Open Access Article
Open Access ArticleCore–shell magnetic nano-powders with an excellent decolorization effect on dye wastewater
Shuai Hea,
Hanzhe Zhub,
Hui Zhao *ac and
Zhenghou Zhu*a
*ac and
Zhenghou Zhu*a
aCollege of Material Science and Engineering, Nanchang University, Honggutan New District University Avenue No. 999, Nanchang 330031, Jiangxi, China. E-mail: candyzhaohui@126.com; z00708@sina.con; Tel: +86-18970946897 Tel: +86-791-83969141
bFaculty of Science, University of Alberta, Edmonton, Canada T6G 2G8
cInstitute of Space Science and Technology, Nanchang University, Nanchang 330031, Jiangxi, China
First published on 3rd December 2019
Abstract
In recent years, zero-valent nano-iron (nZVI) has received extensive attention due to its excellent decolorization effect on dye wastewater. In this paper, zero-valent nano-iron-nickel (nZVIN) powders were prepared by a simple, efficient and non-polluting method. The powder has a unique core–shell structure and excellent oxidation resistance. Hence the problem that nZVI powders are easily oxidized and difficult to store is solved. Due to the addition of Ni, the magnetic properties of the nZVIN powders are enhanced, which facilitates the recycling of the powders using a magnetic field after sewage treatment. In the decolorization treatment of dye wastewater simulated with Congo red (CR) dye, nZVIN powders can maintain a removal rate of more than 90% for CR solutions with different pH values (7.0–11.5) and an initial dye concentration (50–200 mg L−1). The research results show that nZVIN powders have broad application prospects in the treatment of azo dye wastewater.
1. Introduction
In recent years, the large-scale use of synthetic dyes in the paper, textile, cosmetics and other industries has caused serious water pollution problems. Azo dyes are a type of synthetic dye. Such dyes usually have an unsaturated azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bond as the chromophore group, a sulfonic acid group (SO3H), an amino group (NH2) or similar as an auxochrome, and have a complex aromatic hydrocarbon molecular structure. Because of their low cost, good stability and simple use, they are the most widely used dyes and the main contributor of water pollution. First of all, these dyes have a strong coloring effect. Even low concentrations of dye wastewater still have a high chroma, and it would seriously endanger aquatic flora and fauna of the growth and reproduction. Secondly, the hydrogen on the benzene ring of these dyes in the water is easily replaced, which will produce various harmful aromatic compounds, and some may even have a large carcinogenicity. Finally, some toxic pollutants once formed in dye wastewater are difficult to degrade, and will be long-term stability in the environment, which results in irreversible damage on the ecosystem.1–5
N) bond as the chromophore group, a sulfonic acid group (SO3H), an amino group (NH2) or similar as an auxochrome, and have a complex aromatic hydrocarbon molecular structure. Because of their low cost, good stability and simple use, they are the most widely used dyes and the main contributor of water pollution. First of all, these dyes have a strong coloring effect. Even low concentrations of dye wastewater still have a high chroma, and it would seriously endanger aquatic flora and fauna of the growth and reproduction. Secondly, the hydrogen on the benzene ring of these dyes in the water is easily replaced, which will produce various harmful aromatic compounds, and some may even have a large carcinogenicity. Finally, some toxic pollutants once formed in dye wastewater are difficult to degrade, and will be long-term stability in the environment, which results in irreversible damage on the ecosystem.1–5
At present, there are mainly three methods for treating such dye wastewater. The first one is the physical adsorption method, which adsorbs the dye in the water by a specific adsorbent. It is also used in combination with photocatalysis to generate OH free radicals to remove dyes during adsorption.6 However, the adsorption effect of this method is single, and it is effective only for a specific one or several dyes, and it is difficult to cope with complicated and diverse polluted wastewater, meanwhile the adsorbent treatment after adsorption is also a problem.7 The second method is the membrane filtration, but the membrane as a filter is easily contaminated, and requires frequent replacement, and its cost is expensive to use. The third one is the biodegradation method. Bioremediation, as an ecologically friendly method, has attracted extensive attention because of its advantages of degrading dye wastewater and improving soil.8 In practical applications, the degradation effect on dye wastewater is relatively common, and it is not suitable for treating dye wastewater with complex composition because microorganisms are vulnerable to environmental influences and instability.
Zero-valent iron (nZVI) in dye wastewater treatment is increasingly valued because of its chemical nature, low price, and strong degradation and reduction ability for dye wastewater.9 K. V. G. Ravikumar prepared nZVI under anaerobic conditions using anaerobic sludge granules and obtained a 99% removal rate for the methyl orange dye by nZVI obtained.10 Zhang Lian'an prepared S-ZVI by sulfonation, and improved the removal rate of azo dye.11 Akeem Adeyemi Oladipo prepared CoO–NiFe2O4 catalyst by coprecipitation method and calcination method, and studied its degradation effect and decolorization mechanism on dye wastewater in detail.12 Although the above preparation method solves the problem of nZVI preparation, it is still to be solved that nZVI is easy to oxidize in the process of use and storage, difficult to be stored, and difficult to be recovered when dispersed in the solution.
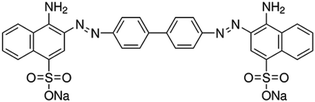
In this paper, in order to solve the above problem of using nZVI, the nickel element was added to the pure iron powders to form FeNi3 alloy phase. Compared with the previous one, the reaction preparation process is simple and environmentally friendly, and its oxidation resistance and magnetic properties have been significantly enhanced to facilitate preservation and recycling. Spherical nano-scale zero-valent iron-nickel (nZVIN) powders were prepared by chemical reduction method, and a series of characterizations were carried out. It was found that the shape of particle size was uniform and controllable. By alloying with the addition of Ni element, oxidation resistance of the powders is enhanced, and magnetic properties are also improved. Meanwhile, the problems of zero-grade iron in storage, transportation and recycling are solved. More importantly, the added nickel element has a catalytic effect on the generation of atomic hydrogen in the solution by zero-valent iron, which can promote the reduction and decolorization of the dye by zero-valent iron.13 In the decolorization treatment of dye wastewater simulated by Congo red (molecular formula C32H22N6Na2O6S2, structural formula shown in Fig. 1), nZVIN powders preserving for one year can maintain a removal rate of more than 90% for CR solutions in a wide pH value (7.0–12.0). And the nZVIN powders in the solution after treatment can be removed by simple magnetic separation.
2. Experiment
2.1 Drugs and characterization
All the solutions in this paper were prepared with deionized water. All chemical materials were ones of analytical grade, such as, ferrous sulfate heptahydrate (FeSO4·7H2O), nickel sulfate hexahydrate (NiSO4·6H2O), hydrazine hydrate (N2H4·H2O), sodium hydroxide (NaOH), hydrochloric acid (HCl), Congo red (CR) and alcohol.The phase identification of nZVIN powders was performed by X-ray diffraction (XRD) on a Bruker-axe D8 ADVANCE X-ray diffract meter with CuKα radiation. The test conditions were that tube voltage was 40 kV, the current was 40 mA, and the step was 0.02°. Particle microstructures and morphologies were observed by employing a field emission scanning electron microscopy (JSM-6701F(JEOL)) and transmission electron microscope (Hitachi 7650B). The hysteresis loop was tested by PPMS-9 physical property test system (American quantum design company), in which the sweep speed was 100 Oe s−1 and the test temperature was 300 K.
The absorbance of the CR dye was measured by an ultraviolet spectrophotometer with a measurement step of 2 nm and a scanning range of 400–600 nm. The infrared spectra of samples before and after treatment were measured by Nicolet 380 Fourier transform spectrometer (resolution 2 cm−1) by KBr compression method.
2.2 Preparation of nZVIN powders
FeSO4·7H2O of 10.5 g and NiSO4·6H2O of 10 g were dissolved in water solution of 200 mL, and the solution was heated in a water bath, and keep the temperature at 85 °C. Then NaOH of 6 g was added into the mixture, and stirred at a high-speed of 1500 rpm. When gray-green precipitates appeared in the mixture, N2H4·H2O of 11.25 g was added, and the solution reacted vigorously with stirring for about 30 min, and the reaction process was associated with colorless and irritating gas volatile out, at last black powders were generated. After completion of the reaction, the resultant was washed three times with deionized water and absolute ethanol to remove residual starting materials and reagents. Finally, the nZVIN powders were obtained by magnetic separation and drying.2.3 Decolorization experiment
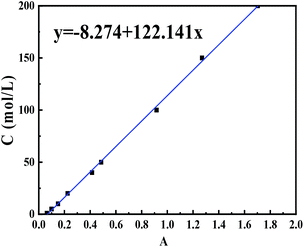
The azo dye wastewater was simulated using CR dye. CR of 1 g L−1 was prepared firstly as a stock solution with deionized water, and then configured to different concentrations of CR standard solution. The absorbance of different concentrations of Congo red solution was measured by a UV spectrophotometer at the characteristic absorption peak of CR (λ = 497 nm) to obtain a CR solution concentration–absorbance curve (Fig. 2). The nZVIN powders of 0.1 g were added into 100 mL CR solution of different concentration or different initial pH value, and the mixture was sonicated in ultrasound. Suspension of 3 mL was taken at regular intervals, and the supernatant was taken after centrifugation, and the absorbance was measured by an ultraviolet spectrophotometer, and then the CR concentration was estimated from the curve of Fig. 2.At time t (min), the removal amount Qt of CR can be calculated by the eqn (1), and the removal rate Rt of CR is calculated by the eqn (2).
| Qt = (C0 − Ct)/mV | (1) |
| Rt = (C0 − Ct)/C0 × 100% | (2) |
3. Results and discussion
Hydrazine hydrate has both oxidizability and reducibility. The oxidation potential of Fe2+ to Fe3+ is 0.66 V, and the reduction potential of reduction to zero valence iron is 0.283 V. Therefore, Fe2+ is difficult to reduce to obtain zero-valent iron generally. But, when Ni2+ and Fe2+ coexist, part of Fe2+ and Ni2+ can be reduced in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
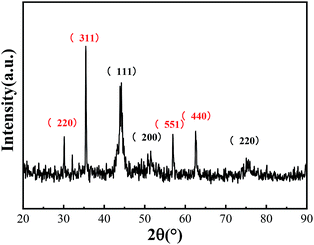
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Hence, nZVIN powders were prepared by adding Ni element in this experiment.14 At the beginning of the reaction, the molar ratio of [Ni] to [Fe] is 1/1, and FeNi3 nuclei were first formed, and gradually grew until Ni element consumed, and a part of the remaining Fe2+ were oxidized to Fe3+, which combined with [O] to form Fe3O4. As can be seen from the XRD pattern (Fig. 3), the phase composition of the nZVIN powder phase is Fe3O4 (represented by the red crystal face index) and FeNi3 (represented by the black crystal face index).
3. Hence, nZVIN powders were prepared by adding Ni element in this experiment.14 At the beginning of the reaction, the molar ratio of [Ni] to [Fe] is 1/1, and FeNi3 nuclei were first formed, and gradually grew until Ni element consumed, and a part of the remaining Fe2+ were oxidized to Fe3+, which combined with [O] to form Fe3O4. As can be seen from the XRD pattern (Fig. 3), the phase composition of the nZVIN powder phase is Fe3O4 (represented by the red crystal face index) and FeNi3 (represented by the black crystal face index).
The unique size and morphology of nZVIN powders can be observed from Fig. 4. The nZVIN powder has a particle size of about 60 nm and is spherical (Fig. 4(a) and (b)). This special size and spherical shape make the nZVIN powders have a large specific surface area and surface energy, which contributes to enhance its reactivity in solution.
It can be seen from the TEM image (Fig. 4(c) and (d)) that the nZVIN powder consists of two layers of different component, the core layer has a high Ni content and a darker color, the outer layer has a lower Ni content and a lower color, which confirms that nZVIN powders have a core–shell structure. In addition, since the [Ni]/[Fe] molar ratio is 1/1 at the reaction, FeNi3 nuclei are formed in the initial stage of the reaction, and as the reaction increases, [Ni] has less residual content than [Fe], the surface of the powders formed at the end of the reaction have a large number of defects and lattice distortion, which is favorable for adsorption and reaction with CR.
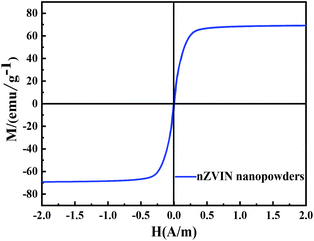
In addition, magnetic properties are also key factors determining the application of nZVIN powders. The magnetic properties can be significantly enhanced by the addition of Ni. The hysteresis loop of the nZVIN powders measured at room temperature and a kind of typical S-type hysteresis loop (Fig. 5). Hence the nZVIN powder is a typical ferromagnetic material. The nZVIN powder has a high Ms (69.8 emu g−1), a low Hc (0.0047 A m−1) and a Mr (4.79 emu g−1). Due to the good magnetic properties, the nZVIN powders can respond quickly under the action of external magnetic field, which can solve the problem of the recovery of powders after actual sewage treatment, which is favorable for adsorption and reaction with CR.
150 mg L−1 CR solution was used to simulate azo dye wastewater with CR as target pollutant. The nZVIN powders of 0.1 g were added for decolorization experiment. The color of the solution was red at the beginning, then the color turned dark red after nZVIN powders had been put in solution. Finally, the solution became colorless after powders were separated by magnetic field (Fig. 6).
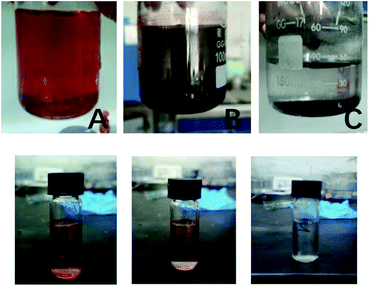 | ||
| Fig. 6 CR solution color change process after nZVIN powders being added ((A–C) pre and post reaction solution. (a–c) Test sample before and after reaction). | ||
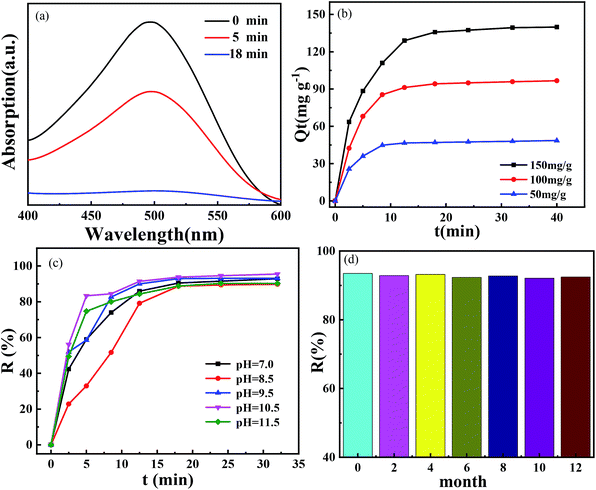
The UV spectrum of the solution reaction process is shown in Fig. 7(a). With the increase of nZVIN powders addition time, the absorbance of CR gradually decreased. At 18 min, the CR absorbance curve became smoother, and the characteristic absorption peak (λ = 497 nm) disappeared. Therefore, it can be considered that the CR in the solution has been removed after adding the nZVIN powders for 18 min, which indicates that nZVIN powders have a good removal effect on CR.
In view of the great advantages of nZVIN powders in the application of azo dyes, such as excellent magnetic properties and obvious decolorization effects, we have carried out a series of studies on the decolorization effect under different conditions, and conducted a preliminary exploration on the decolorization mechanism.
Firstly, we performed the removal test at different initial concentrations of CR. As shown in Fig. 7(b), the unit removal of CR by nZVIN powders increased with the initial concentration of CR, and increased with the increase of the processing time of nZVIN powders, but grew slowly after 15 min. And the maximum removal of CR by nZVIN powders can reach 140 mg g−1. This exceeds the amount of degradation of common nZVI composites (126 mg g−1).15
The pH value of the solution is one of the important factors affecting on the treatment effect of sewage. We studied the removal rate of CR by nZVIN powders under different initial solution pH values (7.0–11.5) of solution. When the initial pH value was in the range of pH 7.0–11.5, the removal rate of CR by nZVIN powders still maintained above 90% (Fig. 7(c)). Compared with the current removal rate of nZVI (86%),16 the common dye wastewater in daily life is generally alkaline. Therefore, a broad ph range is of great significance for the treatment of common dye wastewater in daily life by nZVIN powders.
Fig. 7(d) shows the effect of removing the CR solution by nZVIN powders stored in open air for different times, and the initial concentration of CR is 150 mg L−1. By comparing the CR removal rates, the CR removal ability of nZVIN powders with a storage time of up to one year, has no degradation. Because of the special core–shell structure, the coated ferric oxide can prevent its oxidation and pollution in the air, so that nZVIN powder has a good stability, the use of practical life has a broad prospect.
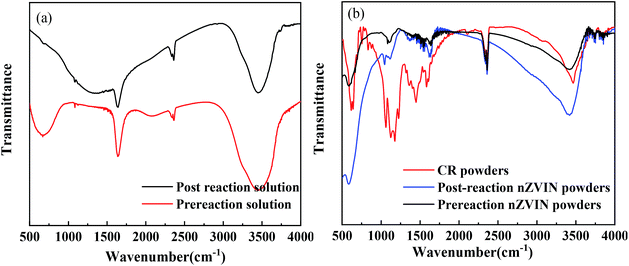
To further investigate the decolorization mechanism of nZVIN powders, we performed infrared spectroscopy on the sample, as shown in Fig. 8. The absorption peak of the chromogenic group N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond at 1630 cm−1 and the sulfonic acid group at 620 cm−1, disappeared after nZVIN powders being put in the solution (Fig. 8(a)), which further proves that nZVIN powders have a good treatment effect on azo dye wastewater. The infrared absorption peak of nZVIN powders before and after being put in the CR solution, did not make any differences (Fig. 8(b)), which indicated that nZVIN powders had degraded the CR.
N bond at 1630 cm−1 and the sulfonic acid group at 620 cm−1, disappeared after nZVIN powders being put in the solution (Fig. 8(a)), which further proves that nZVIN powders have a good treatment effect on azo dye wastewater. The infrared absorption peak of nZVIN powders before and after being put in the CR solution, did not make any differences (Fig. 8(b)), which indicated that nZVIN powders had degraded the CR.
The micro-morphology and structure of nZVIN powders (Fig. 4) and the infrared spectrum of the samples before and after the reaction (Fig. 8), indicates that the decolorization procedures of CR solution go through two processes of adsorption and degradation. Firstly, because the surface layers of powders have a large number of defects and lattice deformity, nZVIN powders have a strong adsorption effect on CR, and the CR in the solution are adsorbed on the surface of the powders. Then, the oxidation of zero-valent iron atoms inside nZVIN powders causes atomic hydrogen to be generated in the water, and [Ni] catalyzes the generation of atomic hydrogen, and finally the azo bond of the CR molecule adsorbed on the surfaces of the powders are reductively cracked by the generated atomic hydrogen, and the CR solution is bleached.17
4. Conclusions
(1) The nZVIN powders were prepared by liquid phase reduction method, which solved the problem that it is difficult to prepare nZVI powders under normal temperature and pressure. The method is simple, efficient and pollution-free. Those nZVIN powders have a unique core–shell structure with a particle size of about 60 nm and excellent oxidation resistance, which solve the problem that the nZVI powders are easily oxidized and difficult to store.(2) The nZVIN powders have good magnetic properties and can be separated and recovered by magnetic field treatment, which solve the problem of recycling powders for sewage treatment.
(3) In the decolorization treatment of dye wastewater simulated by Congo red (CR) dye, nZVIN powders can maintain a removal rate of more than 90% for CR solutions with different pH values (7.0–11.5) and initial dye concentration (50–200 mg L−1). The research results show that nZVIN powders have broad application prospects in the treatment of azo dye wastewater.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by National Natural Science Foundation of China (grant no. 61361008).Notes and references
- P. T. Almazán-Sánchez, I. Linares-Hernández, M. J. Solache-Ríos and V. Martínez-Miranda, Water, Air, Soil Pollut., 2016, 227, 100 CrossRef.
- R. Brindha, P. Muthuselvam, S. Senthilkumar and P. Rajaguru, Chemosphere, 2018, 201, 77–95 CrossRef CAS PubMed.
- S. Dutta, R. Saha, H. Kalita and A. N. Bezbaruah, Environmental Technology & Innovation, 2016, 5, 176–187 Search PubMed.
- J. Fan, Y. Guo, J. Wang and M. Fan, J. Hazard. Mater., 2009, 166, 904–910 CrossRef CAS PubMed.
- D. V. Kerkez, D. D. Tomašević, G. Kozma, M. R. Bečelić-Tomin, M. D. Prica, S. D. Rončević, Á. Kukovecz, B. D. Dalmacija and Z. Kónya, J. Taiwan Inst. Chem. Eng., 2014, 45, 2451–2461 CrossRef CAS.
- S. Ye, M. Yan and X. Tan, et al., Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light, Appl. Catal., B, 2019, 250, 78–88 CrossRef CAS.
- S. Ye, G. Zeng and H. Wu, et al., Biological technologies for the remediation of co-contaminated soil, Crit. Rev. Biotechnol., 2017, 37(8), 1062–1076 CrossRef CAS PubMed.
- Q. Huang, M. Liu, L. Mao, D. Xu, G. Zeng, H. Huang, R. Jiang, F. Deng, X. Zhang and Y. Wei, J. Colloid Interface Sci., 2017, 499, 170–179 CrossRef CAS PubMed.
- V. Kecić, Đ. Kerkez, M. Prica, O. Lužanin, M. Bečelić-Tomin, D. T. Pilipović and B. Dalmacija, J. Cleaner Prod., 2018, 202, 65–80 CrossRef.
- K. V. G. Ravikumar, S. Santhosh, S. V. Sudakaran, Y. V. Nancharaiah, P. Mrudula, N. Chandrasekaran and A. Mukherjee, J. Environ. Chem. Eng., 2018, 6, 1683–1689 CrossRef CAS.
- A. A. Oladipo, A. O. Ifebajo and M. Gazi, Appl. Catal., B, 2019, 243, 243–252 CrossRef CAS.
- L. Zhang, Q. Shao and C. Xu, J. Cleaner Prod., 2019, 213, 753–761 CrossRef CAS.
- H. Dong, Z. Jiang, C. Zhang, J. Deng, K. Hou, Y. Cheng, L. Zhang and G. Zeng, J. Colloid Interface Sci., 2018, 513, 117–125 CrossRef CAS PubMed.
- H. Zhao, Z. H. Zhu, X. L. Zheng, C. Xiong and Q. Y. Lin, RSC Adv., 2016, 20, 16413–16418 RSC.
- R. Kvg, S. Das and J. W. Osborne, et al., Novel nano-bio (nano zerovalent iron and Klebsiella sp.) composite beads for congo red removal using response surface methodology, J. Environ. Chem. Eng., 2019, 7(5), 103413 CrossRef CAS.
- G. B. Jegadeesan, S. Amirthavarshini and J. Divya, et al., Catalytic peroxygen activation by biosynthesized iron nanoparticles for enhanced degradation of Congo red dye, Adv. Powder Technol., 2019, 30, 2890–2899 CrossRef CAS.
- Y. Lin, Z. Chen, M. Megharaj and R. Naidu, J. Colloid Interface Sci., 2012, 381, 30–37 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |