Open Access Article
Open Access ArticleSynthesis and application of amine-containing conjugated small molecules for the automatic formation of an electron transporting layer via spontaneous phase separation from the bulk-heterojunction layer†
Juae
Kim‡
a,
Yong Ryun
Kim‡
b,
Minji
Kim
a,
Jong Sung
Jin
c,
Ji Yeong
Sung
c,
Hyungcheol
Back
b,
Heejoo
Kim
*d,
Kwanghee
Lee
 *e and
Hongsuk
Suh
*e and
Hongsuk
Suh
 *a
*a
aDepartment of Chemistry, Chemistry Institute for Functional Materials, Pusan National University, Busan 609-735, Republic of Korea. E-mail: hssuh@pusan.ac.kr
bResearch Institute for Solar and Sustainable Energies, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea
cBusan Center, Korea Basic Science Institute (KBSI), Busan 46742, Republic of Korea
dInstitute of Integrated Technology, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea. E-mail: heejook@gist.ac.kr
eSchool of Materials Science and Engineering, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea. E-mail: klee@gist.ac.kr
First published on 9th October 2019
Abstract
Carbazole-based conjugated small molecule electrolytes (CSEs) containing different numbers of amine groups were synthesized and applied to bulk-heterojunction (BHJ) organic solar cells for the formation of a spontaneous self-assembled electron transporting layer (ETL). The active layer was spin-coated with a mixture solution containing the BHJ materials and a small amount of CSE, and a thin layer of CSE was formed underneath the active layer (CSE/BHJ bi-layer) via spontaneous phase separation, which is confirmed by the depth profile of the time of flight secondary ion mass spectroscopy (ToF-SIMS) spectrum. The amino groups in the CSEs form hydrogen-bonds with the surface of indium tin oxide (ITO), which acts as an ETL in BHJ solar cells. Moreover, the formed CSE layer is capable of changing the effective work function (WF) of ITO. An increasing number of amino groups in the CSEs (from Cz1N to Cz3N) provides more reduction of the effective WF of ITO, which results in a lower internal resistance and a higher power conversion efficiency (PCE). Furthermore, the enhanced hydrogen bonding between the amines and ITO with an increased number of amine groups has been studied by XPS. This result suggests that one-step processing provides a reduction of the manufacturing cost, which can provide an attractive design concept for ETL fabrication.
Introduction
Over the past decade, bulk-heterojunction (BHJ) solar cells comprising conjugated polymer donors and fullerene acceptors have attained significant attention due to their advantages, such as solution processability, large area fabrication, and applicability for roll-to-roll processes.1–4 The improvement of the power conversion efficiency (PCE) of BHJ solar cells has been achieved through several strategies, such as control of BHJ morphology, new molecular design of the semiconducting materials, and modification of the interfaces.5–12 Among them, introduction of interlayer materials between the indium tin oxide (ITO) bottom electrode and the photoactive layer in an inverted BHJ solar cell is regarded as one of the facile and efficient methods of improving the PCE of BHJ solar cells.13–16 The introduced layer can generate an interfacial dipole at the interface to change the WF of the electrode, leading to ohmic contact between the BHJ layer and ITO. As a result, the electron transfer from the photoactive layer to the ITO electrode is facilitated, increasing the PCE.13,15,17Solution processable metal oxides such as ZnO18 and TiO2ref. 19 and 20 are the most popular electron transport layer (ETL) materials. However, their complicated fabrication method, with the application of a high annealing temperature (>200 °C) for the crystallization of the metal oxides, is not compatible with roll-to-roll manufacturing processes for high throughput.18,21 To solve this problem, there is an alternative route without thermal annealing that is afforded by a ZnO nanoparticle (ZnO NP) emulsion, which allows applications in roll-to-roll deposition.22,23 However, this sequential process for fabricating the ETL/BHJ bi-layered structure increases the number of fabrication steps for multi-layered devices, and thus is also unfavorable for the mass production of BHJ solar cells. As a low temperature processable ETL, polyelectrolytes such as poly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9-dioctylfluorene)] (PFN)24 and polyethyleneimine ethoxylated (PEIE)25 have been shown to be alternative candidates. Furthermore, their electronic structure can be easily tailored for ohmic contact at the interface by changing the polymer backbone or side chains.26–35 However, polyelectrolytes/BHJ bi-layer structures are also fabricated with a sequential process.
Recently, Lee et al. reported a one-step process for fabricating an ETL/BHJ bi-layered structure, which is enabled by using a mixed BHJ solution including a small amount of non-conjugated polyethyleneimine (PEI). The amines in the PEI could induce spontaneous phase migration to ITO during the spin-casting due to the hydrogen bonding between PEI and ITO, thereby forming an ETL/BHJ bi-layered structure simply by using one spin-casting process.36–38 Subsequently, a dipole layer is formed between the migrated PEI molecules and the surface of ITO, resulting in similar device performance to that of sequentially processed devices. However, as observed in the sequentially processed bi-layered devices, the optimized thickness of the PEI layer for the one-step processed BHJ solar cells was less than 5 nm.39 Because the backbone of PEI is an insulating material causing thickness sensitivity, a thicker PEI layer (>5 nm) causes poor charge transport, decreasing both FF and JSC significantly. This result can be ascribed to a lack of conjugation portion in the polymer backbone of PEI.40–43 Recently, Kim et al. reported self-organization of polymer additive poly(2-vinylpyridine) (P2VP) via one-step solution processing to increase the device performance. But P2VP was used in addition to a metal oxide (ZnO) layer, and could not reduce the processing steps of the device.44 In contrast to the aforementioned works, Cao et al. reported a good device performance with a thicker ETL (thickness ∼ 40 nm)/BHJ bi-layered structure when they used a conjugated polyelectrolyte (CPE) as the ETL.45 Although this bi-layered structure was fabricated by a sequential process, this result implies that CPEs comprising a conjugated portion and hydrophilic amine groups made it possible to keep the charge transport property.
Here, we report the design, syntheses and properties of novel conjugated small molecule electrolytes (CSEs), which have not only a conjugated portion for efficient electron transport but also amine groups, essential functional group of PEI for spontaneous phase separation. These CSEs with a carbazole moiety and amine groups have a simple synthetic method, batch-to-batch reproducibility, a well-defined molecule structure and absolutely fixed molecular weight. Carbazole was used as the conjugated moiety because it has been widely utilized as an ETL material. The hydrophobic carbazole portion will provide reasonable solubility of the CSEs in the solvent that is being used for the spin-coating of one solution of electron donor/electron acceptor/CSE. The amine groups provide the driving force for spontaneous phase separation to form a CSE layer on top of ITO.
Furthermore, we systemically studied the spontaneous phase separation of CzxN as an ETL in inverted organic solar cells by using one spin-coating method using one solution of PTB7-Th/PC71BM/CzxN. With the aim of achieving better device performance, we chose to vary the number of amines to provide Cz1N, Cz2N, and Cz3N, since the variation of the number of amine groups can improve the hydrophilicity of the CSE. Furthermore, Kelvin probe (KP) measurement was used to check how the number of amines varies the WF of ITO cathodes. In addition to this, the hydrogen bonding between the amine and ITO after spontaneous phase separation has been confirmed by XPS.
The KP measurements on CzxN/ITO showed that the WF of ITO decreased on increasing the number of amine groups. The water contact angle measurements on CzxN and BHJ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CzxN films showed that the hydrophilic properties of CSEs were improved on increasing the number of amine groups in the CSEs. A higher performance was obtained with the device with an increased number of amine groups (Cz3N), which provided a lower WF of ITO, lower water contact angle and more efficient hydrogen bonding with ITO as confirmed by XPS. Therefore, our results will provide a way of developing effective ETL materials inducing spontaneous phase separation to realize a printing process of large area BHJ solar cells with reduced steps.
CzxN films showed that the hydrophilic properties of CSEs were improved on increasing the number of amine groups in the CSEs. A higher performance was obtained with the device with an increased number of amine groups (Cz3N), which provided a lower WF of ITO, lower water contact angle and more efficient hydrogen bonding with ITO as confirmed by XPS. Therefore, our results will provide a way of developing effective ETL materials inducing spontaneous phase separation to realize a printing process of large area BHJ solar cells with reduced steps.
Results and discussion
Material characterization
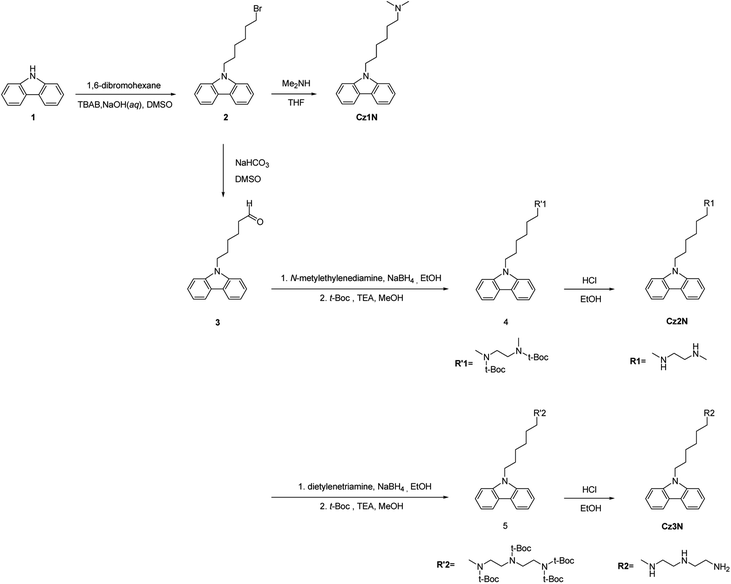
The general synthetic routes toward the intermediates and small molecules are outlined in Scheme 1. The detail synthetic routes are described in the experimental section and the ESI.† 9-(6-Bromo-hexyl)-9H-carbazole (2) was synthesized by alkylation of 9H-carbazole (1). Cz1N was prepared by amination of compound 2. After the Kornblum oxidation of compound 2, 6-carbazol-9-yl-hexanal (3) was obtained. Cz2N and Cz3N were synthesized by reductive amination of the aldehyde with a carbazole moiety using the corresponding amine-containing reagents. All of the final compounds, which have simple synthetic routes and can be easily purified, dissolve in polar solvents such as MeOH. The clear identifications of the synthesized molecules were performed by using 1H NMR, 13C NMR and mass spectrometry as shown in the ESI, Fig. S1–S3.†The UV-vis absorption spectra of the solutions (in MeOH) and solid films of CzxNs are presented in Fig. S4† and the λmax values both for solutions and solid films are summarized in Table S1.† As shown in Fig. S4(a),† the solutions of CzxNs in MeOH exhibited almost identical absorption spectra with peaks at 330 nm and 345 nm. In solid films, the wavelength of the optical absorption onset was slightly red-shifted from 356 nm to 358 nm on increasing the number of amine groups. Therefore, the optical band gaps for Cz1N, Cz2N, and Cz3N were estimated to be 3.48, 3.47 and 3.46 eV, respectively. Nevertheless, the shapes of the absorption spectra for all solid films were almost identical, indicating that the change of the side chains does not disturb the electronic structure of the carbazole moiety. The electrochemical properties of the CzxN molecules, such as the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels, were characterized by using cyclic voltammetry (CV) (see Fig. S5† and Table S2†). The HOMO and LUMO energy levels of Cz1N, Cz2N and Cz3N were estimated to be −6.99, −7.03, −7.04 eV and −3.19, −3.05, −3.04 eV, respectively. The estimated band gaps of Cz1N, Cz2N, and Cz3N were 3.80, 3.98, and 4.00 eV, respectively. Although the band gaps of each material from the optical absorption spectra and CV measurements were marginally different, typically it is accepted that there is a difference between the optical band gap and electrochemical band gap of organic materials.46,47 As observed in the absorption spectra of solid films, the difference of the HOMO for each material was also very small (see Fig. S5† and Table S2†).
Device characterization
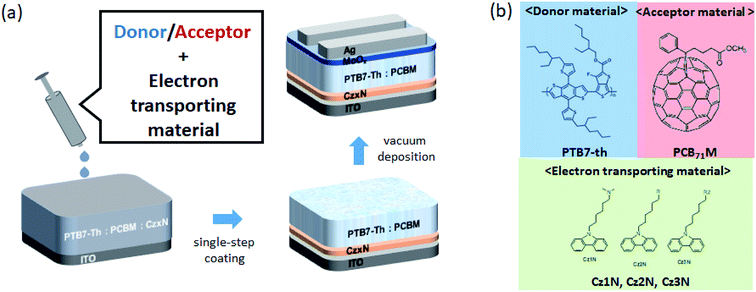
Fig. 1(a) illustrates the single-step fabrication method of the BHJ/CzxN layer on an ITO substrate, and Fig. 1(b) exhibits the chemical structures of the electron donor, poly([2,6′-4,8-di(5-ethylhexylthienyl)benzo[1,2-b;3,3-b]dithiophene]{3-fluoro-2[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl}) (PTB7-Th), electron acceptor, phenyl-C71 butyric acid methyl ester (PC71BM), and electron transporting materials, Cz1N, Cz2N and Cz3N, used in this study. Inverted BHJ organic solar cells were prepared with the configuration of ITO/PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM
PC71BM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CzxN/MoOx/Ag, in which the CzxN layer with a different number of amine groups (1 to 3) was introduced, via spontaneous phase separation, to examine the effect on the device performance. To clarify the function of CzxNs in the BHJ solar cells, we also fabricated the reference device: ITO/PTB7-Th
CzxN/MoOx/Ag, in which the CzxN layer with a different number of amine groups (1 to 3) was introduced, via spontaneous phase separation, to examine the effect on the device performance. To clarify the function of CzxNs in the BHJ solar cells, we also fabricated the reference device: ITO/PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM/MoOx/Ag. Fig. 2(a) exhibits the current density–voltage (J–V) characteristic curves of devices containing different CzxNs, and the corresponding device performances are summarized in Table 1. The device without CzxN yielded an average PCE of less than 1% with an extremely low VOC of 0.13 V. This low VOC value can be ascribed to the high-energy barrier between the LUMO value of PC71BM and the work function of ITO, which hinders the formation of ohmic contact at the interface. Interestingly, on adding a small amount of CzxNs, including Cz1N, Cz2N, and Cz3N, the PCE is increased from 0.24% to 4.91%. (For the best champion device, the PCE increased from 0.53% to 5.67%). This is attributed to the formation of an ETL, which induces a substantially decreased WF of ITO via the development of interfacial dipoles and reduces the energy barrier between the BHJ and ITO substrate as shown in Fig. 2(b). Additionally, we also performed impedance spectroscopy exhibiting a significantly decreased internal resistance on increasing the number of amine groups in the CzxNs: Cz3N (145 Ω) < Cz2N (158 kΩ) < Cz1N (200 kΩ) as shown in Fig. 2(d). Typically, a reduced internal resistance in BHJ solar cells can be ascribed to a decrease of the bulk resistance of BHJ films or a decrease of the series resistance at the interface between the ETL and active layers. The bulk resistance of BHJ films is also significantly related to the change of surface morphology of the BHJ films. Therefore, we observed the surface morphology of the CzxN
PC71BM/MoOx/Ag. Fig. 2(a) exhibits the current density–voltage (J–V) characteristic curves of devices containing different CzxNs, and the corresponding device performances are summarized in Table 1. The device without CzxN yielded an average PCE of less than 1% with an extremely low VOC of 0.13 V. This low VOC value can be ascribed to the high-energy barrier between the LUMO value of PC71BM and the work function of ITO, which hinders the formation of ohmic contact at the interface. Interestingly, on adding a small amount of CzxNs, including Cz1N, Cz2N, and Cz3N, the PCE is increased from 0.24% to 4.91%. (For the best champion device, the PCE increased from 0.53% to 5.67%). This is attributed to the formation of an ETL, which induces a substantially decreased WF of ITO via the development of interfacial dipoles and reduces the energy barrier between the BHJ and ITO substrate as shown in Fig. 2(b). Additionally, we also performed impedance spectroscopy exhibiting a significantly decreased internal resistance on increasing the number of amine groups in the CzxNs: Cz3N (145 Ω) < Cz2N (158 kΩ) < Cz1N (200 kΩ) as shown in Fig. 2(d). Typically, a reduced internal resistance in BHJ solar cells can be ascribed to a decrease of the bulk resistance of BHJ films or a decrease of the series resistance at the interface between the ETL and active layers. The bulk resistance of BHJ films is also significantly related to the change of surface morphology of the BHJ films. Therefore, we observed the surface morphology of the CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ films by using the tapping mode of atomic force microscopy (AFM). As shown in Fig. S6(a)–(c),† despite slightly different surface morphologies, it can be noticed that the small grains in the BHJ films were almost similar in the AFM images. This result is well consistent with the trend of JSC values obtained in the J–V characteristics (small change of JSC value from Cz1N to Cz3N, see Table 1); the surface morphology of the BHJ films is not significantly influenced by changing the number of amine groups on the CSEs. With this result and KP measurements for CzxNs, it can be concluded that the effect of the interface is more dominant for the decrease of the resistance in the device. Therefore, the charge recombination in the CzxN
BHJ films by using the tapping mode of atomic force microscopy (AFM). As shown in Fig. S6(a)–(c),† despite slightly different surface morphologies, it can be noticed that the small grains in the BHJ films were almost similar in the AFM images. This result is well consistent with the trend of JSC values obtained in the J–V characteristics (small change of JSC value from Cz1N to Cz3N, see Table 1); the surface morphology of the BHJ films is not significantly influenced by changing the number of amine groups on the CSEs. With this result and KP measurements for CzxNs, it can be concluded that the effect of the interface is more dominant for the decrease of the resistance in the device. Therefore, the charge recombination in the CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ devices is reduced. Even though the best PCE values of PTB7-Th/PC71BM with the standard ETL system are higher than our resulting data, our method has the merit of eliminating one of the processing steps. So, this type of material, for the formation of an ETL by using spontaneous phase separation, should be consistently researched, especially for roll-to-roll processes. The photovoltaic properties of the PTB7-Th-based BHJ solar cells with a standard ETL are included in the ESI.†
BHJ devices is reduced. Even though the best PCE values of PTB7-Th/PC71BM with the standard ETL system are higher than our resulting data, our method has the merit of eliminating one of the processing steps. So, this type of material, for the formation of an ETL by using spontaneous phase separation, should be consistently researched, especially for roll-to-roll processes. The photovoltaic properties of the PTB7-Th-based BHJ solar cells with a standard ETL are included in the ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ blend based solar cells. The mean values of each parameter (Voc, Jsc, FF, and PCE) with the standard deviation were calculated on the basis of 20 devices
BHJ blend based solar cells. The mean values of each parameter (Voc, Jsc, FF, and PCE) with the standard deviation were calculated on the basis of 20 devices
| Interlayer | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%) | J SC a (mA cm−2)IPCE |
|---|---|---|---|---|---|
| a is integrated JSC from the IPCE spectra. | |||||
| None | 0.13 ± 0.04 | 6.70 ± 0.60 | 0.28 ± 0.02 | 0.24 ± 0.12 | |
| Cz1N | 0.50 ± 0.01 | 14.90 ± 0.52 | 0.48 ± 0.02 | 3.81 ± 0.37 | 14.00 |
| Cz2N | 0.55 ± 0.07 | 15.10 ± 0.86 | 0.51 ± 0.05 | 4.21 ± 0.41 | 16.05 |
| Cz3N | 0.60 ± 0.04 | 15.50 ± 0.89 | 0.53 ± 0.02 | 4.91 ± 0.35 | 15.46 |
Mechanism
In order to gain an insight into the formation of the vertically self-assembled ETL of CzxNs via a single-step coating process, we investigated the location of the CzxN layer in the ITO/CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ structure using time-of-flight secondary-ion mass spectroscopy (ToF-SIMS), which allowed the distribution of the composition along the depth of the film to be detected. Fig. 3(a)–(c) exhibit the ToF-SIMS depth profiles of the ITO/CzxN
BHJ structure using time-of-flight secondary-ion mass spectroscopy (ToF-SIMS), which allowed the distribution of the composition along the depth of the film to be detected. Fig. 3(a)–(c) exhibit the ToF-SIMS depth profiles of the ITO/CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ structure. Each material, including ITO, CzxN and PTB7-Th, has been tracked down with their unique ions. The S− and InO− are assigned to the PTB7-Th and ITO, respectively, and the CN− ion is attributed to the existence of CzxNs. The accumulation of CN− ions, with high intensity, at the interface between the ITO and BHJ layer was monitored, which shows the formation of the CzxN layer by spontaneous vertical phase separation during the single-step coating process of the CzxN
BHJ structure. Each material, including ITO, CzxN and PTB7-Th, has been tracked down with their unique ions. The S− and InO− are assigned to the PTB7-Th and ITO, respectively, and the CN− ion is attributed to the existence of CzxNs. The accumulation of CN− ions, with high intensity, at the interface between the ITO and BHJ layer was monitored, which shows the formation of the CzxN layer by spontaneous vertical phase separation during the single-step coating process of the CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ blend solutions. This phenomenon of spontaneous vertical phase separation may be attributed to the high surface energies of the CzxNs relative to the BHJ components. Therefore, these results show that the introduction of CzxNs, which have a conjugated portion and amine groups in one molecules, in a one-step process has the advantage of reduction of the processing steps.
BHJ blend solutions. This phenomenon of spontaneous vertical phase separation may be attributed to the high surface energies of the CzxNs relative to the BHJ components. Therefore, these results show that the introduction of CzxNs, which have a conjugated portion and amine groups in one molecules, in a one-step process has the advantage of reduction of the processing steps.
To support the formation of a CzxN self-assembled layer on top of the ITO substrate and the absence of CzxN in the self-assembled BHJ layer, water contact angle measurements of the samples (described in methods) have been carried out. Fig. 4(a) shows snapshots of a water droplet on the bare ITO substrate, BHJ film, CzxN films and CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ films on ITO, respectively. From this data, it can be concluded that the hydrophilic property of the films increased with the increase of the number of amine groups (Cz1N < Cz2N < Cz3N, 55° > 50° > 43°), whereas the CzxN
BHJ films on ITO, respectively. From this data, it can be concluded that the hydrophilic property of the films increased with the increase of the number of amine groups (Cz1N < Cz2N < Cz3N, 55° > 50° > 43°), whereas the CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ films show strong hydrophobic properties with the increase of the number of amine groups (Cz3N
BHJ films show strong hydrophobic properties with the increase of the number of amine groups (Cz3N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ > Cz2N
BHJ > Cz2N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ > Cz1N
BHJ > Cz1N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ, 92° > 90° > 86°). This is because of not only the presence of amine groups in the CzxNs, which lead to hydrophilicity, but also the non-polar chemical composition from the BHJ materials having hydrophobicity. We also note that the hydrophobicity of the CzxN
BHJ, 92° > 90° > 86°). This is because of not only the presence of amine groups in the CzxNs, which lead to hydrophilicity, but also the non-polar chemical composition from the BHJ materials having hydrophobicity. We also note that the hydrophobicity of the CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ films is relatively similar to that of the BHJ film itself. That is, the uppermost surface contains no CzxNs, indicating that the vertical self-assembly process for the BHJ/CzxN bi-layered structure occurred during film formation (Table 2).
BHJ films is relatively similar to that of the BHJ film itself. That is, the uppermost surface contains no CzxNs, indicating that the vertical self-assembly process for the BHJ/CzxN bi-layered structure occurred during film formation (Table 2).
 | ||
| Fig. 4 (a) Water contact angle measurement, (b) high-resolution XPS deconvoluted spectra of O 1s for ITO/CzxNs and (c) the concept of spontaneous phase separation of conjugated small molecules. | ||
| Interlayer | Position (eV) | % Relative intensity | FWHM (eV) | GLa (%) | Area (P) CPS (eV) | Assignment |
|---|---|---|---|---|---|---|
| a GL: % Lorentzian–Gaussian. | ||||||
| Cz1N | 529.63 | 58.63 | 1.17 | 41 | 124139 | In–O |
| 530.68 | 34.31 | 1.64 | 0 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 642 642 |
Sn–O | |
| 532.03 | 7.07 | 1.51 | 0 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 962 962 |
O–H, O–C and (O2)2− | |
| Cz2N | 529.68 | 58.14 | 1.18 | 36 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434 |
In–O |
| 530.76 | 31.24 | 1.52 | 2 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 222 |
Sn–O | |
| 531.93 | 9.77 | 1.48 | 0 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 522 |
O–H, O–C and (O2)2− | |
| Cz3N | 529.49 | 54.17 | 1.24 | 34 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 857 |
In–O |
| 530.75 | 28.83 | 1.50 | 0 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 597 597 |
Sn–O | |
| 531.94 | 17.00 | 1.67 | 0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279 279 |
O–H, O–C and (O2)2− | |
To understand the interaction between the CzxNs and ITO substrate during the single-coating process, we carried out X-ray photoelectron spectroscopy (XPS) measurements on the three different CzxN layers (Cz1N, Cz2N and Cz3N)/ITO substrates. As shown in Fig. 4(b), the O 1s core level XPS spectra are presented as solid lines, while the deconvolutions of the O 1s spectra with Gaussian peaks are presented as dashed lines. The O 1s core level signals were successfully fitted to three peaks corresponding to the In–O (BE = 529.5 eV), Sn–O (BE = 530.7 eV) and chemically adsorbed O–H bonding (BE = 531.9 eV), respectively.48–51 As compared to Cz1N and Cz2N, Cz3N exhibited higher intensity of the peak at 531.9 eV corresponding to the chemically adsorbed O–H bonds, indicating that the increase of the number of amine groups effectively provides more chemically adsorbed O–H bonds, which implies that the interfacial interaction between the amine groups and the ITO was increased. The CzxNs have protons attached to the electro-negative nitrogen atoms, which work as the hydrogen bonding donors toward the electro-negative oxygen atoms, which work as the hydrogen bonding acceptors, at the surface of ITO.
Conclusions
We designed and synthesized carbazole-based CSEs, Cz1N, Cz2N and Cz3N, with different numbers of amine groups, by reductive amination reaction to achieve the spontaneous phase separation of the amine-containing CSEs for the automatic formation of an ETL layer, out of one solution containing the bulk-heterojunction materials and CSEs. A strong interfacial dipole caused by the hydrogen bonding between the amine groups of the small molecules and ITO is formed at the interface of ITO/CzxNs, leading to a decrease of the effective WF of ITO. Cz3N showed the lowest WF of ITO and series resistance at the interface caused by more hydrogen bonding interaction with ITO as confirmed by XPS.Spontaneous phase separation generated the automatic formation of the bulk-heterojunction layer and electron transporting CzxN interlayer when one solution containing CzxN and BHJ materials was spin-casted on ITO, which was verified by performing ToF-SIMS elemental depth profiles. When the number of amine groups was increased, the device performance was enhanced caused by more effective spontaneous phase separation, and lower WF of ITO and impedance. It was demonstrated that increasing the number of amine groups in the CSEs (from Cz1N to Cz3N) enhanced the hydrogen bonding between the CzxNs and ITO, as confirmed by XPS, and provides higher PCE. These results show that the introduction of CzxNs in one-step processing by using spontaneous phase separation has the advantage of enabling the reduction of the processing steps. By optimizing the structure of the CSE via the introduction of a more efficient electron transporting conjugated moiety and modification of the side chain, it could be possible to realize a more practical procedure for device fabrication.
Experimental section
Materials
All reagents were purchased from Aldrich or TCI, and used without further purification. 1H/13C NMR spectra were recorded by a Varian Gemini-300 spectrometer (recorded in ppm units). High resolution mass spectra (HRMS) were recorded on a JEOL JMS-700 mass spectrometer under electron impact (EI) or fast atom bombardment (FAB) conditions at the Korea Basic Science Institute (Daegu).Synthesis of monomers and small molecules
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 122.77
122.77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120.32, 118.70, 108.66, 82.13, 57.85, 55.54, 43.87, 42.98, 42.58, 28.99 28.07, 27.37, 27.29, 27.17. HRMS (m/z, EI+) calcd for C31H45N3O4, 523.3410, found 523.3412.
120.32, 118.70, 108.66, 82.13, 57.85, 55.54, 43.87, 42.98, 42.58, 28.99 28.07, 27.37, 27.29, 27.17. HRMS (m/z, EI+) calcd for C31H45N3O4, 523.3410, found 523.3412.
Device fabrication and characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM was prepared by dissolving 7 mg of PTB7-Th and 10.5 mg of PC71BM (1
PC71BM was prepared by dissolving 7 mg of PTB7-Th and 10.5 mg of PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 by weight) in 1 ml of chlorobenzene (CB) with a 1,8-diiodooctane (DIO) additive (3% by volume) and stirred overnight at 60 °C. The CzxN
1.5 by weight) in 1 ml of chlorobenzene (CB) with a 1,8-diiodooctane (DIO) additive (3% by volume) and stirred overnight at 60 °C. The CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ solutions were prepared by mixing CzxN and the blend solutions (1
BHJ solutions were prepared by mixing CzxN and the blend solutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 by volume).
9 by volume).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ/MoOx/Ag or ITO/BHJ/MoOx/Ag. The coated ITO substrates were ultra-sonicated with deionized water, acetone and IPA for 20 minutes each, and then dried overnight in an oven at 85 °C. The ultra-sonicated ITO glass substrates were UV-Ozone treated for 20 minutes prior to device fabrication. For the i-OPV structures, the CzxN
BHJ/MoOx/Ag or ITO/BHJ/MoOx/Ag. The coated ITO substrates were ultra-sonicated with deionized water, acetone and IPA for 20 minutes each, and then dried overnight in an oven at 85 °C. The ultra-sonicated ITO glass substrates were UV-Ozone treated for 20 minutes prior to device fabrication. For the i-OPV structures, the CzxN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BHJ or BHJ were spin-cast onto the ITO at 800 rpm for 30 seconds, followed by 5000 rpm for 10 seconds. Finally, MoOx (2 nm) and Ag (120 nm) layers were deposited on the blend films via thermal evaporation under a high vacuum of 1 × 10−6 torr with a shadow mask. The active area of the devices was defined by the patterned Ag electrode (0.0464 cm2).
BHJ or BHJ were spin-cast onto the ITO at 800 rpm for 30 seconds, followed by 5000 rpm for 10 seconds. Finally, MoOx (2 nm) and Ag (120 nm) layers were deposited on the blend films via thermal evaporation under a high vacuum of 1 × 10−6 torr with a shadow mask. The active area of the devices was defined by the patterned Ag electrode (0.0464 cm2).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015M1A2A2057510). H. K. acknowledges the financial support from the National Foundation of Korea (NRF) grant funded by the Korea Government (the Ministry of Science and ICT, MSIT) (NRF-2017R1A2B4012490). Also, this work was supported by a GIST Research Institute (GRI) grant funded by GIST in 2018.References
- R. Søndergaard, M. Hösel, D. Angmo, T. T. Larsen-Olsen and F. C. Krebs, Mater. Today, 2012, 15, 36–49 CrossRef.
- W. Liu, S. Liu, N. K. Zawacka, T. R. Andersen, P. Cheng, L. Fu, M. Chen, W. Fu, E. Bundgaard, M. Jørgensen, X. Zhan, F. C. Krebs and H. Chen, J. Mater. Chem. A, 2014, 2, 19809–19814 RSC.
- S. Khan, L. Lorenzelli and R. S. Dahiya, IEEE Sens. J., 2015, 15, 3164–3185 Search PubMed.
- Y.-W. Su, S.-C. Lan and K.-H. Wei, Mater. Today, 2012, 15, 554–562 CrossRef CAS.
- P. M. Beaujuge and J. M. J. Frechet, J. Am. Chem. Soc., 2011, 133, 20009–20029 CrossRef CAS.
- H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397–7457 CrossRef CAS.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS.
- M. Tanveer, A. Habi and M. B. Khan, NUST J. eng. sci., 2013, 6, 15–20 Search PubMed.
- H.-C. Liao, C.-C. Ho, C.-Y. Chang, M.-H. Jao, S. B. Darling and W.-F. Su, Mater. Today, 2013, 16, 326–336 CrossRef CAS.
- F. Zhao, C. Wang and X. Zhan, Adv. Energy Mater., 2018, 1703147 CrossRef.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS.
- J. Xiong, B. Yang, C. Cao, R. Wu, Y. Huang, J. Sun, J. Zhang, C. Liu, S. Tao, Y. Gao and J. Yang, Org. Electron., 2016, 30, 30–35 CrossRef CAS.
- S. Nam, J. Seo, S. Woo, W. H. Kim, H. Kim, D. D. C. Bradley and Y. Kim, Nat. Commun., 2015, 6, 8929 CrossRef CAS.
- Y.-J. Noh, S.-I. Na and S.-S. Kim, Sol. Energy Mater. Sol. Cells, 2013, 117, 139–144 CrossRef CAS.
- H.-C. Chen, S.-W. Lin, J.-M. Jiang, Y.-W. Su and K.-H. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 6273–6281 CrossRef CAS PubMed.
- H.-K. Lin, Y.-W. Su, H.-C. Chen, Y.-J. Huang and K.-H. Wei, ACS Appl. Mater. Interfaces, 2016, 8, 24603–24611 CrossRef CAS.
- C. V. Hoven, A. Garcia, G. C. Bazan and T.-Q. Nguyen, Adv. Mater., 2008, 20, 3793–3810 CrossRef CAS.
- Y. Sun, J. H. Seo, C. J. Takacs, J. Seifter and A. J. Heeger, Adv. Mater., 2011, 23, 1679–1683 CrossRef CAS.
- A. Guerrero, S. Chambon, L. Hirsch and G. Garcia-Belmonte, Adv. Funct. Mater., 2014, 24, 6234–6240 CrossRef CAS.
- J. Kong, J. Lee, Y. Jeong, M. Kim, S.-O. Kang and K. Lee, Appl. Phys. Lett., 2012, 100, 213305 CrossRef.
- X. Bulliard, S. Ihn, S. Yun, Y. Kim, D. Choi, J. Choi, M. Kim, M. Sim, J. Park, W. Choi and K. Cho, Adv. Funct. Mater., 2010, 20, 4381–4387 CrossRef CAS.
- W. Beek, M. Wienk, M. Kemerink, X. Yang and R. Janssen, J. Phys. Chem. B, 2005, 109, 9505–9516 CrossRef CAS PubMed.
- S. Dkhil, D. Duché, M. Gaceur, A. Thakur, F. Aboura, L. Escoubas, J. Simon, A. Guerrero, J. Bisquert, G. Belmonte, Q. Bao, M. Fahlman, C. Ackermann, O. Margeat and J. Ackermann, Adv. Energy Mater., 2014, 4, 1400805–1400816 CrossRef.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 CrossRef.
- Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A. J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun, J. Kim, M. Fenoll, A. Dindar, W. Haske, E. Najafabadi, T. M. Khan, H. Sojoudi, S. Barlow, S. Graham, J.-L. Brédas, S. R. Marder, A. Kahn and B. A. Kippelen, Science, 2012, 336, 327–332 CrossRef CAS.
- M. Song, J.-W. Kang, D.-H. Kim, J.-D. Kwon, S.-G. Park, S. Nam, S. Jo, S. Y. Ryu and C. S. Kim, Appl. Phys. Lett., 2013, 102, 143303 CrossRef.
- D. Ma, M. Lv, M. Lei, J. Zhu, H. Wang and X. Chen, ACS Nano, 2014, 8, 1601–1608 CrossRef CAS.
- J. Wang, K. Lin, K. Zhang, X.-F. Jiang, K. Mahmood, L. Ying, F. Huang and Y. Cao, Adv. Energy Mater., 2016, 6, 1502563 CrossRef.
- J. Lee, H. Kang, S. Kee, S. H. Lee, S. Y. Jeong, G. Kim, J. Kim, S. Hong, H. Back and K. Lee, ACS Appl. Mater. Interfaces, 2016, 8, 6144–6151 CrossRef CAS PubMed.
- Z. Li, Y. Liu, K. Zhang, Z. Wang, P. Huang, D. Li, Y. Zhou and B. Song, Langmuir, 2017, 33, 8679–8685 CrossRef CAS.
- H. Zhao, Y. Luo, L. Liu, Z. Xie and Y. Ma, Mater. Chem. Front., 2017, 1, 1087–1092 RSC.
- Y.-M. Chang, R. Zhu, E. Richard, C.-C. Chen, G. Li and Y. Yang, Adv. Funct. Mater., 2012, 22, 3284–3289 CrossRef CAS.
- S. Dong, Z. Hu, K. Zhang, Q. Yin, X. Jiang, F. Huang and Y. Cao, Adv. Mater., 2017, 29, 1701507 CrossRef.
- T. Schmaltz, G. Sforazzini, T. Reichert and H. Frauenrath, Adv. Mater., 2017, 29, 1605286 CrossRef.
- J. Vinokur, B. Shamieh, I. Deckman, A. Singhal and G. L. Frey, Chem. Mater., 2016, 28, 8851–8870 CrossRef CAS.
- J. Lee, J. Kim, C.-L. Lee, G. Kim, T. K. Kim, H. Back, S. Jung, K. Yu, S. Hong, S. Lee, S. Kim, S. Jeong, H. Kang and K. Lee, Adv. Energy Mater., 2017, 7, 1700226 CrossRef.
- H. Kang, J. Lee, S. Jung, K. Yu, S. Kwon, S. Hong, S. Kee, S. Lee, D. Kim and K. Lee, Nanoscale, 2013, 5, 11587–11591 RSC.
- H. Kang, S. Kee, K. Yu, J. Lee, G. Kim, J. Kim, J.-R. Kim, J. Kong and K. Lee, Adv. Mater., 2015, 27, 1408–1413 CrossRef CAS.
- H. Kang, S. Hong, J. Lee and K. Lee, Adv. Mater., 2012, 24, 3005–3009 CrossRef CAS.
- S. Kim, H. Kang, S. Hong, J. Lee, S. Lee, B. Park, J. Kim and K. Lee, Adv. Funct. Mater., 2016, 26, 3563–3569 CrossRef CAS.
- Z. Gao, Y. Zheng, Z. Wang and J. Yu, J. Lumin., 2018, 201, 359–363 CrossRef CAS.
- F.-C. Wu, K.-C. Tung, W.-Y. Chou, F.-C. Tang and H.-L. Cheng, Org. Electron., 2016, 29, 120–126 CrossRef CAS.
- L. Qiu, L. K. Ono, Y. Jiang, M. R. Leyden, S. R. Raga, S. Wang and Y. Qi, J. Phys. Chem. B, 2018, 122, 511–520 CrossRef CAS.
- W. Lee, S. Jeong, C. Lee, G. Han, C. Cho, J.-Y. Lee and B. J. Kim, Adv. Energy Mater., 2017, 7, 1602812 CrossRef.
- Z. Wu, C. Sun, S. Dong, X. F. Jiang, S. Wu, H. Wu, H. L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2016, 138, 2004–2013 CrossRef CAS.
- A. Misra, P. Kumar, R. Srivastava, S. K. Dhawan, M. N. Kamalasanan and S. Chandra, Indian J. Pure Appl. Phys., 2005, 43, 921–925 CAS.
- R. Heuvel, J. J. Franeker and R. A. J. Janssen, Macromol. Chem. Phys., 2017, 218, 1600502 CrossRef.
- J. F. Moulder, W. F. Strickle, P. E. Sobol and K. D. Bomben, Handbook of X-Ray Photoelectron Spectroscopy, Perkin-Elmer, Eden Prairie, MN, 1992 Search PubMed.
- K. H. Lee, H. W. Jang, K.-B. Kim, Y.-H. Tak and J.-L. Lee, J. Appl. Phys., 2004, 95, 586–590 CrossRef CAS.
- H. Estrade-Szwarckopf, B. Rousseau, C. Herold and P. Lagrange, Mol. Cryst. Liq. Cryst., 1998, 310, 231–236 CrossRef CAS.
- K.-W. Tsai, S.-N. Hsieh, T.-F. Guo, Y.-J. Hsu, A. K.-Y. Jene and T.-C. J. Wen, J. Mater. Chem. C, 2013, 1, 531–535 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: DSC and TGA curves, electrochemical characteristics, XRD powder patterns, device performance, solubility studies, and 1H and 13C NMR spectra. See DOI: 10.1039/c9ra06293a |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |