Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Analysis of volatile compounds in fresh sturgeon with different preservation methods using electronic nose and gas chromatography/mass spectrometry
Wenfu Hou ab,
Qianhui Han
ab,
Qianhui Han b,
Heng Gongb,
Wen Liub,
Hongxun Wangc,
Min Zhoub,
Ting Minb and
Siyi Pan
b,
Heng Gongb,
Wen Liub,
Hongxun Wangc,
Min Zhoub,
Ting Minb and
Siyi Pan *a
*a
aCollege of Food Science and Technology, Huazhong Agricultural University, 1st Shizishan Road, Wuhan, Hubei 430070, P. R. China. E-mail: pansiyi303@163.com
bCollege of Food Science and Engineering, Wuhan Polytechnic University, Wuhan, Hubei 430023, P. R. China
cSchool of Biological and Pharmaceutical Engineering, Wuhan Polytechnic University, Wuhan, Hubei 430023, P. R. China
First published on 28th November 2019
Abstract
Contamination of microorganisms causes a rapid deterioration in the quality of fresh sturgeon meat, which results in the shortening of the shelf-life and increase in the health risk. In this paper, two preservation treatments based on microbial control were considered. During the chilling storage (0–6 days) period, the sensory analysis and the volatile compound (VOC) evaluation were performed by electronic nose and SPME-GC/MS. Results showed that washing with acidic oxidized electrolyzed water and the addition of ε-PL influences the sensitive VOCs of the fresh sturgeon by inhibiting the spoilage of microbes or introducing the chemical agents like free chlorine and reactive oxygen species. Furthermore, GC/MS analysis detected more than 40 kinds of VOCs, mainly aldehydes and ketones, in the fresh sturgeon during the chilling storage period. The relative content of heptanal, nonanal, and acetophenone increased linearly with the storage time in all the groups, where R2 of all the groups was larger than 0.9. However, the content of hexanal and octanal decreased simultaneously. This indicated that the present work discovered the potential biomarkers acting as indicators for rapidly evaluating the quality of sturgeon products.
1. Introduction
The sturgeon belongs to one of the oldest families of bony fish. It is a native of subtropical, temperate, and sub-Arctic rivers, lakes, and coastlines of Eurasia and North America.1 The sturgeon not only has important economic value, such as food, nutrition, nourishment, health care, medicinal, and ornamental, but also has great significance in scientific research.2 China's sturgeon resources are very rich, especially the artificial breeding, which feeds the sturgeons with the commercially available diets. Until now, the edible sturgeon products are still dominated by dried, smoked or salted versions. Product development of the fresh sturgeon is less, so the methods for the preservation and quality control of the fresh sturgeon products are urgent problems to be solved.Living, fresh or refrigerated fishes are the most popular forms for human consumption, accounting for the largest proportion (45%) in 2016. The spoilage of fresh fish after slaughter is mainly dependent on various biochemical reactions (including protein hydrolysis and lipid oxidation by endogenous enzymes) and the growth of bacteria.3 Numerous methods like freezing, adding antiseptic substances, and modified atmosphere packaging (MAP) have been focused on to maintain the freshness of the aquatic products by inhibiting the reactions. The acidic electrolyzed oxidizing water (AEOW) is synthesized by electrolyzing the NaCl solution (<0.1 g per 100 mL) in an electrolysis chamber and has been recognized as an environmentally friendly and highly effective antimicrobial agent to reduce the foodborne pathogens on the fresh food.4,5 Generally, AEOW has a low pH (<2.8), high oxidation–reduction potential (ORP > 1050 mV), and high available chlorine concentration (ACC is greater than 5 mg L−1).6 Park et al. found that AEOW treatment completely eliminates Listeria monocytogenes, Salmonella, and Escherichia coli (O157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H7) from lettuce and spinach leaves.7 However, AEOW might get altered with the pH or induced in the oxidative substrates. On the other hand, the addition of natural antibacterial compounds does not change the chemical environment of the food. ε-Polylysine (ε-PL) is a natural polypeptide comprising 25–35 L-lysine units.8 It has high chemical stability and exhibits wide-spectrum antimicrobial activity against the Gram-positive and Gram-negative bacteria, yeast, and fungi.9 No toxicity of ε-PL on rats has been demonstrated,10 and it is approved for usage as a food preservative in Japan.
H7) from lettuce and spinach leaves.7 However, AEOW might get altered with the pH or induced in the oxidative substrates. On the other hand, the addition of natural antibacterial compounds does not change the chemical environment of the food. ε-Polylysine (ε-PL) is a natural polypeptide comprising 25–35 L-lysine units.8 It has high chemical stability and exhibits wide-spectrum antimicrobial activity against the Gram-positive and Gram-negative bacteria, yeast, and fungi.9 No toxicity of ε-PL on rats has been demonstrated,10 and it is approved for usage as a food preservative in Japan.
Sensory detection, which is induced by volatile substances generated during the storage, is a direct way to evaluate the quality of the aquatic products.11 An electronic nose (enose, EN) and solid phase microextraction-gas chromatography-mass spectrometry (SPME-GC/MS) are effective ways to characterize the changes in the volatile components.12,13 The advantages of EN for detecting and distinguishing odors in food are simple operation, good repeatability, and high sensitivity.14,15 The EN could be utilized for the early detection of contamination and defects in the foodstuffs.16 Wang et al. used an EN to predict the total viable counts (TVC) in chilled pork.17 In contrast, SPME-GC/MS is a quantitative analytical method,18 which has been used to study detailed volatile organic compounds (VOCs) in foods, such as prawns,19 crabs,20 and fish.21,22 Parlapani et al. found that some VOCs were associated with the metabolic activity of a particular microbial group, e.g., ethyl esters were linked with Pseudomonas, while 2,3-methylbutanal and 3-hydroxy-2-butanone were associated with Carnobacterium and Lactobacillus. Therefore, GC/MS serves as a possible application for rapid freshness assessment.23,24 In this study, both the EN and SPME-GC/MS were combined to detect and analyze the volatile organic compounds (VOCs) of the fresh sturgeon under different processing treatments during storage in the refrigerator. The EN was used to judge the comprehensive sensory changes in the sturgeon products (qualitative analysis), while SPME-GC/MS was employed for the quantitative analysis of the VOCs. Finally, the results obtained could help to assess the freshness of the fresh sturgeon quality with different preservation methods (AEOW and addition of ε-PL), and at the same time, allow to pick the better way for maintaining the quality of fresh sturgeon.
2. Materials and methods
2.1. Materials
A total of 21 tail sturgeon (Acipenser gueldenstaedti, cultured in Qingjiang River, Yichang City, Hubei Province, China), with an average body weight of 1500 ± 300 g, was purchased from the local fisheries market. Chemicals used in the experiments, such as sodium chloride (analytic grade), acidic electrolyzed oxidized water (AEOW), and ε-polylysine (ε-PL), were purchased and used directly without any purification. All the animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Huazhong Agricultural University, and all the experiments were approved by the Animal Ethics Committee of Huazhong Agricultural University (approval ID: SYXK2015-0084).2.2. Sample preparation and preservation treatments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/v). After 10 min, the pieces of sturgeon were drained and placed in a sterile sample box, and the box was placed in a refrigerator at 4 °C.
2 (w/v). After 10 min, the pieces of sturgeon were drained and placed in a sterile sample box, and the box was placed in a refrigerator at 4 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v) for 3 min. Then, they were drained and placed in a sterile sample box, and the box was placed in a refrigerator at 4 °C.
3 (w/v) for 3 min. Then, they were drained and placed in a sterile sample box, and the box was placed in a refrigerator at 4 °C.2.3. Test of electronic nose (EN)
In the experiment, each group of the sturgeon samples (4 g) for the EN test was accurately weighed and placed in 10 mL sterile vials suitable for the EN test. The data were detected using a metal oxide semiconductor (MOS) based on the gas analyzer array electronic nose detector combined with a headspace auto-sampler (Alpha M. O. S., FOX 4000, France). The measurement started after 300 s of equilibration at 60 °C under agitation (500 rpm). The injection volume was 3500 μL with an injection speed of 2500 μL s−1. The data acquisition lasted for 180 s. Each sample was measured for 4 readings per sample, but the first reading was discarded for the certainty of sensor stabilization. The EN signal response of the samples was calculated using the following expression:| R = (R0 − Rt)/R0 |
2.4. SPME-GC/MS
The sampling protocol was designed as follows: 5 g of each group of the sturgeon samples were weighed accurately. 15 mL of the saturated NaCl solution was then added in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v), and was then homogenized. Then, 5 g of the mixtures were placed in 10 mL glass vials; each sample was prepared in triplicate.
3 (w/v), and was then homogenized. Then, 5 g of the mixtures were placed in 10 mL glass vials; each sample was prepared in triplicate.
VOCs were extracted by SPME (Supelco, Bellefonte, PA, USA) with a coated fiber of PDMS/DVB (polydimethylsiloxane/divinylbenzene, coating thickness was 65 μm). The SPME fiber was exposed to the headspace of the sample for 30 min at 60 °C. The GC-MS analysis was performed by 7890A-5975C (Agilent Technologies Inc., USA) with a DB-5 column (30 m × 0.25 mm, 0.25 μm). The oven temperature was set at 40 °C for 4 min, programmed by an increase in the temperature at a rate of 6 °C min−1 to 200 °C for 5 min, and then an increase of 10 °C min−1 to 250 °C for 5 min. Helium was employed as the carrier gas at a constant flow rate of 1.0 mL min−1 (splitless mode). The temperature of the mass spectrometry source and quadrupole was set at 230 and 150 °C, respectively. All the analyses were performed by setting the ionization energy at 70 eV, with the mass scan range being 50–400 m/z.
Analytical methods: (1) qualitative analysis: the compounds were searched by computer and matched with NIST11 (107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds) and Wiley Library (320
000 compounds) and Wiley Library (320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds, Version 6.0), with a matching degree of 80% or more; the literature qualitatively analyzes the substances detected in the experiment. (2) Quantitative analysis: the relative percentage content was calculated by the peak area normalization method. The preliminary experiments were undertaken to test the repeatability of these analytical methods by analyzing the control group 3 times under the proposed conditions. The relative standard deviation (%RSD) presented in the study was in the range of 5–19% with an average value of 9%.
000 compounds, Version 6.0), with a matching degree of 80% or more; the literature qualitatively analyzes the substances detected in the experiment. (2) Quantitative analysis: the relative percentage content was calculated by the peak area normalization method. The preliminary experiments were undertaken to test the repeatability of these analytical methods by analyzing the control group 3 times under the proposed conditions. The relative standard deviation (%RSD) presented in the study was in the range of 5–19% with an average value of 9%.
2.5. Statistical data analysis
The electronic nose data was collected by the MOS sensor, converted into a data table by the fingerprint analysis, and then, the software Unscrambler X 10.4 (64 bit, CAMO Software Inc. USA) was used for the calculations. Multivariate analysis methods include principal component analysis (PCA) and radar image analysis. PCA was performed using the SPSS 19.0 software, and the data were statistically processed and plotted with Microsoft Excel 2010. The GC-MS data used the NIST 11 library and the Wiley library for the qualitative analysis of volatile components (matching greater than 80, maximum 100). Eventually, the total area of the volatile flavor components was calculated by Microsoft Excel 2010.3. Results and discussion
3.1. Sensory evaluations of sturgeon chilling storage under different treatments by EN
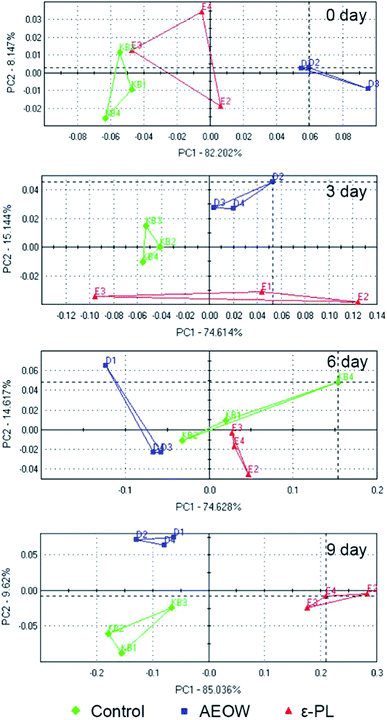
An electronic nose (EN) and electronic tongue25 have been designed to simulate human senses of smell and taste in a great possible way.26 The principal component analysis (PCA) graph was obtained to identify the patterns of correlation with individual composition variables among the fresh sturgeon during the chilled storage with different treatments (Fig. 1). A sum of the contribution percentages of PC1 and PC2 for all the groups was determined to be above 90%, indicating that PC1 and PC2 contained most of the volatile characters' information. A clearly different distribution of the volatile odor of the sturgeon under different treatments in the PCA graph confirmed that the MOS module has the ability to respond accurately.27 According to Fig. 1, all the datasets were located in three non-overlapping regions, indicating that the samples of control, AROW, and ε-PL were easily differentiated. For example, at the initial stage, an obvious separation between the AEOW treatment and the control group occurred along the PC1 (82.20%). At the same time, the spider plots provided a graphical representation of the flavor profiles for different sensory detectors (Fig. 2). The characteristic fingerprint identified by 18 sensors of the initial treatments illustrated that the sensor response at T70/2, PA/2, P30/1, P30/2, TA/2, and T40/1 showed differences. However, in the case of AEOW, the reason for getting combined with the chemical reagent in the treating groups might be attributed to the free chlorine and reactive oxygen species present in AEOW.28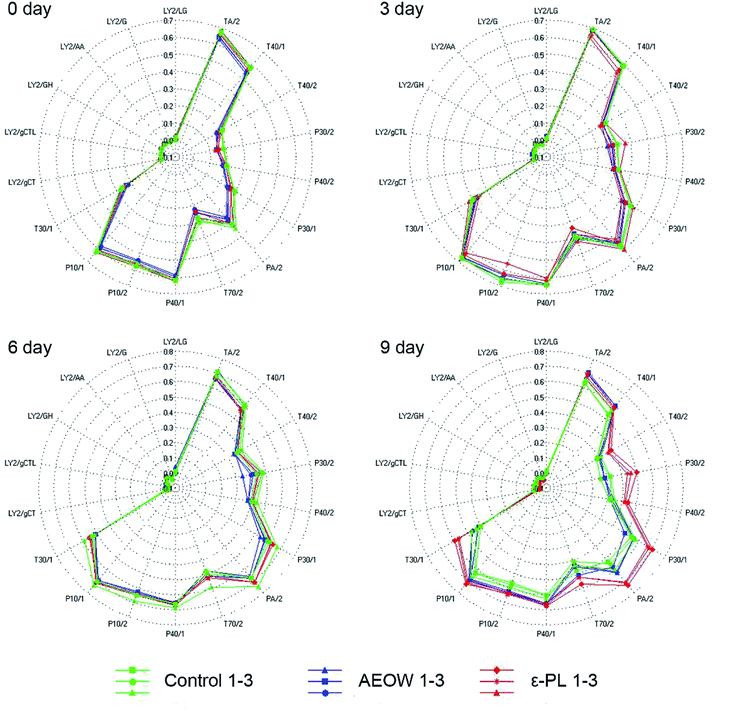 | ||
| Fig. 2 The radical plots of the chilling sturgeon treated with different preservation methods for 0, 3, 6, and 9 days, respectively. | ||
From 0 to 9 days, the plots in the PCA chart of these three groups were distributed significantly different with the prolonged storage time. At 0 day storage, all the radial curves of each group were similar, and the signal response intensity for all the 18 attributes was also very close, indicating that the difference in flavorings between the samples was not obvious. Further, the radial plots displayed differences when the sturgeon was stored after 6 d, especially in channel T30/1, P10/2, T70/2, PA/2, P30/1, and PA/2. Particularly, at the 9 day storage, from the visible check of the naked eyes, the control group tended to be spoiled. A separation between the control and ε-PL groups appeared in PC1 (85.04%), whereas the difference with AEOW was observed in PC2 (9.62%). The response strength on the sensor T30/1, T70/2, PA/2, P30/1, P40/2, P30/2, and T40/2 became weak in the control group, indicating that the spoilage might induce the weaker response strength. On the other hand, the radial plot of the control and AEOW groups at the 9 day storage seemed quite similar, and it should be noticed that the distance between these two flavorings was relatively less.17 Moreover, it has been demonstrated by the former researches that the EN system cluster analyzed by PCA could be a simple and rapid technique for monitoring the shelf-life of Tuber magnatum Pico during the storage,29 and thus, it can serve as a specialized gas-sensing instrument for fruit identifications, ripeness assessments etc.30 Therefore, the EN could be used for the assessment of freshness, thereby detecting the presence of the off-flavor compounds and discriminating different fish species.31
3.2. Volatile components of sturgeon during different treatments measured by SPME-GC/MS
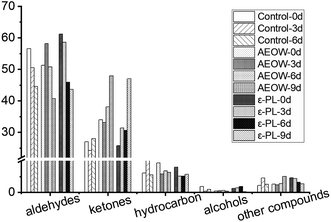
After killing the sturgeon, a strong fishy smell, grassy smell, and fatty scent could be smelled. These odors were mainly attributed to aldehydes, ketones, alcohols, hydrocarbons, and other substances. During the storage, the various biochemical reactions, including degradation of ATP and protein, oxidation of the unsaturated fatty acid, and the growth of bacteria, would generate more volatile substrates to change the smell of the sturgeon products. All of these volatile substrates were named as volatile organic compounds (VOCs). Here, the results showed that more than 40 kinds of VOCs, mainly aldehydes, ketones, alcohols, hydrocarbons, and a small amount of benzene compounds, were characterized by GC/MS (Fig. 3). Among the 40 kinds of VOCs, twelve kinds of VOCs were picked as the key indicators because their changes in the relative content (decrease or increase) varied evidently during the chilling storage period with different preservation treatments. According to the database of mass spectrometry, the 12 kinds of VOCs were identified as hexanal, heptanal, benzaldehyde, octanal, nonanal, decanal, acacia aldehyde, 2,5-dioctylketone, acetophenone, 3,5-octadien-2-one, 5,8-undecadien-2-one, 6,10-dimethyl-E, and 1,2-octadecane. The detailed illustrations of the influence of preservation treatments on the VOCs of sturgeon under different storage time are as follows:| Aldehydes | Retention time/min | Relative percentage % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | AEOW | ε-PL | ||||||||||
| 0 d | 3 d | 6 d | 0 d | 3 d | 6 d | 9 d | 0 d | 3 d | 6 d | 9 d | ||
| Hexanal | 5.62 | 26.72 | 17.74 | 15.73 | 17.76 | 24.39 | 21.33 | 8.13 | 25.58 | 23.21 | 15.16 | 10.64 |
| Heptanal | 8.69 | 2.92 | 4.07 | 6.90 | 1.20 | 1.68 | 3.69 | 4.44 | 2.54 | 3.45 | 4.60 | 5.92 |
| Benzaldehyde | 10.49 | 5.20 | 5.73 | 6.08 | 6.46 | 6.63 | 7.37 | 7.07 | 6.75 | 6.77 | 6.86 | 7.03 |
| Octanal | 11.77 | 6.41 | 2.78 | 2.09 | 4.89 | 3.06 | 1.40 | 1.51 | 5.44 | 3.53 | 1.52 | 2.07 |
| (E,E)-2,4-Heptadienal | 11.99 | 1.01 | 0.35 | 0.57 | 0.92 | 0.80 | 0.23 | 0.73 | 0.88 | 1.35 | 1.18 | 0.81 |
| 2-Octenal | 13.33 | 1.17 | 0.61 | 0.12 | 0.58 | 0.65 | 0.52 | 0.61 | 1.04 | 1.07 | 0.77 | 0.50 |
| Nonanal | 14.60 | 6.39 | 9.80 | 11.32 | 10.85 | 11.98 | 12.99 | 14.09 | 10.55 | 12.24 | 12.49 | 12.70 |
| 2-Hydroxynonenal | 16.05 | 0.29 | 0.19 | 0.12 | — | 0.27 | 0.11 | — | — | 0.28 | 0.14 | — |
| 3-Ethylbenzaldehyde | 16.14 | 0.60 | 0.55 | 0.26 | 0.52 | 0.63 | 0.47 | 0.98 | 0.54 | 0.71 | 0.34 | 0.29 |
| Decanal | 17.21 | 2.07 | 4.55 | 0.72 | 4.50 | 5.30 | 1.65 | 1.89 | 5.17 | 3.55 | 2.14 | 2.21 |
| 2,4-Nonadienal | 17.46 | 0.20 | — | — | 0.14 | 0.32 | — | — | 0.14 | 0.19 | — | — |
| 2-Decenal | 18.57 | 0.30 | 0.75 | 0.22 | 0.42 | 0.30 | 0.22 | 0.22 | 0.47 | 0.44 | 0.16 | — |
| Undecanal | 19.65 | 0.50 | 0.92 | 0.16 | 0.58 | 0.64 | 0.22 | 0.20 | 0.67 | 0.48 | 0.18 | 0.54 |
| 2,4-Decadienal | 19.90 | 0.73 | 1.12 | 0.07 | 0.47 | 0.35 | 0.22 | 0.37 | 0.41 | 0.69 | 0.08 | 0.19 |
| Dodecanal | 21.93 | 0.28 | 0.73 | 0.10 | 0.40 | 0.50 | 0.10 | 0.2 | 0.58 | 0.36 | 0.10 | 0.26 |
| Tridecylic aldehyde | 24.07 | 0.16 | 0.32 | — | — | 0.30 | — | — | — | — | — | 0.21 |
| Acacia aldehyde | 30.11 | 1.65 | 0.31 | 0.13 | 1.55 | 0.36 | 0.26 | 0.25 | 0.43 | 0.24 | 0.15 | 0.22 |
| Sum | 56.6 | 50.52 | 44.59 | 51.24 | 58.16 | 50.78 | 40.69 | 61.19 | 58.56 | 45.87 | 43.59 | |
In the initial stage of treatment (0 day), the content of hexanal, heptanal, and nonanal for the control and ε-PL addition groups had a similar value. Nevertheless, the AEOW wash directly decreased them, indicating that AEOW wash would decrease the grassy odor of the fresh sturgeon. On the other hand, the immediate changes in the flavor components were attributed to the strong odor of the acidic electrolyzed water itself. Upon increasing the storage time (3–9 days), the relative percentage of hexanal and octanal in the samples of each group generally showed a downward trend with varying degrees. The reason might be the inhibition of the degradation of oleic acid by the preservation method. The octanal had a good effect on the flavor (aroma component) of sturgeon. The proportion of octanal decreased in the samples during the shelf life of each group (the control, AEOW group, and addition of ε-PL group), and the retention rates were determined to be 32.61%, 30.88%, and 38.05%, respectively. Thus, the results showed that the addition of ε-PL would help in maintaining the aroma flavor of sturgeon.
The relative percentages of heptanal, nonanal, and benzaldehyde exhibited an upward trend during the chilling storage, resulting from the deep oxidation of unsaturated lipids, such as oleic acid, linoleic acid, and arachidonic acid. The preservation treatments inhibited the generation of heptanal effectively, thereby revealing that they assist in lowering the oxidative degradation of fatty acids. Other unsaturated aldehydes also contributed to the comprehensive flavor of the sturgeon, like 2,4-decadienal, 2,4-heptane aldehyde, 2-octenal aldehyde, and 2-decenal. They all belonged to the linoleic acid degradation products. 2-Nonenal is a degradation product of oleic acid and linoleic acid, often with oily rancid and fishy smell. Further, 2,4-heptanedialdehyde has a grass odor, while 2,4-nonadienal has a fatty taste.36
The data presented in Table 2 showed that the relative percentage of 2,5-octanedione in each group of sturgeon increased significantly during the storage time. It might be caused by the oxidation of fatty acids and the microbial growth and reproduction. The increase in the 2,5-octanedione content in the AEOW group and the ε-PL addition group was found to be 70.28% and 61.72%, respectively. For the AEOW group, the free radical in the AEOW could promote the oxidation of lipids and generate more 2,5-octanedione. Apart from 2,5-octanedione, the content of acetophenone was also relatively increased. Moreover, the studies showed that acetophenone had a mushroom odor, but due to the high threshold, acetophenone had a little effect on the odor characteristics of the fish. A degradation product of n-3 polyunsaturated fatty acids, 3,5-octadiene-2-one, which was observed in the volatile fraction of fresh oysters, decreased with an increase in the storage time.39
| Compounds | Retention time/min | Relative percentage % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | AEOW | ε-PL | ||||||||||
| 0 d | 3 d | 6 d | 0 d | 3 d | 6 d | 9 d | 0 d | 3 d | 6 d | 9 d | ||
| Ketones | ||||||||||||
| 2,5-Dioctylketone | 11.26 | 14.86 | 15.78 | 15.48 | 16.59 | 20.00 | 22.69 | 28.25 | 16.85 | 18.16 | 14.15 | 27.25 |
| Acetophenone | 13.52 | 4.51 | 6.82 | 11.35 | 7.22 | 5.42 | 12.02 | 16.84 | 3.13 | 4.55 | 13.03 | 16.42 |
| 3,5-Octadien-2-one | 13.63 | 6.93 | 1.06 | 1.10 | 9.10 | 7.24 | 3.22 | 2.40 | 5.20 | 8.50 | 3.30 | 3.05 |
| 5,8-Undecadien-2-one, 6,10-dimethyl-, (E)- | 22.73 | 0.68 | 0.59 | 0.11 | 1.09 | 0.48 | 0.14 | 0.39 | 0.59 | 0.14 | 0.14 | 0.22 |
| Sum | 26.98 | 24.25 | 28.04 | 34.00 | 33.14 | 38.07 | 47.88 | 25.77 | 31.35 | 30.62 | 46.94 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Hydrocarbon | ||||||||||||
| 1-Ethylene cyclohexene | 11.61 | 0.73 | — | — | 0.64 | — | — | — | 0.47 | 0.55 | — | — |
| 1,4-Cyclooctadiene | 16.97 | 0.46 | 0.59 | 0.20 | 0.50 | 0.50 | 0.17 | — | — | 0.33 | 0.11 | 0.22 |
| Tetradecane | 21.73 | 0.46 | 1.50 | 0.19 | 0.58 | 0.59 | 0.18 | 0.13 | 0.40 | 0.35 | 0.63 | 0.50 |
| Octacosane | 23.58 | 0.72 | 2.10 | 0.36 | 1.13 | 0.51 | 0.11 | 0.60 | 1.11 | 0.17 | 0.38 | 0.18 |
| Pentadecane | 23.79 | 0.58 | 2.53 | 0.45 | 0.72 | 0.77 | 0.48 | 1.60 | 0.62 | 0.54 | 0.87 | 0.81 |
| Nonadecane | 24.51 | 0.25 | 0.66 | 0.15 | 0.62 | 0.18 | 0.07 | 0.24 | 0.55 | 0.09 | 0.07 | — |
| Cyclopentadecane | 24.93 | 0.10 | 0.19 | 0.26 | 0.34 | 0.22 | 0.46 | 0.18 | 0.30 | 0.16 | 0.22 | 0.22 |
| Hexadecane | 25.85 | 0.53 | 0.96 | 0.58 | 0.89 | 0.53 | 0.99 | 0.53 | 0.52 | 0.38 | 0.24 | 1.07 |
| 1,2-Octadecane | 26.10 | 0.36 | 0.10 | — | 0.24 | 0.67 | — | — | 0.62 | 0.40 | — | — |
| Heptadecane | 27.76 | 0.61 | 0.68 | 1.08 | 1.50 | 0.74 | 1.40 | 0.85 | 1.66 | 0.71 | 0.65 | 1.17 |
| 2,6,10,14-Tetramethylpentadecane | 27.83 | 0.91 | 0.63 | 1.56 | 1.89 | 0.96 | 2.69 | 1.95 | 1.51 | 1.10 | 1.66 | 1.27 |
| Octadecane | 29.56 | 0.11 | 0.16 | 0.74 | 0.25 | 0.15 | 0.25 | 0.23 | 0.23 | 0.13 | 0.28 | 0.23 |
| Sum | 6.14 | 10.31 | 5.57 | 9.30 | 5.82 | 6.80 | 6.31 | 7.99 | 5.13 | 5.11 | 5.67 | |
With the extension of storage time, then among all the hydrocarbon compounds, 2,6,10,14-tetramethylpentadecane, which smells fresh and sweet, was found to exist in the highest concentration. On the 6th day, the relative percentage of 2,6,10,14-tetramethylpentadecane in each group was found to increase, which might be caused by an increase in the lipid oxidation and carotenoid decomposition. Until the 9th day, the relative percentage of 2,6,10,14-tetramethylpentadecane reduced, indicating that the sturgeon was deteriorated.
| Compounds | Retention time/min | Relative percentage % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | AEOW | ε-PL | ||||||||||
| 0 d | 3 d | 6 d | 0 d | 3 d | 6 d | 9 d | 0 d | 3 d | 6 d | 9 d | ||
| Alcohols | ||||||||||||
| 3,6-Nonadiene-1-ol | 12.52 | 0.98 | 0.18 | 0.22 | 0.18 | 0.19 | 0.19 | 0.36 | 1.03 | 1.14 | 0.31 | 0.19 |
| Eucalyptol | 12.57 | — | — | 0.66 | — | — | 0.39 | — | — | — | 1.55 | — |
| 3,5-Octadoene-2-ol | 12.74 | 0.34 | 0.18 | — | 0.20 | 0.33 | — | — | 0.23 | 0.28 | — | — |
| 2-Octen-1-ol | 16.31 | 0.54 | — | — | — | — | — | — | — | — | — | — |
| Sum | 1.86 | 0.36 | 0.88 | 0.38 | 0.52 | 0.58 | 0.36 | 1.26 | 1.42 | 1.86 | 0.19 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Other compounds | ||||||||||||
| Naphthalene | 16.72 | 0.50 | 1.09 | 0.92 | 0.64 | 0.77 | 1.33 | 1.88 | 0.76 | 0.92 | 1.03 | 1.78 |
| 2,3,5,6-Tetramethylphenol | 17.56 | 0.12 | 0.14 | 0.36 | 0.11 | 0.13 | — | — | 0.14 | 0.11 | — | — |
| Methoxybenzene | 19.18 | 0.52 | 1.94 | 1.11 | 0.89 | 0.63 | 0.84 | 1.81 | 0.15 | 0.83 | 0.56 | 0.32 |
| 2,6-Butylated hydroxytoluene | 23.92 | 0.3 | 0.38 | — | 0.19 | 0.37 | 0.31 | 1.17 | 0.95 | 0.67 | 1.50 | 0.40 |
| Isobutyl phthalate | 30.57 | 0.78 | 0.41 | 0.15 | 0.78 | 0.48 | 0.25 | 0.22 | 2.51 | 1.86 | 0.13 | 0.18 |
| Sum | 2.22 | 4.50 | 2.54 | 2.61 | 2.38 | 2.73 | 5.08 | 4.66 | 4.39 | 3.22 | 2.68 | |
In addition to the above-mentioned main types of volatile components, there were some other kinds of VOCs like benzenes, phenols, and lipids (Table 3). Naphthalene is a diphenyl ring compound and has been reported to be responsible for an unpleasant smell of fish. Naphthalene is always detected in lobsters, scallops, and crabs. However, it is generally believed that naphthalene has a great relationship with environmental contaminants.43 In this experiment, 2,6-butylated hydroxytoluene was detected, which has been reported in the VOCs of herbs and spices. Ester compounds, like isooctyl phthalate, are generally condensed by the esterification of acid and alcohol and are an important source of meat flavor characteristics. The relative content of other components was generally less throughout the shelf life.
3.3. Analysis of critical VOCs content for quality evaluation
Lots of work offer EN as a potential tool in multisensory systems for spoilage examination in foods.44 Changes in the generated fingerprint, which result from the metabolism of spoilage bacteria, includes the appearance of new chemical compounds or variations in the quantity of certain volatile compound. So, the EN provides a simple and convenient method for detecting the origin of food contaminants like microbiological, chemical or physical. However, EN and GC/MS have several differences regarding reproducibility, quantitative analysis, and real-time analysis. The reproducibility of EN depends on the structure and training data set.Researchers reported that the type and content of polyunsaturated lipids in various aquatic products are highly dependent on the origin, zone of culture, and processing or preservation treatments, with the detailed categories of VOCs of the aquatic products showing some differences.32,43 Upon analyzing all the VOCs in the sturgeon products during the chilling storage with different preservation treatments, 12 kinds of VOCs were picked for correlated fitting. It was found that six of them showed a strong and simple relationship with the storage time (Fig. 4). Further, the data of the control group were collected within 6 days because after 6 days, the sturgeon was totally spoiled and lost the edible characteristics. Moreover, the content of hexanal and octanal decreased with increasing storage time. Within 0–6 days, the linear relationship between the content of octanal and the storage days was clear. However, as the number of days continued to increase, the content of octanal reached a certain low level without any obvious change. Since octanal has a fruity aroma and grassy scent, the spoilage of the sturgeon meat would result in the loss of this flavor. To fit the trends by linear fitting, it was found that the R2 reached 0.9 or more, indicating that the content of heptanal (R2: 0.94–0.99), nonanal (R2: 0.77–0.99), and acetophenone (R2: 0.80–0.97) showed a linear correlation with the storage time. Therefore, these 3 kinds of compounds were used to quickly determine the quality change of sturgeon.
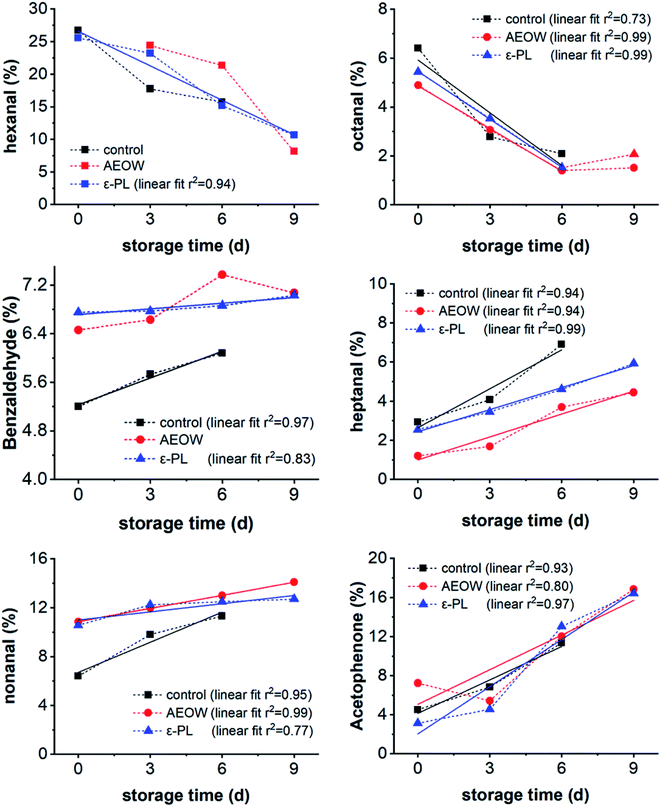 | ||
| Fig. 4 Changes of hexanal, octanal, benzaldehyde, heptanal, nonanal, and acetophenone for fresh sturgeon vs. the chilling storage time. | ||
4. Conclusion
In the traditional method, the microbial test and training of the expert team for identifying the VOCs for food spoilage detection is complex and time-consuming.26 Further, the determination of the microbiological status, using the biochemical tests, relies mainly on the total viable counts (TVCs) and phenotyping microbial isolates. These methods sometimes provide limited information.45 Additionally, the chemical methods can also be utilized to detect the microbial contamination of the food based on the analysis of certain chemical markers. The amounts of total volatile basic nitrogen (TVBN) and trimethylamine can be indicative of fish spoilage,46 but these markers increase in fish only during the late stages of storage.47GC/MS focuses on the quantitative analysis of VOCs instead of the EN, but the EN would finish the analysis in real-time. So, we combined both of them together. The EN successfully distinguished the flavor characteristics of the sturgeon treated with different preservation methods during the chilling storage. A total of 40 substances were detected by GC/MS, with these substances mainly belonging to aldehydes, alcohols, ketones, and hydrocarbons. During the whole storage period, the relative content of aldehydes in each sample continued to decrease, but the content of ketones increased. VOCs were commonly generated from the degradation catalyzed by the endogenous enzymes (like lipoxygenase, lipase enzymes), or due to the growth of bacteria. AEOW and the addition of ε-PL exerted an influence on the VOCs of the sturgeon by at least two ways: (1) inhibiting the spoilage of microbes and (2) giving rise to the odors of added chemical agents like free chlorine and reactive oxygen species. The content of heptanal, nonanal, and acetophenone increased linearly with the storage time in all the groups, but the content of hexanal and octanal decreased. Thus, the present work provides the potential biomarkers for sensory and qualitative evaluation during the commercial sturgeon storage.
Abbreviations used
| SPME | Solid-phase microextraction |
| GC-MS | Gas chromatography/mass spectrometry |
| EN | Electronic nose |
| VOCs | Volatile organic compounds |
| ε-PL | ε-Polylysine |
| AEOW | Acidic electrolyzed oxidizing water |
| ORP | Oxidation reduction potential |
| MOS | Metal oxide semiconductor |
| PCA | Principal component analysis |
| MAP | Modified atmosphere packaging |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work financially supported by the National Key R&D Program of China (2016YFD0401202), The Sci-tech Innovation Project for Excellent Young and Middle-Aged University Teachers of Hubei Province [T201809].References
- W. E. Bemis, E. K. Findeis and L. Grande, Comp. Parasitol., 1997, 48, 25–71 Search PubMed.
- F. Maccari, F. Ferrarini and N. Volpi, Carbohydr. Res., 2010, 345, 1575–1580 CrossRef CAS PubMed.
- L. Fei, R. J. Huang, L. Liu, X. Zhou and Y. T. Ding, J. Food Sci. Technol., 2015, 52, 1742–1747 CrossRef PubMed.
- J. Fang, J. L. Cannon and Y. C. Hung, Food Control, 2016, 61, 13–19 CrossRef CAS.
- C. Zhang, C. Wei, Y. C. Hung and B. Li, Food Control, 2016, 67, 177–182 CrossRef CAS.
- M. A. Alholy and B. A. Rasco, Food Control, 2015, 54, 317–321 CrossRef CAS.
- P. Eun-Jin, A. Edward, G. A. Taylor, C. Roy and K. Dong-Hyun, Food Microbiol., 2009, 26, 386–390 CrossRef PubMed.
- M. R. Zahi, H. M. El, H. Liang and Q. Yuan, Food Chem., 2016, 221, 18–23 CrossRef PubMed.
- L. Cai, A. Cao, F. Bai and J. Li, LWT–Food Sci. Technol., 2015, 62, 1053–1059 CrossRef CAS.
- H. Jun, I. Takafumi, N. Shin-Ichi, S. Hideaki, U. Katsumi, S. Hiroshi, K. Shigemi, Y. Yukio and J. W. Barnett, Regul. Toxicol. Pharmacol., 2003, 37, 328–340 CrossRef.
- S. Giannoukos, M. J. A. Joseph and S. Taylor, Anal. Methods, 2017, 9, 910–920 RSC.
- W. Yang, J. Yu, F. Pei, A. M. Mariga, N. Ma, Y. Fang and Q. Hu, Food Chem., 2016, 196, 860–866 CrossRef CAS PubMed.
- Y. Q. Yang, H. X. Yin, H. B. Yuan, Y. W. Jiang, C. W. Dong and Y. L. Deng, PLoS One, 2018, 13, e0193393 CrossRef PubMed.
- M. Peris and L. Escudergilabert, Anal. Chim. Acta, 2009, 638, 1–15 CrossRef CAS PubMed.
- M. Li, H. Wang, L. Sun, G. Zhao and X. Huang, J. Food Sci., 2016, 81, M906–M912 CrossRef CAS PubMed.
- A. Sanaeifar, H. Zakidizaji, A. Jafari and M. D. L. Guardia, TrAC, Trends Anal. Chem., 2017, 97, S0165993617302005 CrossRef.
- D. Wang, X. Wang, T. Liu and Y. Liu, Meat Sci., 2012, 90, 373–377 CrossRef PubMed.
- M. Kupska, T. Wasilewski, R. Jędrkiewicz, J. Gromadzka and J. Namieśnik, Int. J. Food Microbiol., 2016, 19, 2726–2738 CAS.
- Z. Zhang, L. I. Gongke, L. Luo and G. Chen, Anal. Chim. Acta, 2010, 659, 151–158 CrossRef CAS PubMed.
- S. Gu, X. Wang, N. Tao and N. Wu, Food Res. Int., 2013, 54, 81–92 CrossRef CAS.
- M. C. Erickson, L. M. Ma and M. P. Doyle, J. Food Prot., 2015, 78, 2156 CrossRef PubMed.
- F. F. Parlapani, A. Mallouchos, S. A. Haroutounian and I. S. Boziaris, LWT–Food Sci. Technol., 2017, 78, 54–62 CrossRef CAS.
- F. F. Parlapani, A. Mallouchos, S. A. Haroutounian and I. S. Boziaris, LWT--Food Sci. Technol., 2017, 78, 54–62 CrossRef CAS.
- A. A. Argyri, A. Mallouchos, E. Z. Panagou and G. J. Nychas, Int. J. Food Prop., 2015, 193, 51–58 CAS.
- M. Podrażka, E. Bączyńska, M. Kundys, P. S. Jeleń and E. Witkowska Nery, Biosensors, 2018, 8, 3 CrossRef PubMed.
- T. Wasilewski, D. Migoń, J. Gębicki and W. Kamysz, Anal. Chim. Acta, 2019, 1077, 14–29 CrossRef CAS PubMed.
- B. Wang, S. Xu and D.-W. Sun, Food Res. Int., 2010, 43, 255–262 CrossRef CAS.
- M. A. Alholy and B. A. Rasco, Sensors, 2015, 54, 317–321 CAS.
- G. Pennazza, C. Fanali, M. Santonico, L. Dugo, L. Cucchiarini, M. Dachà, A. D'Amico, R. Costa, P. Dugo and L. Mondello, Food Chem., 2013, 136, 668–674 CrossRef CAS PubMed.
- M. Baietto and A. D. Wilson, Sensors, 2015, 15, 899–931 CrossRef CAS PubMed.
- R. Ambra Rita Di and L. Francesco, in Electronic Nose Technologies and Advances in Machine Olfaction, ed. A. Yousif Abdullatif and A. Fatema, IGI Global, Hershey, PA, USA, 2018, pp. 151–174, DOI:10.4018/978-1-5225-3862-2.ch008.
- G. Fratini, S. Lois, M. Pazos, G. Parisi and I. Medina, Food Res. Int., 2012, 48, 856–865 CrossRef CAS.
- G. M. Turchini, T. Mentasti, F. Caprino, I. Giani, S. Panseri, F. Bellagamba, V. M. Moretti and F. Valfré, Ital. J. Anim. Sci., 2010, 4, 241–252 CrossRef.
- F. Piveteau, G. S. Le, G. Gandemer, J. P. Baud, C. Prost and M. Demaimay, J. Agric. Food Chem., 2000, 48, 4851 CrossRef CAS PubMed.
- B. S. Pan and J. M. Kuo, Flavour of shellfish and kamaboko flavorants, Seafoods: Chemistry, Processing Technology and Quality, ed. F. Shahidi and J. R. Botta, Springer, Boston, MA, 1994, pp. 85–114 Search PubMed.
- J. L. Wee, S. A. Harris, J. P. Smith, C. P. Dionigi and D. F. Millie, J. Appl. Phycol., 1994, 6, 365–369 CrossRef CAS.
- E. J Stephen, M. M. Campo, E. Michael and D. S. Mottram, J. Agric. Food Chem., 2002, 50, 1126–1132 CrossRef PubMed.
- L. Wu, M. Mascal, T. Farmer, S. P. Arnaud and M. A. W. Chang, ChemSusChem, 2016, 10, 166–170 CrossRef PubMed.
- M. C. Cruz-Romero, J. P. Kerry and A. L. Kelly, Innovative Food Sci. Emerging Technol., 2008, 9, 0–61 CAS.
- Z. Zhang, T. Li, W. Dan, Z. Lan and G. Chen, Food Chem., 2009, 115, 1150–1157 CrossRef CAS.
- C. Alasalvar, M. Al-Farsi, P. C. Quantick, F. Shahidi and R. Wiktorowicz, ACS Symp. Ser., 2005, 89, 69–76 CAS.
- J. B. German, H. Zhang and R. Berger, ACS Symp. Ser., 1992, 74–92 CrossRef CAS.
- P. J. Sarnoski, S. F. O'Keefe, M. L. Jahncke, P. Mallikarjunan and G. J. Flick, Food Chem., 2017, 122, 930–935 CrossRef.
- M. Ghasemi-Varnamkhasti, C. Apetrei, J. Lozano and A. Anyogu, Trends Food Sci. Technol., 2018, 80, 71–92 CrossRef CAS.
- S. Ramírez-Guízar, H. Sykes, J. D. Perry, E. C. Schwalbe, S. P. Stanforth, M. C. I. Perez-Perez and J. R. Dean, J. Chromatogr. A, 2017, 1501, 79 CrossRef PubMed.
- E. Jaffrès, D. Sohier, F. Leroi, M. F. Pilet, H. Prévost, J. J. Joffraud and X. Dousset, Int. J. Food Prop., 2009, 131, 20–29 Search PubMed.
- I. S. Boziaris, Seafood Processing: Technology, Quality and Safety, 2013 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |