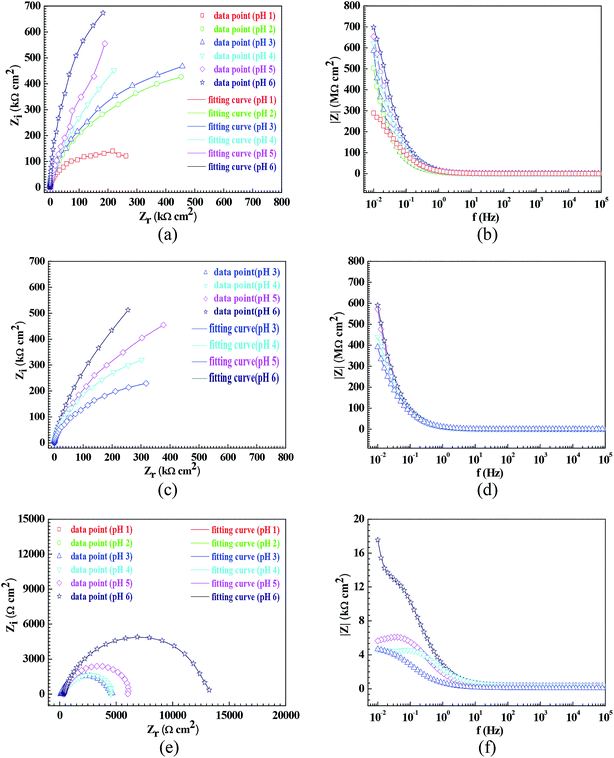
Fig. 3 shows the pH influences on the values of the corrosion current density (icorr). From Fig. 3, the icorr value in each solution decreased with increasing pH value, indicating the pH influence on the corrosion rate. The chemical equilibriums of H+ reduction (eqn (3)) and O2 reduction (eqn (4)) moved toward the left direction with increasing pH value, resulting in a decrease of the corrosion rate.30 At the same time, the icorr value gradually increased in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions at the same pH value (Fig. 3), which is attributed to the inhibitive and aggressive properties of NO3−,31 and Cl−,32 respectively.In order to further confirm the influence of the pH value on the corrosion rate, EIS tests were also carried out. Fig. 4 shows the Nyquist and Bode plots of the Q235 samples in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions at the applied potential of OCP. From the Nyquist plots shown in Fig. 4a, c and e, all Nyquist plots for the Q235 carbon steel in the three solutions at OCP are composed of a single depressed capacitive semicircle in the whole applied frequency range, which is independent of the pH value. It was reported that for carbon steels in acidic electrolytes, the single capacitive semicircle reflected the electrochemical process of a charge transfer between electron double layer.33 At the same time, the radius of the capacitive semicircle expanded with increasing pH value in each solution, indicating a decrease of the corrosion rate.34 On the other hand, from the Bode plots shown in Fig. 4b, d and f, the modulus at the low frequency limit of 0.01 Hz increased with increasing pH value in each solution, confirming the decrease of the corrosion rate. This result is in agreement with the icorr result. Furthermore, the EIS results were interpreted with the EEC model shown in Fig. 5a, where RS represents the solution resistance, CPEdl represents the double layer capacitance, and Rct represents the charge transfer resistance. It was reported that the Rct value indicated the corrosion rate of the metals and alloys in electrolytic environments.35 Fig. 6 shows the pH influences on the values of Rct. Comparing Fig. 6 with Fig. 3, the variations of Rct and icorr are very similar: in each solution, the Rct value increased with increasing pH value. At the same pH value, the Rct value in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions gradually decreased in turn.
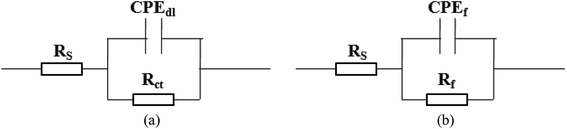 | ||
| Fig. 5 Equivalent electrical circuit (EEC) models for EIS interpretation: (a) EIS shown in Fig. 4 and (b) EIS shown in Fig. 8. | ||
3.3 Passivation behavior (passivation capability)
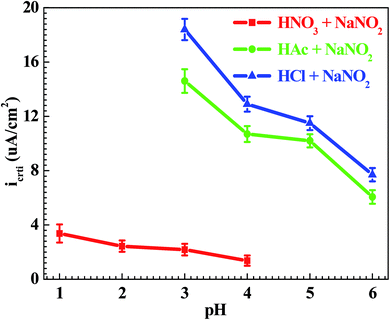
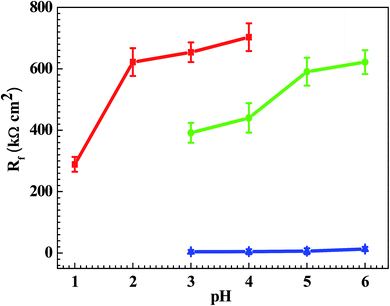
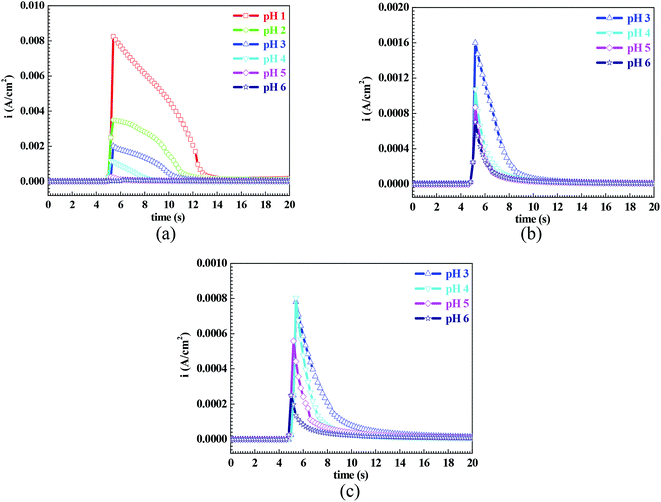
From Fig. 1, the pH range for the surface passivation, including anodic passivation and spontaneous passivation, of the Q235 carbon steel in HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions was from pH 1 to pH 6, from pH 3 and pH 6 and from pH 3 to pH 6, respectively. On the one hand, the pH range of passivation occurrence in the HNO3–NaNO2 solutions was more extensive than that in the HAc–NaNO2 and HCl–NaNO2 solutions. On the other hand, the spontaneous passivation occurred in the HNO3–NaNO2 solutions at pH 5 and pH 6 only. The above two aspects indicate that for the Q235 carbon steel in the three solutions, the passivation capability in HNO3–NaNO2 solutions was strongest. Furthermore, the passivation capability of the Q235 steel in the HAc–NaNO2 solutions took second place, and the relatively narrow pH range of passivation occurrence was due to the weak acidic property of HAc.18 Although the surface passivation occurred in the HCl–NaNO2 solutions at pH 3 to pH 6, the formed passive film would inevitably be attacked by the aggressive Cl− ions,36 so the passivation capability of the Q235 carbon steel in HCl–NaNO2 solutions would be the weakest. The above discussion will be confirmed by the icrit results, passive film resistance (Rf) and passivation power (Qpass) later. The influences of pH values on the main passivation parameters are discussed as follows, which only involve the electrochemical characteristics of A–P–T and A–P–P.Fig. 7 shows the pH influences on the values of icrit. From Fig. 7, the icrit value decreases in each solution with increasing pH value, suggesting the pH influence on the passivation capability. In order to further confirm the pH influence on the passivation capability, the initial passivation potential (Einit) was added on the Q235 sample as an applied potential, and the electrochemical tests of EIS and potential step were carried out. Fig. 8 shows the Nyquist and Bode plots of the Q235 samples in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions at the applied Einit potential. From the Nyquist plots shown in Fig. 8a, c and e, the Nyquist plots of the Q235 carbon steel in the HNO3–NaNO2 and HAc–NaNO2 solutions were composed of an elevated capacitive semicircle, but those in the HCl–NaNO2 solutions were composed of a depressed capacitive semicircle, which may be attributed to the presence or absence of an aggressive species.37 At the same time, the radius of the capacitive semicircle in each solution expanded with increasing pH value, indicating the reinforcement of the passivation capability.38 On the other hand, from the Bode plots shown in Fig. 8b, d and f, the modulus at the low frequency limit of 0.01 Hz in each solution increased with increasing pH value, confirming the reinforcement of the passivation capability. Furthermore, the EEC model shown in Fig. 5b was used to interpret the EIS results, in which CPEf and Rf represent the capacitance and resistance of the passive film, respectively. Fig. 9 shows the pH influences on the values of Rf. From Fig. 9, the Rf value in each solution increased with increasing pH value, further confirming the reinforcement of the passivation capability. Fig. 10 shows the current transients of the Q235 samples in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions at the applied Einit potential. From Fig. 10, the area of the current transient in each solution decreases with increasing pH value, indicating a decrease in the passivation power (Qpass).39 In addition, the icrit and Rf values at the same pH value in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions increased and decreased in turn, respectively (Fig. 7 and 9).
However, it is worth noting that the imain value shown in Fig. 1 and the background current shown in Fig. 10 were independent of the pH value, suggesting the pH value only affected the passivation capability, but did not affect the actual passivation effectiveness.
4. Conclusions
For the Q235 carbon steel in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions, the influences of pH values on the manifestations of electrochemical characteristics and the variations of electrochemical parameters were discussed, and the following conclusions were obtained:(1) The Q235 steel showed the A–P–T characteristic in the HNO3–NaNO2 solutions at pH 1 to pH 4 and in the HAc–NaNO2 solutions at pH 3 to pH 6, the sP-T characteristic in the HNO3–NaNO2 solutions at pH 5 and pH 6, the A characteristic in the HCl–NaNO2 solutions at pH 1 and pH 2, and the A–P–P characteristic in the HCl–NaNO2 solutions at pH 3 to pH 6.
(2) Except in the HCl–NaNO2 solutions at pH 1 and pH 2, the corrosion occurred on the Q235 surface with the positive shift of applied potential, followed by the occurrence of passivation. The influences of pH values on the corrosion and passivation behaviors were closely associated with the cathodic reactions of H+ reduction, O2 reduction and NO2− reduction.
(3) In each solution, the corrosion rate decreased and the passivation capability strengthened with increasing pH value. At the same pH value, the corrosion rate increased and the passivation capability weakened in the HNO3–NaNO2, HAc–NaNO2 and HCl–NaNO2 solutions in turn, respectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (contract 51601133).References
- J. Shi, J. F. Shi, H. X. Chen, Y. B. He, Q. J. Wang and Y. Zhang, J. Pressure Vessel Technol., 2018, 140, 031404 CrossRef.
- X. T. Zheng, K. W. Wu, W. Wang, J. Y. Yu, J. M. Xu and L. W. Ma, Nucl. Eng. Des., 2017, 314, 285–292 CrossRef CAS.
- B. Feng, Y. H. Kang, Y. H. Sun, Y. Yang and X. Z. Yan, Int. J. Appl. Electromagn. Mech., 2016, 52, 357–362 Search PubMed.
- S. Y. Jiang, H. Q. Ding and J. Xu, J. Tribol., 2017, 139, 014501 CrossRef.
- H. L. Huang, Z. Q. Pan, Y. B. Qiu and X. P. Guo, Microelectron. Reliab., 2013, 53, 1149–1158 CrossRef CAS.
- P. Zhong, K. F. Ping, X. H. Qiu and F. X. Chen, Desalin. Water Treat., 2017, 93, 109–119 CrossRef CAS.
- Y. Zhou, H. J. Huang, P. Zhang, D. Liu and F. A. Yan, Surf. Rev. Lett., 2019, 26, 1850218 CrossRef CAS.
- D. Lee, W. C. Kim and J. G. Kim, Corros. Sci., 2012, 64, 105–114 CrossRef CAS.
- Z. H. Dong, W. Shi, G. A. Zhang and X. P. Guo, Electrochim. Acta, 2011, 56, 5890–5897 CrossRef CAS.
- Z. H. Dong, W. Shi and X. P. Guo, Corros. Sci., 2011, 53, 1322–1330 CrossRef CAS.
- M. B. Valcarce and M. Vazquez, Electrochim. Acta, 2008, 53, 5007–5015 CrossRef CAS.
- M. Reffass, R. Sabot, M. Jeannin, C. Berziou and Ph. Refait, Electrochim. Acta, 2007, 52, 7599–7606 CrossRef CAS.
- Y. Zhou and Y. Zuo, J. Electrochem. Soc., 2015, 162, C47–C54 CrossRef CAS.
- Y. Zuo, L. Yang, Y. J. Tan, Y. S. Wang and J. M. Zhao, Corros. Sci., 2017, 120, 99–106 CrossRef CAS.
- R. J. Deng, P. Zhang, X. Y. Zhao, G. Y. Cai, H. Liu, J. P. Xiong and Y. Zhou, J. Braz. Chem. Soc. DOI:10.21577/0103-5053.20190237.
- P. Garces, P. Saura, A. Mendez, E. Zornoza and C. Andrade, Corros. Sci., 2008, 50, 498–509 CrossRef CAS.
- Y. Zhou, P. Zhang, H. J. Huang, J. P. Xiong and F. A. Yan, J. Braz. Chem. Soc., 2019, 30, 1688–1696 CAS.
- Y. Zhou, P. Zhang, J. P. Xiong and F. A. Yan, RSC Adv., 2019, 9, 23589–23597 RSC.
- Y. Zhou, P. Zhang, J. P. Xiong and F. A. Yan, Anti-Corros. Methods Mater., 2019, 66, 879–887 CrossRef.
- Y. Zhou, P. Zhang, Y. Zuo, D. Liu and F. A. Yan, J. Braz. Chem. Soc., 2017, 28, 2490–2499 CAS.
- J. Li, C. Y. Xiong, J. Li, D. Yan, J. Pu, B. Chi and L. Jian, Int. J. Hydrogen Energy, 2017, 42, 16752–16759 CrossRef CAS.
- Y. Zhou and F. A. Yan, Int. J. Electrochem. Sci., 2016, 11, 3976–3986 CrossRef CAS.
- Y. Zhou and Y. Zuo, Appl. Surf. Sci., 2015, 352, 924–932 CrossRef.
- F. X. Chen, S. L. Xie, X. L. Huang and X. H. Qiu, J. Hazard. Mater., 2017, 322, 152–162 CrossRef CAS PubMed.
- P. Zhang, Y. J. Chen, H. J. Huang, Y. Zhou, F. A. Yan and G. C. Nie, Surf. Rev. Lett., 2020, 27, 1950179 Search PubMed.
- X. J. Li, F. Gui, H. B. Cong, C. S. Brossia and G. S. Frankel, Electrochim. Acta, 2014, 117, 299–309 CrossRef CAS.
- L. Yohai, M. Vazquez and M. B. Valcarce, Electrochim. Acta, 2013, 102, 88–96 CrossRef CAS.
- W. Xiong, L. Zhou and S. T. Liu, Chem. Eng. J., 2016, 284, 650–656 CrossRef CAS.
- F. R. Foulkes and P. McGrath, Cem. Concr. Res., 1999, 29, 873–883 CrossRef CAS.
- H. L. Huang and J. Tian, Microelectron. Reliab., 2017, 78, 131–142 CrossRef CAS.
- Y. Zuo, H. T. Wang, J. M. Zhao and J. P. Xiong, Corros. Sci., 2002, 44, 13 CrossRef CAS.
- Q. Y. Xiong, J. P. Xiong, Y. Zhou and F. A. Yan, Int. J. Electrochem. Sci., 2017, 12, 4238–4250 CrossRef CAS.
- F. G. Deng, L. S. Wang, Y. Zhou, X. H. Gong, X. P. Zhao, T. Hu and C. G. Wu, RSC Adv., 2017, 7, 48876–48893 RSC.
- X. Chen, Q. Y. Xiong, F. Zhu, H. Li, D. Liu, J. P. Xiong and Y. Zhou, Int. J. Electrochem. Sci., 2018, 13, 1656–1665 CrossRef CAS.
- Y. Zhou, J. P. Xiong and F. A. Yan, Surf. Coat. Technol., 2017, 328, 335–343 CrossRef CAS.
- L. C. Chen, P. Zhang, Q. Y. Xiong, P. Zhao, J. P. Xiong and Y. Zhou, Int. J. Electrochem. Sci., 2019, 14, 919–928 CrossRef CAS.
- Q. Y. Xiong, Y. Zhou and J. P. Xiong, Int. J. Electrochem. Sci., 2015, 10, 8454–8464 CAS.
- J. Yang, P. Zhang, Y. Zhou and F. A. Yan, Int. J. Electrochem. Sci., 2019, 14, 11349–11357 CrossRef.
- Y. F. Cheng and J. L. Luo, Electrochim. Acta, 1999, 44, 2947–2957 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |