Open Access Article
Open Access ArticleDesigning dithienonaphthalene based acceptor materials with promising photovoltaic parameters for organic solar cells†
Muhammad Ans *a,
Javed Iqbal
*a,
Javed Iqbal *ab,
Ijaz Ahmad Bhattia and
Khurshid Ayub*c
*ab,
Ijaz Ahmad Bhattia and
Khurshid Ayub*c
aDepartment of Chemistry, University of Agriculture Faisalabad, Faisalabad, 38000, Pakistan. E-mail: ansbhatti24@gmail.com
bPunjab Bio-energy Institute, University of Agriculture, Faisalabad, 38040, Pakistan. E-mail: javedkhattak79@gmail.com
cDepartment of Chemistry, COMSAT University, Abbottabad Campus, Abbottabad, KPK 22060, Pakistan. E-mail: khurshid@cuiatd.edu.pk
First published on 28th October 2019
Abstract
Scientists are focusing on non-fullerene based acceptors due to their efficient photovoltaic properties. Here, we have designed four novel dithienonaphthalene based acceptors with better photovoltaic properties through structural modification of a well-known experimentally synthesized reference compound R. The newly designed molecules have a dithienonaphthalene core attached with different acceptors (end-capped). The acceptor moieties are 2-(5,6-difluoro-2-methylene-3-oxo-2,3-dihydroinden-1-ylidene)malononitrile (H1), 2-(5,6-dicyano-2-methylene-3-oxo-2,3-dihydroinden-1-ylidene)-malononitrile (H2), 2-(5-methylene-6-oxo-5,6-dihydrocylopenta[c]thiophe-4-ylidene)-malononitrile (H3) and 2-(3-(dicyanomethylene)-2,3-dihydroinden-1-yliden)malononitrile (H4). The photovoltaic parameters of the designed molecules are discussed in comparison with those of the reference R. All newly designed molecules show a reduced HOMO–LUMO energy gap (2.17 eV to 2.28 eV), compared to the reference R (2.31 eV). Charger transfer from donor to acceptor is confirmed by a frontier molecular orbital (FMO) diagram. All studied molecules show extensive absorption in the visible region and absorption maxima are red-shifted compared to R. All investigated molecules have lower excitation energies which reveal high charge transfer rates, as compared to R. To evaluate the open circuit voltage, the designed acceptor molecules are blended with a well-known donor PBDB-T. The molecule H3 has the highest Voc value (1.88 V). TDM has been performed to show the behaviour of electronic excitation processes and electron hole location between the donor and acceptor unit. The binding energies of all molecules are lower than that of R. The lowest is calculated for H3 (0.24 eV) which reflects the highest charge transfer. The reorganization energy value for both the electrons and holes of H2 is lower than R which is indicative of the highest charge transfer rate.
Introduction
The energy crisis is an ever-intensifying major challenge faced by the world today.1 According to the World Energy Council, the energy demand in 2020 will rise by 50–80% over the energy demands the world had back in 1990.2 Fossil fuels, although depleting rapidly, have been the major energy sources since ancient times. Fossil fuels also have serious environmental impact in the form of global warming, health issues and climate change etc. These environmental issues have driven the attention of scientists towards clean and environmental friendly renewable energy sources.3 The renewable energy sources include solar energy, hydro-power energy, wind energy, bio-mass energy.4 Among these, solar energy is a more abundant, reliable and sustainable energy source with a negligible effect on the environment. Solar cells work on the principle of the photoelectric effect (sunlight directly converts into electricity).5Silicon is used as conducting material in the commercially available solar cells due to its high power conversion efficiency (PCE), lower toxicity, large abundance and high stability.6 Although silicon has several advantages but it also has some limitations as well such as non-tuneable energy level, brittleness, and high cost. Therefore, scientists are continuously searching for alternatives. Organic solar cells offer certain advantages such as tuneable energy levels, intensive absorption, low cost, mechanical flexibility, high reproducibility and easy processability.7–14 Organic solar cells (OSCs) contain electron donor and electron acceptors where photoexcited electron from donor shifts towards electron acceptor.13,15 From the last two decades, fullerene based acceptors have been routinely used due to high charge mobility, high photo-induce electron and isotropic charge transfer.16–18 Although fullerene based acceptor have encouraging results, but it is tough to improve the PCE because of limited absorption of fullerene in visible region and less tuneability of energy levels.19,20 Recently, many non-fullerene based acceptor have been explored21–24 for their use in efficient photovoltaic materials.25–38 The acceptor molecules are rationally designed to meet the practical requirements. Among different design strategies, A–D–A (acceptor–donor–acceptor) design principle has gained much more attention. The A–D–A type acceptor molecules have tune-able energy levels, broader absorption in visible region and ease in fabrication.39–43 For A–D–A strategy, the ladder type fused ring donor unit should be used in order to prevent the rotation and help in co-planarity which lowers the reorganization energy and thereby increase the charge transfer ability.44–46 Furthermore, sp3 hybridized methyl group on donor unit help in easy processability to avoid self-aggregation in blended form. Based on above strategy Zhan et al., reported A–D–A based non-fullerene acceptor, 3,9-bis-2-methylene-(3-1-dicyanomethylene)-indanone-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2,3-d]-s-indaceno[1,2-b:5,6-b]dithiophene (ITIC), in which indacenodithienothiophene (IDTT) is used as a ladder type donor material.24 Here, we have designed four new A–D–A type acceptor molecules (H1–H4) based on IDTT donor for better performance in photovoltaic cells. The molecular structure of all molecules are illustrated in Fig. 1.
Our designed molecules contain dithienonaphthalene with methyl group as donor moiety with different end capped acceptor units. Optical properties calculated with DFT method are compared with the recently reported reference compound (DTNIC) R.47
Computational details
All calculation were performed with Gaussian 09 software.48 Gauss view 5.0 (ref. 49) was used for visualization of results. First, reference compound R was optimized with five different functionals of DFT including CAM-B3LYP,50 B3LYP,51 ωB97XD,52 PBEPBE,53 and MPW1PW91 (ref. 54) with 6-31G(d,p) basis set. For the selection of the best functional to study the electronic and optical properties of the IDTT based solar cells, absorption maxima (λmax) of R are calculated with time dependent DFT with the above mentioned functionals (B3LYP, PBEPBE, wB97XD, CAM-B3LYP, and mPW1PW91). The λmax values calculated with above functionals were compared with experimental λmax value. A good agreement of λmax value was achieved by B3LYP at 6-31G(d,p) basis set. The observation is consistent with many of our previous studies where we have shown that electronic properties are best studied with B3LYP functional. Based on this small benchmarking, all remaining calculations of the designed molecules (H1–H4) were performed with B3LYP functional. The absorption spectra were plotted in both gas and solvent phase. IEFPC model is used for calculations in the solvent phase.55 Origin 6.0 software was used for plotting UV/visible absorption spectra. For the calculation of charge transfer, frontier molecular orbital, density of state and transition density matrix of R and designed molecules (H1–H4), the selected functional B3LYP was used. Density of state (DOS) spectra were plotted with Pymolyze software.The reorganization energies were calculated for estimating charge mobilities. The reorganization energy has two parts; internal (λi) and external (λext) reorganization energy. The λi deal with quick changes in internal structure while external λext deals with effect of polarization and environmental relaxation. In the current study, external (λext) reorganization energy is neglected and we only dealt with internal reorganization energy. The mathematical equations56,57 for the calculations of reorganization energies of electron (λe) and hole (λh) are:
| λe = [E0− − E−] + [E0− − E0] | (I) |
| λh = [E0+ − E+] + [E0+ − E0] | (II) |
Results and discussion
The focus of this study is to design new A–D–A type acceptor molecules based on dithienonaphthalene donor unit for better performance in OSCs. The end-capped acceptors are 2-(5,6-difluoro-2-methylene-3-oxo-2,3-dihydroinden-1-ylidene)malononitrile (H1), 2-(5,6-dicyano-2-methylene-3-oxo-2,3-dihydroinden-1-ylidene)-malononitrile (H2), 2-(5-methylene-6-oxo-5,6-(dihydrocylopenta[c]thiophe-4-ylidene)malononitrile (H3) and 2-(3-(dicyanomethylene)-2,3-dihydroinden-1-yliden)malononitrile (H4). Initially the absorption maxima of R was evaluated with five different functionals (B3LYP, mPW1PW91, PBEPBE, ωB97XD and CAM-B3LYP) at 6-31G(d,p) basis set. The λmax values of R at B3LYP, ωB97XD, PBEPBE, CAM-B3LYP and mPW1PW91 are 607.8 nm, 470.82 nm, 781.0 nm, 484.16 nm, and 575.50 nm, respectively. The reported value for R is 634.0 nm.47 The comparison bar chart of all functionals with experimental value is given in Fig. 2.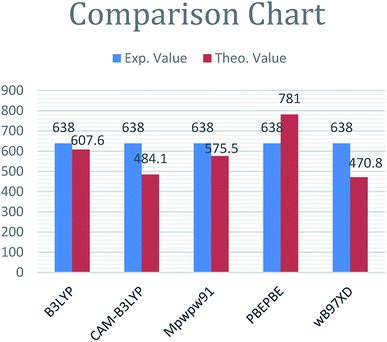 | ||
| Fig. 2 Comparison diagram of λmax value of R with ωB97XD, B3LYP, CAM-B3LYP, PBEPBE and MPW1PW91 at 6-31G(d,p). | ||
From Fig. 2, it is evident that the absorption maximum calculated at B3LYP/6-31G(d,p) is in best agreement with experimental value. Therefore, B3LYP/6-31G(d,p) is selected for all remaining calculations.
Frontier molecular orbital diagram
The molecular structures of designed molecules (H1, H2, H3 and H4) and the reference compound R are illustrated in Fig. 1, and their optimized geometries are presented in Fig. 3.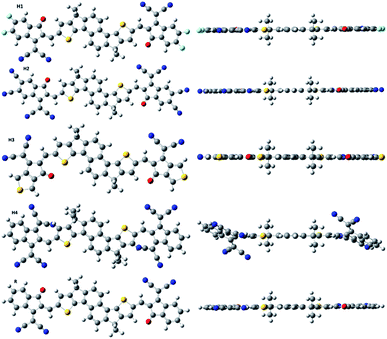 | ||
| Fig. 3 Systematic optimized geometry of H1–H4 including model R at B3LYP with 6-31G(d,p) level of DFT. | ||
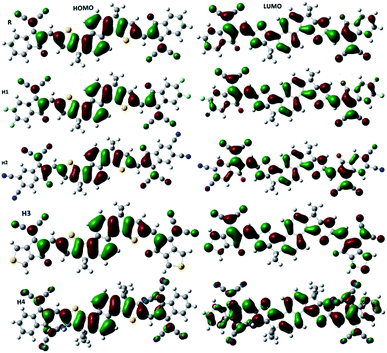
From the optimized geometries, it is apparent that the acceptor and the dithienonaphthalene donor unit are in one plane (see side view in Fig. 3). Two methyl groups on donor part helps to avoid self-aggregation. It is clear from Fig. 3, all studied molecules exhibits planar structure which is much preferred for high charge mobilities.58 Charger transfer is greatly influenced by the distribution pattern of frontier molecular orbitals (HOMO and LUMO). The distribution of densities in HOMO and LUMO of the reference and designed molecules are illustrated in Fig. 4. In photovoltaic materials, the energy of HOMO, energy of LUMO and HOMO–LUMO energy gap (energy gap = ELUMO − EHOMO) of the organic material play key role. The energy gap dictates the power conversion efficiency, stability, chemical hardness, softness, lower dissociation energy, and binding energy which help in charger transfer analysis.59 The computed HOMO and LUMO values of R are −5.68 eV and −3.37 eV, respectively which lead to H–L gap of 2.31 eV. The energies of HOMO of H1, H2, H3 and H4 are −5.82, −6.23, −5.71 and −5.86 eV, respectively. The energies of LUMO of H1, H2, H3 and H4 are −3.54, −4.05, −3.45 and −3.69 eV, respectively. Form above results, it is obvious that the reference R has higher energies of HOMO and LUMO with respect to the designed molecules.
Among all studied molecules (R, H1–H4), H2 exhibits the lowest energies of HOMO and LUMO. The stabilized HOMO and LUMO in H2 are due to strong electron withdrawing effect of the acceptor moiety namely 5,6-dicyano-2-methylene-3-oxo-2,3-dihydroinden-1-yliden-malononitrile. The HOMO and LUMO energies of H4 are lower than those of R, H1 and H3, which illustrates the strong electron withdrawing effect of acceptor in H4. Among all designed molecules, H3 shows high energies of HOMO and LUMO values but these values are still lower than HOMO and LUMO values of R, which reveal that the acceptor in H3 has strong electron withdrawing effect than that of the reference R.
The HOMO values of all designed molecules lie in the order of R > H3 > H1 > H4 > H2 whereas the order for the corresponding LUMO energies is R > H3 > H1 > H4 > H2. Another promising key factor which effect the efficiency of organic solar cells is energy gap (Eg). The HOMO–LUMO energy values and H–L energy gap are illustrated in Table 1. The highest energy gap is observed for R (2.31 eV). It is clear from Table 1 that the energy gaps of the designed molecules (H1–H4) are lower than that of the reference R. The energy gaps of all studied molecules are in the range of 2.17 to 2.28 eV. The energy gap of H1, H2, H3 and H4 are 2.28, 2.18, 2.25 and 2.17 eV, respectively. The designed molecules H2 and H4 have comparable energy gaps. The lower energy gap of H3 than that of H1 indicates that H3 may have red shifts in the absorption spectrum, which mean possible better photo-absorption ability and higher short-circuit current density. The graph of HOMO–LUMO with their energy gap have been illustrated in Fig. 5.
| Molecules | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|
| a Eg = energy band gap. | |||
| R | −5.68 | −3.37 | 2.31 |
| H1 | −5.82 | −3.54 | 2.28 |
| H2 | −6.23 | −4.05 | 2.18 |
| H3 | −5.71 | −3.45 | 2.25 |
| H4 | −5.86 | −3.69 | 2.17 |
Furthermore, partial density of state (PDOS) were calculated to explore the optical and electronic properties of OSCs. PDOS support the facts (frontier molecular orbital diagram) which are described in Fig. 4. It is clear form Fig. 4 that the end-capped acceptor unit in the designed molecules effect the distribution pattern around HOMO and LUMO. In case of R, the HOMO is primarily distributed on donor unit while LUMO is spread on the entire molecule. A similar type of pattern is observed for H1 and H2, where the HOMO is spread on donor core unit with small densities on the acceptor moieties whereas the LUMO is populated mostly on end capped acceptor groups with small densities present on the central donor group. For H3, the distribution of density in HOMO is comparable to those of H1 and H2 (distributed on the donor part). The distribution of density in LUMO of H3 is different in the sense that it is spread on the entire molecule. Finally the HOMO of H4 is strongly distributed on donor unit without acceptor moiety, while LUMO is strongly available on acceptor unit with less spread on donor core unit. The calculated PDOS are illustrated in Fig. 6.
 | ||
| Fig. 6 Density of states around HOMOs and LUMOs of model R and H1–H4 at B3LYP/6-31G(d,p) level of theory. | ||
Optical properties
To evaluate the optical properties of R and (H1–H4), UV/visible absorption spectra are calculated in gas and solvent phases. The absorption maxima λmax, oscillator strength, excitation energy, and orbitals involved in the transition are shown in Table 2 for gas phase calculations.The absorption maxima of R, H1–H4 lie in the range of 607.6 to 657.4 nm. As shown in Table 2, it is apparent that all designed molecules (H1–H4) show absorbance in the visible region. The λmax value of R calculated with B3LYP functional (607.6 nm) agrees nicely with the experimental value (634.0 nm). It is clearly seen that the strong electron withdrawing acceptor moieties particularly in H4 cause significant red shift in the absorption spectra. Among all molecules studied, H4 shows the highest λmax value (657.4 nm). The next highest absorption maximum is seen for H2 (642.7 nm). The λmax value of H1 and H3 are slightly higher than the absorption maxima of the reference compound R.
The red shifts in the absorption maxima of the designed molecules are attributed to extended conjugation between donor and end-capped acceptor groups. The absorption maxima are in decreasing order of H4 > H2 > H3 > H1 > R. The absorption maxima of the designed molecules H1, H2, H3 and H4 are 5.4 nm, 35.1 nm, 8.2 nm and 49.8 nm red shifted when compared with the λmax value of R. The absorption spectra are illustrated in Fig. 7.
For charge transfer, the excitation energy is an important tool. The lower the excitation energy, the greater is the charge transfer rate which ultimately increases the PCE. All studied molecules show lower excitation energy as equated to the reference R. The excitation energy of R is 2.04 eV. The excitation energies of H1, H2, H3 and H4 are 2.02, 1.93, 2.01 and 1.89 eV, respectively. The incorporation of electron withdrawing group with the donor moiety in designed molecules causes lowering of excitation energies. The lowest excitation energy is calculated for H4 which is attributed to extended conjugation between donor and acceptor units, which leads to enhanced charge mobility. The order of excitation energies is R > H1 > H3 > H2 > H4. The λmax value, excitation energy, oscillator strength and transition of all molecules are also investigated in chloroform solvent with IEFPCM model and results are illustrated in Table 3.
From Table 3 it is obvious that the absorption pattern of the reference R and designed H1–H4 molecules in chloroform solvent is very similar to that in the gas phase. The λmax values of all molecules (H1–H4) show red shift compared to the reference R. Moreover, the absorption maxima of all compounds in chloroform solvent are red shifted compared to those in the gas phase. For example, the absorption maximum of R in chloroform solvent is 652.2 nm compared to 607.6 nm in the gas phase. Similarly, the λmax value of H1, H2, H3 and H4 are 659.7 nm, 721 nm, 659.0 nm and 704.9 nm, respectively. The λmax values of H1, H2, H3 and H4 are red shifted (from those in the gas phase) by 46.7 nm, 78.3 nm, 44.1 nm, and 47.7 nm, respectively (Fig. 8).
 | ||
| Fig. 8 UV/visible spectra of all molecules in solvent (chloroform) at TD-B3LYP/6-31G(d,p) level of DFT. | ||
In brief, the absorption maxima of our designed molecules are more red shifted (in both gas as well as in chloroform solvent) than the reference compound R which is highly beneficial for their application in photovoltaic cells.
Charge mobilities
The performance of OSCs is directly related to charge mobilities which can be evaluated through reorganization energies of electron (λe) and hole (λh). Reorganization energy is calculated with selected functional and the results are summarized in Table 4.Charge mobilities and reorganization energies are in inverse relationship. There is inverse relationship between reorganization energies and charge mobilities. The lower the reorganization energy, the higher is the charge mobility.60 Our designed molecules shows lower reorganization energies value as compared to recently reported molecule namely SBF1 (ref. 61) with highest absorption value 400 nm. The reorganization energy for SBF1 is 0.144 eV. It is clear from above Table 4 our designed molecules have high charge mobilities due to lower value of reorganization energies.
Reorganization energies are divided into two parts; internal reorganization and external reorganization energy. External reorganization energy deals with environmental relaxation while internal reorganization energy deals with changes in the geometries of cation and anion. Cationic geometry describes with the mobility of cation while anionic geometry deals with the mobility of electron from donor material. In the current study, we are only dealing with internal reorganization energy.
Intra molecular reorganization energy is estimated with the aid of Marcus equation.62 It is the energy cost due to geometry form charge to neutral and neutral to charge molecules. Charge mobilities of organic solar cells is control by reorganization energies. The Marcus equation is given below
The λi in this equation can be explain in term of reorganization energy. This equation shows that, smaller is the geometry relaxation causes high electron transfer rate (kET). If both geometries such as geometry of final state (neutral state) and geometry of initial state (ionized state) are same then electron can easily be passes without any occurring of vibrations. If both geometries are different then the molecule has to wait until vibrations bring the acceptor into a shape that is more similar to the donor. Charge mobility is an activated process because it requires vibration that will prepare the system so that the geometries are equivalent and the electron move.
The λe value of R is 0.005939 eV. The designed molecules H1 and H4 have higher value of electron reorganization energies which is indicative of lower charge transfer rate in these molecules. The lower value of λe for H2 and H3 means high charge transfer rate from donor to acceptor unit. Among all, the lowest value of H2 is due to extending conjugation in acceptor unit. The λe values are in order of H4 > H1 > R > H3 > H2.
Secondly, the hole reorganization (λh) value of R is 0.004648 eV. A similar pattern is observed in hole reorganization energy; H1 and H4 have higher hole reorganization energies which mean low charge transfer rates. Among all the designed molecules, H2 and H3 exhibit lower reorganization energy with respect to R, which reflects high charge transfer mobilities. The designed molecules H1 and H4 have high reorganization energies which indicate low charge transfer rate as compared to H2 and H3. Among designed molecules, the highest hole mobility is calculated for H2 which is attributed to lower reorganization energy value. The λh value of all investigated acceptors are in the order of H4 > H1 > H2 > R > H3. By comparing hole and electron reorganization energies, the lower value of hole reorganization energy with respect to electron shows that these molecules are good for hole carrier. Above discussion indicates that the investigated molecules are efficient photovoltaic material in OSCs.
Open circuit voltage (Voc)
To evaluate the efficiency of solar cells, the most important factor is open circuit voltage (Voc).20 Voc describes the maximum current which can be taken out from any devices. Voc depends upon many factors such as charge carrier combination, external fluorescence, light source, temperature of device, and energy level of solar cells. However, Voc primarily depends on light generated and saturation current. The Voc is approximately equal to the difference of LUMO (acceptor) and HOMO (donor) moiety. The value of Voc is calculated with the aid of Scharber equation,63 as eqn (III).| Voc = (EHOMO (PBDB-T) − ELUMO (acceptor)) − 0.3 | (III) |
In above equation 0.3 is the empirical factor for efficient charge separation.64 To obtain the high value of Voc the HOMO of donor unit should be low in energy while LUMO of acceptor unit should have high energy. In the current study, we designed non-fullerene based acceptor so we blended our molecules with well-known PBDB-T donor. The calculate Voc with PBDB-T are shown in Fig. 9.
 | ||
| Fig. 9 The open circuit voltages (Voc) of reference R and H1–H4 with respect to donor material PBDB-T donor. | ||
The Voc value of R with respect to PBDB-T is 1.96 V. The Voc values of H1, H2, H3 and H4 are 1.79 V, 1.28 V, 1.88 V and 1.64 V respectively. The Voc value of H1 is 0.51 V higher than H2. The computed Voc value for H3 is 0.24 V higher than that of H4. The designed molecules H4 has Voc 0.36 V higher value with respect to H2. The highest Voc value is shown by H3. The Voc values for H1, H2, H3 and H4 lies in decreasing order of H3 > H1 > H4 > H2.
Exciton binding energy (Eb) and transition density matrix
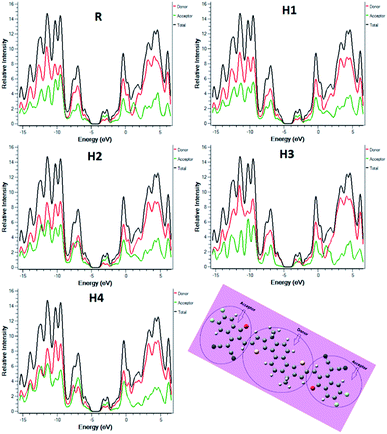
The nature of transition from S0–S1 is evaluated with the help of transition density matrix (TDM)65–69 with the selected functional. In our study, the contribution of hydrogen atom is very small therefore, it is neglected. The TDM illustrates the localization of electron and hole in the excited state, electronic excitation and also helps to understand the effect of acceptor and donor in the excited state. To evaluate the TDM, we divided our designed molecules into two parts one is donor denoted as D and other is end-capped acceptor moiety which is denoted as A. TDM results are shown in Fig. 10.The designed and reference molecules show similar behaviour; the electron consistency is diagonal to donor, and on acceptor group. The electron coherence is available on donor as well as on acceptor unit. From TDM diagram, is it confirmed that the electron is transferred from donor part to acceptor unit. Additionally, the coherence interaction between donor to acceptor unit in excited state is in the decreasing order of R = H4 > H1 > H2 > H3. All designed molecules have lower coefficient of interaction which means that these molecules have high charge transfer rate with respect to R. Among all, the highest charge density is observed for H3.
The charge dissociation ability of all molecule is higher than R which increases current charge density Jsc.
To enhance the efficiency of OSCs, the binding energy is a promising tool. Binding energy describes the coulombic interaction between positive and negative charges. Higher value of Eb mean high coulombic interaction between positive and negative charges which mean lower will be the exciton dissociation in excited state. The Eb value for R and designed molecules can be calculated by using following eqn (IV).
| Eb = EH–L − Eopt | (IV) |
In above equation Eb is the binding energy, EH–L is the energy gap and Eopt is the single point energy and results are shown in Table 5.
| Molecules | EH–L (eV) | Eopt (eV) | Eb (eV) |
|---|---|---|---|
| a Eb = binding energy. | |||
| R | 2.31 | 2.04 | 0.27 |
| H1 | 2.28 | 2.02 | 0.26 |
| H2 | 2.18 | 1.92 | 0.26 |
| H3 | 2.25 | 2.01 | 0.24 |
| H4 | 2.17 | 1.90 | 0.27 |
Binding energy analysis support the facts described by transition density matrix in Fig. 10. The binding energy of R, H1, H2, H3, and H4 are 0.27, 0.26, 0.26, 0.24 and 0.27 eV, respectively. R has highest binding energy which mean lower charge transfer. Among all studied molecules, H3 has the lowest binding energy which mean the highest charge transfer ability. Binding energy of reference R and H4 have same value, hence both R and H4 exhibit same charge mobilities. The binding energy of H1 is comparable to H2 however, these values are lower than that of R which reveals that both H1 and H2 show same charge transfer rate (higher than reference R). Furthermore, the order of binding energies of all molecules is R = H4 > H1 = H2 > H3.
Charge transfer analysis
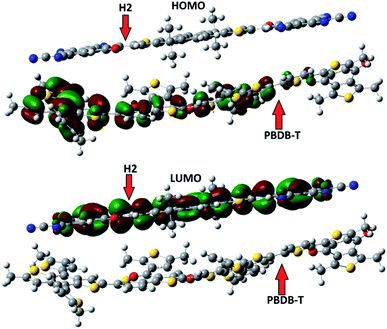
To evaluate the nature of transition, we made a complex between a designed molecules (H2) and PBDB-T donor. H2 molecule is chosen due to low reorganization energy and high charge mobility. The complex H2/PBDB-T is optimized with the selected functional. The literature reveals that the dipole moment from donor to acceptor is responsible for charge transfer.70–73 The side view of optimized geometry of complex in shown in Fig. 11.The dipole moment μ of H2/PBDB-T arises due to permanent dipole from donor to acceptor unit. To further check the nature of charge transfer (electronic properties) and distribution pattern from donor to acceptor unit, the FMOs analyses has been performed with selected functional and results are illustrated in Fig. 12. It is clear from Fig. 11 that the charge density is shifted from donor to acceptor unit which is in great agreement to show the nature of charges. In the complex, the HOMO is mainly distributed on donor unit (PBDB-T) while LUMO is spread on acceptor moiety.
Conclusions
In this study, we have designed four novel dithienonaphthalene based acceptor molecules (H1–H4) with different acceptor moieties to promote the opto-electronic properties of OSCs. By increasing extending conjugation in acceptor unit with central donor skeleton enhances, opto-electronic properties of OSCs are enhanced. A suitable functional for all further calculations is selected through a benchmark study where UV-vis spectrum of the reference compound R is calculated with five different functionals and compared with the experimental data. After careful assessment, B3LYP/6-31G(d,p) is chosen for further calculations. All molecules shows red shift in visible region with respect to reference molecule R. Among all, H2 has maximum red shift in the absorption spectrum (721 nm) in chloroform solvent. All designed molecules have reduced HOMO–LUMO energy gap however, the molecule H4 has the lowest HOMO–LUMO energy gap which is attributed to extended conjugation in acceptor moiety. Moreover, all designed molecules have higher charge transfer rate with respect to R, (lower value of excitation energy) where H2 has the highest charge transfer rate. For Voc, the designed molecules are scaled up with donor PBDB-T. The highest Voc value is calculated for H3. To calculate the charge transfer, binding energies are calculated which illustrate that all newly designed molecules have lower value of binding energies (high excitation and high charge transfer rate). The highest charge mobility is calculated for H2 which is due to lowest value of binding energy. To show the nature of charge transfer from donor to acceptor, the designed molecule H2 is blended with PBDB-T donor group. In summary, all designed molecules have outstanding opto-electronic properties with respect to reference molecule R.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Computations/simulations/SIMILAR were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) at Umeå University, 901 87, Umeå, Sweden. The authors acknowledge the financial and technical support from Punjab Bio-energy Institute (PBI), University of Agriculture Faisalabad (UAF).Notes and references
- D. M. Chapin, C. Fuller and G. Pearson, J. Appl. Phys., 1954, 25, 676–677 CrossRef CAS.
- A. M. Omer, Renewable Sustainable Energy Rev., 2008, 12, 2265–2300 CrossRef CAS.
- M. D. Archer and M. A. Green, Clean electricity from photovoltaics, World Scientific, 2001 Search PubMed.
- R. Prakash and I. K. Bhat, Renewable Sustainable Energy Rev., 2009, 13, 2716–2721 CrossRef.
- G. P. Smestad, Sol. Energy Mater. Sol. Cells, 2004, 82, 227–240 CrossRef CAS.
- A. McEvoy and T. Markvart, Solar cells: materials, manufacture and operation, Academic Press, 2012 Search PubMed.
- A. Facchetti, Chem. Mater., 2010, 23, 733–758 CrossRef.
- H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397–7457 CrossRef CAS.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS.
- J. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709–1718 CrossRef CAS.
- M. Ans, J. Iqbal, B. Eliasson and K. Ayub, Comput. Mater. Sci., 2019, 159, 150–159 CrossRef CAS.
- Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4272 RSC.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645–2655 CrossRef CAS.
- A. J. Heeger, Adv. Mater., 2014, 26, 10–28 CrossRef CAS.
- Y. Ma, Z. Kang and Q. Zheng, J. Mater. Chem. A, 2017, 5, 1860–1872 RSC.
- M. Ans, J. Iqbal, K. Ayub, E. Ali and B. Eliasson, Mater. Sci. Semicond. Process., 2019, 94, 97–106 CrossRef CAS.
- X. Zhan, A. Facchetti, S. Barlow, T. J. Marks, M. A. Ratner, M. R. Wasielewski and S. R. Marder, Adv. Mater., 2011, 23, 268–284 CrossRef CAS.
- M. Ans, K. Ayub, I. A. Bhatti and J. Iqbal, RSC Adv., 2019, 9, 3605–3617 RSC.
- C. B. Nielsen, S. Holliday, H.-Y. Chen, S. J. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803–2812 CrossRef CAS.
- C. Zhan and J. Yao, Chem. Mater., 2016, 28, 1948–1964 CrossRef CAS.
- M. Ans, J. Iqbal, Z. Ahmad, S. Muhammad, R. Hussain, B. Eliasson and K. Ayub, ChemistrySelect, 2018, 3, 12797–12804 CrossRef CAS.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS.
- Y. Zhong, M. T. Trinh, R. Chen, G. E. Purdum, P. P. Khlyabich, M. Sezen, S. Oh, H. Zhu, B. Fowler and B. Zhang, Nat. Commun., 2015, 6, 8242 CrossRef CAS.
- Y. J. Hwang, H. Li, B. A. Courtright, S. Subramaniyan and S. A. Jenekhe, Adv. Mater., 2016, 28, 124–131 CrossRef CAS.
- D. Meng, D. Sun, C. Zhong, T. Liu, B. Fan, L. Huo, Y. Li, W. Jiang, H. Choi and T. Kim, J. Am. Chem. Soc., 2015, 138, 375–380 CrossRef.
- S. Holliday, R. S. Ashraf, A. Wadsworth, D. Baran, S. A. Yousaf, C. B. Nielsen, C.-H. Tan, S. D. Dimitrov, Z. Shang and N. Gasparini, Nat. Commun., 2016, 7, 11585 CrossRef CAS.
- F. Liu, Z. Zhou, C. Zhang, T. Vergote, H. Fan, F. Liu and X. Zhu, J. Am. Chem. Soc., 2016, 138, 15523–15526 CrossRef CAS.
- S. Holliday, R. S. Ashraf, C. B. Nielsen, M. Kirkus, J. A. Röhr, C.-H. Tan, E. Collado-Fregoso, A.-C. Knall, J. R. Durrant and J. Nelson, J. Am. Chem. Soc., 2015, 137, 898–904 CrossRef CAS.
- X. F. Wu, W. F. Fu, Z. Xu, M. Shi, F. Liu, H. Z. Chen, J. H. Wan and T. P. Russell, Adv. Funct. Mater., 2015, 25, 5954–5966 CrossRef CAS.
- M. Ans, J. Iqbal, B. Eliasson, M. J. Saif, H. M. A. Javed and K. Ayub, J. Mol. Model., 2019, 25, 129 CrossRef.
- Y. Kim, C. Song, E.-J. Ko, D. Kim, S.-J. Moon and E. Lim, RSC Adv., 2015, 5, 4811–4821 RSC.
- P. Sonar, G.-M. Ng, T. T. Lin, A. Dodabalapur and Z.-K. Chen, J. Mater. Chem., 2010, 20, 3626–3636 RSC.
- D. Meng, H. Fu, C. Xiao, X. Meng, T. Winands, W. Ma, W. Wei, B. Fan, L. Huo and N. L. Doltsinis, J. Am. Chem. Soc., 2016, 138, 10184–10190 CrossRef CAS.
- Y. Liu, C. Mu, K. Jiang, J. Zhao, Y. Li, L. Zhang, Z. Li, J. Y. L. Lai, H. Hu and T. Ma, Adv. Mater., 2015, 27, 1015–1020 CrossRef CAS.
- P. E. Hartnett, A. Timalsina, H. R. Matte, N. Zhou, X. Guo, W. Zhao, A. Facchetti, R. P. Chang, M. C. Hersam and M. R. Wasielewski, J. Am. Chem. Soc., 2014, 136, 16345–16356 CrossRef CAS.
- B. Xiao, A. Tang, J. Zhang, A. Mahmood, Z. Wei and E. Zhou, Adv. Energy Mater., 2017, 7, 1602269 CrossRef.
- M. Cheng, C. Chen, X. Yang, J. Huang, F. Zhang, B. Xu and L. Sun, Chem. Mater., 2015, 27, 1808–1814 CrossRef CAS.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Röhr, S. Holliday, A. Wadsworth, S. Lockett and M. Neophytou, Nat. Mater., 2017, 16, 363 CrossRef CAS.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma and W. You, Adv. Mater., 2017, 29, 1700144 CrossRef.
- B. Kan, H. Feng, X. Wan, F. Liu, X. Ke, Y. Wang, Y. Wang, H. Zhang, C. Li and J. Hou, J. Am. Chem. Soc., 2017, 139, 4929–4934 CrossRef CAS.
- N. Qiu, H. Zhang, X. Wan, C. Li, X. Ke, H. Feng, B. Kan, H. Zhang, Q. Zhang and Y. Lu, Adv. Mater., 2017, 29, 1604964 CrossRef.
- Y. Ma, Q. Zheng, Z. Yin, D. Cai, S.-C. Chen and C. Tang, Macromolecules, 2013, 46, 4813–4821 CrossRef CAS.
- Y. Ma, S.-C. Chen, Z. Wang, W. Ma, J. Wang, Z. Yin, C. Tang, D. Cai and Q. Zheng, Nano Energy, 2017, 33, 313–324 CrossRef CAS.
- J. Lee, A. J. Kalin, T. Yuan, M. Al-Hashimi and L. Fang, Chem. Sci., 2017, 8, 2503–2521 RSC.
- Y. Ma, M. Zhang, Y. Yan, J. Xin, T. Wang, W. Ma, C. Tang and Q. Zheng, Chem. Mater., 2017, 29, 7942–7952 CrossRef CAS.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. Petersson, J. Exp. Clin. Med., 2009, 4(3), 165–169 Search PubMed.
- R. D. Dennington, T. A. Keith and J. M. Millam, Gaussian Inc, 2008.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- B. Civalleri, C. M. Zicovich-Wilson, L. Valenzano and P. Ugliengo, CrystEngComm, 2008, 10, 405–410 RSC.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- R. M. El-Shishtawy, A. M. Asiri, S. G. Aziz and S. A. Elroby, J. Mol. Model., 2014, 20, 2241 CrossRef.
- C. Adamo and V. Barone, J. Chem. Phys., 1998, 108, 664–675 CrossRef CAS.
- M. Cossi, V. Barone, B. Mennucci and J. Tomasi, Chem. Phys. Lett., 1998, 286, 253–260 CrossRef CAS.
- M. Irfan, J. Iqbal, S. Sadaf, B. Eliasson, U. A. Rana, S. Ud-din Khan and K. Ayub, Int. J. Quantum Chem., 2017, 117, e25363 CrossRef.
- M. E. Köse, W. J. Mitchell, N. Kopidakis, C. H. Chang, S. E. Shaheen, K. Kim and G. Rumbles, J. Am. Chem. Soc., 2007, 129, 14257–14270 CrossRef.
- G. Zhang, G. Yang, H. Yan, J. H. Kim, H. Ade, W. Wu, X. Xu, Y. Duan and Q. Peng, Adv. Mater., 2017, 29, 1606054 CrossRef.
- S. S. Amiri, S. Makarem, H. Ahmar and S. Ashenagar, J. Mol. Struct., 2016, 1119, 18–24 CrossRef.
- B. Xu, D. Bi, Y. Hua, P. Liu, M. Cheng, M. Grätzel, L. Kloo, A. Hagfeldt and L. Sun, Energy Environ. Sci., 2016, 9, 873–877 RSC.
- B. Zhang, Y. Xu, J. Yang, Y. Liao, L. Tong and S. Zhou, Comput. Theor. Chem., 2019, 112575 CrossRef CAS.
- R. A. Marcus, Annu. Rev. Phys. Chem., 1964, 15, 155–196 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS.
- J.-L. Brédas, D. Beljonne, V. Coropceanu and J. Cornil, Chem. Rev., 2004, 104, 4971–5004 CrossRef.
- M. E. Köse, J. Phys. Chem. A, 2012, 116, 12503–12509 CrossRef PubMed.
- A. Dkhissi, Synth. Met., 2011, 161, 1441–1443 CrossRef CAS.
- B. G. Kim, C. G. Zhen, E. J. Jeong, J. Kieffer and J. Kim, Adv. Funct. Mater., 2012, 22, 1606–1612 CrossRef CAS.
- M. Ans, K. Ayub, S. Muhammad and J. Iqbal, Comput. Theor. Chem., 2019, 1161, 26–38 CrossRef CAS.
- S. K. Gangala, M. Paramasivam, B. Dyaga and V. J. Rao, New J. Chem., 2019, 43(13), 5173–5186 RSC.
- V. Arkhipov, P. Heremans and H. Bässler, Appl. Phys. Lett., 2003, 82, 4605–4607 CrossRef CAS.
- C. Marchiori and M. Koehler, Synth. Met., 2010, 160, 643–650 CrossRef CAS.
- M. Koehler, M. Santos and M. Da Luz, J. Appl. Phys., 2006, 99, 053702 CrossRef.
- S. Baranovskii, M. Wiemer, A. Nenashev, F. Jansson and F. Gebhard, J. Phys. Chem. Lett., 2012, 3, 1214–1221 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06345e |
| This journal is © The Royal Society of Chemistry 2019 |