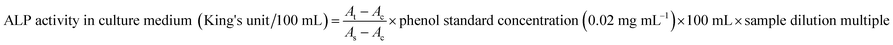Open Access Article
Open Access ArticleImpact of deoxynivalenol and kaempferol on expression of tight junction proteins at different stages of Caco-2 cell proliferation and differentiation†
Xiaojie Wang,
Li Li and
Genyi Zhang *
*
State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China. E-mail: gzhang@jiangnan.edu.cn; Tel: +86-510-85328726
First published on 28th October 2019
Abstract
The barrier function of intestinal tract is essential to gut health, and the expression of tight junction (TJ) proteins in human epithelial colorectal adenocarcinoma (Caco-2) cells, mimicking the intestinal stem cell proliferation and differentiation, was investigated after treatment by the mycotoxin of deoxynivalenol (DON) and phenolic compound of kaempferol (KAM). The results showed that DON (5 μM) significantly reduced the expression of claudin-4 while kaempferol (100 μM) increased the expression of claudin-3 during Caco-2 cell proliferation. After being cultured for 11 days, the DON treatment, unexpectedly, augmented the expression of claudin-4 with an increased TEER. For differentiated Caco-2 cells after a 21 day culture, both the TEER and claudin-4 levels were significantly reduced by DON while KAM pretreatment alleviated the damage caused by DON accompanying an increase of TEER, claudin-3, and ZO-1. Thus, kaempferol and DON differentially affected the expression of tight junction proteins at different stages of Caco-2 cell proliferation and differentiation, and an increase of the integrity of TJ after KAM pretreatment indicates KAM has the potential to protect the integrity of the barrier function of the intestinal epithelium for improved health.
1. Introduction
The mycotoxin deoxynivalenol (DON) has become a major human health concern since it is widely present in contaminated barley, wheat, corn, and oats with high concentrations.1–4 Within the scope of toxicity caused by DON exposure, significant damages to the tight junction of the intestinal epithelium,5–8 which is one recently emerged target in the toxicological assessment of mycotoxin, is one important mechanism. Due to the importance of the barrier function of the intestinal epithelium to human health, strategies to reduce the exposure of DON or to reduce its in vivo toxicity are essential to the prevention of DON-induced chronic intestinal inflammation and related diseases.The tight junction (TJ) between epithelium cells is a network formed by various proteins including integral transmembrane proteins of occludin, claudins, and peripheral adaptor proteins such as zonula occludens-1 (ZO-1) associated with the actin cytoskeleton and other signaling proteins.9–12 The TJ is one critical functional component to the barrier function of epithelium cells, and the expression levels of TJ proteins and their distribution contribute to the TJ barrier function. However, literature study using porcine intestinal epithelial cell line showed that mycotoxin of DON decreased the expression of claudin-4 with impaired intestinal barrier function.13 Similar result was also observed in differentiated Caco-2 cell monolayer with increased membrane permeability after exposure to DON.5 Accordingly, strategies by employment of various bioactive substances to regulate tight junction proteins have become a hot research area.14–18 The repair effect of galacto-oligosaccharide on DON-induced damage in Caco-2 monolayer and mice intestine showed that GOSs stabilized the expression and cellular distribution of claudin-3 in the monolayer Caco-2 cells.19 The protective effects of quercetin, naringenin, berberine and curcumin on the integrity of tight junction have all been reported.20–23 Gut microbiota fermented product of butyrate also enhances the intestinal barrier function through facilitating the assembly of TJ proteins.24 Thus, bioactive compounds might be an effective strategy to combat the toxicity of DON for improved barrier functions of intestinal epithelium to protect the body against luminal bacteria and toxic materials. Kaempferol is a polyphenol antioxidant with a rich content in fruits and vegetables, and its beneficial effects in reducing the risk of chronic diseases have been extensively studied.25
Kaempferol can not only augment the body's antioxidant defense system, and modulate key elements of signaling pathways related to cellular apoptosis and inflammation, it also exerts protective effect against toxic materials.26–28 As a preserving agent, kaempferol could activate the MAPK pathway and to increase the antioxidant capacity such as the expression of antioxidant enzyme of HO-1. Concerning the protective effect on cellular tight junction, kaempferol could substantially increase the transepithelial electrical resistance (TEER, tight junction marker) in a Caco-2 cell line, promote the protein expression of ZO-1 and -2, occludin, and claudin-1, -3, and -4, and the actin cytoskeletal association of the tight junction proteins in cholesterol-rich lipid microdomain.29 However, to our knowledge, there is no literature reports on the protective effect of kaempferol on the DON-induced TJ damage. Additionally, literature study showed that the TJ complexes of Caco-2 cells at different stages of differentiation had different sensitivity to the bioactive compounds.16 Thus, the impact of kaempferol on DON-induced TJ changes was studied at different stages of proliferation and differentiation of Caco-2 cells so that a deeper understanding of the physiological function of kaempferol in protecting the intestinal barrier function will be achieved.
2. Materials and methods
2.1. Chemicals and reagents
Deoxynivalenol (DON, C15H20O6, ≥98% purity) was purchased from Sigma Aldrich (St Louis, MO, USA). Kaempferol (C15H10O6, ≥98% purity) was purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Antibodies of β-actin, claudin-3, claudin-4, occludin and ZO-1 were obtained from Sigma Aldrich (St Louis, MO, USA). Secondary antibodies were obtained from Abcam Co., Ltd (Shanghai, China). Alkaline phosphatase (AKP) activity measurement kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The NuPAGE® MOPS SDS Buffer Kit, NuPAGE® Bis-Tis Mini Gels and iBlot® 2 Transfer Stacks were purchased from Thermo Fisher Scientific (Waltham, MA, USA).2.2. Culture of Caco-2 cells
Human epithelial colorectal adenocarcinoma cells (Caco-2 line, passages 22) obtained from Chinese Academy of Sciences Cell Bank (Shanghai, China) were cultured in 25 cm2 Corning culture flasks (Corning Incorporated, Corning, New York, USA) in Minimum Essential Medium (MEM) (Life Technologies, Inc., Invitrogen, Shanghai, China), and supplemented with 20% fetal bovine serum (FBS), 1% penicillin–streptomycin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1), 1% Glutamax, 1% sodium pyruvate, and 1% nonessential amino acids (Life Technologies). Cells were kept at 37 °C in a humid atmosphere of 5% CO2–95% air. The trypsinization at cell density of 80% confluence was performed with 0.25% trypsin–EDTA (Life Technologies) for about 4 minutes, and a new passage of Caco-2 cell will be cultured in flasks. The cells between 25–35 passages were used for the experiments.
000 U mL−1), 1% Glutamax, 1% sodium pyruvate, and 1% nonessential amino acids (Life Technologies). Cells were kept at 37 °C in a humid atmosphere of 5% CO2–95% air. The trypsinization at cell density of 80% confluence was performed with 0.25% trypsin–EDTA (Life Technologies) for about 4 minutes, and a new passage of Caco-2 cell will be cultured in flasks. The cells between 25–35 passages were used for the experiments.
2.3. Establishment of Caco-2 cell monolayer model
Add 600 μL and 50 μL of culture medium to a 24 well plate and collagen-coated polytetrafluoroethylene (PTFE) insert (0.33 cm2 membrane area with 0.4 μm pores, Corning Incorporated, Corning, New York, USA), respectively. Then place them in a 3 °C incubator for at least 1 h, and digest the fused cells to achieve more than 90% of single cells. The transwell plate was taken out from the incubator, and 50 μL of the cell suspension was added to the inside of the insert to reach a cell density of 1.3 × 105 cells per cm2. The first medium change will be finished within 16 hours. Medium was then refreshed every other day within the first week, and every day after a week. After 21 day of culture, a confluent monolayer was achieved with a mean transepithelial electrical resistance (TEER) about 260 ± 65 Ω cm2 measured by a EVOM2 epithelial cell transmembrane resistance meter (WPI, Shanghai, China). The experiment can be carried out within one week thereafter, and the medium needs to be changed 12–24 hours before the experiment.2.4. TEER measurement
The AP side and the BL side of the cultured Caco-2 cells were washed with a Hank's solution (pH 7.4) to remove cell monolayer surface attachments. Then 0.1 mL and 0.6 mL Hank's solution were added to inside and outside of the insert, and after 20 min incubation at 37 °C and 5% CO2, the TEER value was measured with an EVOM2 resistance meter (WPI). Before the measurement, the STX2 electrode was immersed in a 75% ethanol solution for 15 min, and then washed with Hank's solution and equilibrated for 5 min. The electrodes were sequentially inserted into the well of a 24 well transwell culture plates to detect the resistance value, and each of the three reserved positions of each well was measured. The calculation formula is as follows:| TEER = (R − R0) × S |
2.5. Permeability test of transmembrane transport marker
Fluorescein isothiocyanate (FITC)-dextran (FD-4, Sigma-Aldrich) (1 mg mL−1) or 200 μM propranolol (Sigma-Aldrich) were dissolved in Hank's solution. The above permeability indicator solution (0.1 mL) was added to the AP side with 0.6 mL Hank's solution in the BL side. After 2 hours incubation, the fluorescence intensity of FD-4 was measured by a microplate reader (Molecular Devices Incorporated, Sunnyvale, USA) at 480/520 nm.30 Propranolol content was determined by high performance liquid chromatography (HPLC), the column was a Macherey-Nagel C-18 reversed-phase column (250 mm × 4.6 mm, 5 μm). The chromatographic conditions were as follows: column temperature 37 °C, mobile phase was phosphate solution (25 mM, pH 6.00): acetonitrile (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), the flow rate is 1 mL min−1, the injection volume is 10 μL, and the detection wavelength is 225 nm. Apparent permeability coefficient (Papp) was calculated according to the following equation:
30), the flow rate is 1 mL min−1, the injection volume is 10 μL, and the detection wavelength is 225 nm. Apparent permeability coefficient (Papp) was calculated according to the following equation:| Papp = ΔQ × V/(ΔtAC0) |
2.6. Determination of alkaline phosphatase (ALP) activity
The test was performed according to the ALP kit instruction and samples were taken from both sides of the transwell membrane on days of 3, 7, 11, 16 and 21 after Caco-2 cell inoculation. The absorbance at 520 nm was measured by a microplate reader (Molecular Devices), and the ALP activity at different time points was calculated as follows:where King's unit is defined as product of 1 mg of phenol in 100 mL of liquid reacted at 37 °C for 15 min; At is the measured OD value; As is the standard OD value, and Ac is the OD value of the blank.
2.7. Determination of proliferation inhibition rate and survival rate of Caco-2 cells
Cell proliferation inhibition rate and survival rate were determined by CCK-8 method.31 The cell density was adjusted to 4 × 104 cells per mL, and 200 μL per well was seeded in a 96 well plate. When the cells were in the logarithmic phase after 24 hours of adherent growth, the medium was discarded, and 200 μL of the medium containing DON/kaempferol was added to continue the culture for another 24 hours. One hour before the end of the culture, the medium was also discarded, and 10% CCK-8 solution (Beyotime Biotechnology, Shanghai, China) was added to 100 μL. After incubation at 37 °C for 1 h, the absorbance A was measured at 450 nm. Cell proliferation inhibition rate Y = [1 − (Ay − Abx)/(Ac − Abz)] × 100%, wherein bx and bz are the corresponding blank group, c is the negative control group, and y is the drug treatment group.Kaempferol pretreatment refers to the cells pre-incubated with kaempferol for 24 h, followed by treatment with DON for another 24 h. Method for determining the survival rate is the same as the inhibition rate of proliferation. The calculation formula is cell survival rate = 100% − cell proliferation inhibition rate.
2.8. Western blot
The culture medium was removed, and the cells were washed twice with 4 °C pre-cooled PBS, then 80 μL of RIPA potent lysate (Beyotime) containing 1 mM protease inhibitor PMSF (Beyotime) was added to each insert of the 24 well transwell plate. After lysed for 2 min, the cells were scraped down into 1.5 mL pre-cooled centrifuge tubes. Centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 5 min at 4 °C, the supernatant was stored at −80 °C for testing. All of the above operations were performed on ice. The protein concentration was determined using a BCA protein concentration assay kit (Beyotime).
000 × g for 5 min at 4 °C, the supernatant was stored at −80 °C for testing. All of the above operations were performed on ice. The protein concentration was determined using a BCA protein concentration assay kit (Beyotime).
Prepare the sample solution according to the instructions of the NuPAGE® MOPS SDS Buffer Kit. After mixing, the protein in solution was denaturated at 70 °C for 10 min. The preformed gel was placed in an electrophoresis tank, and then 200 mL of 5% NuPAGE® MOPS SDS loading buffer was added to each of the front and rear tanks, and 500 μL of NuPAGE® antioxidant was added to the front tank. Pull out the comb and add 20 μL of each sample. Electrophoresis lasts about 75 minutes at a voltage of 200 V and an electric current of 80 mA. When the electrophoresis was completed, the gel was taken out and transferred at 20 V, 1 min; 23 V, 4 min; 25 V, 2 min, and then blocked with a TBST containing 5% BSA at room temperature for 2 h under shaking.
After incubating with a suitable concentration of primary antibody for claudin-3, claudin-4, occludin and ZO-1 at 4 °C overnight, the primary antibody dilution was discarded and washed 6 times with TBST on a shaker for 10–20 min each time. The secondary antibody dilution was added and incubated for 2 h at room temperature, then the secondary antibody dilution was removed and washed again with TBST on a shaker for 6 times. After color developed, exposed and photographed, grayscale analysis was performed using Image J software (Bethesda softworks LLT, Bethesda, USA).
2.9. Statistical analysis
Significant analysis of differences between groups were performed using SPSS software (IBM Corporation, Armonk, USA), and p < 0.05 was set as the level of statistical difference. Charts in the article were plotted using Origin 8.5 (OriginLab Corp., MA, USA). The data were presented as mean ± standard deviation. The heatmap analysis was drew with R package of ggplot2.3. Results
3.1. Characteristics of Caco-2 cell model in different stages
Caco-2 cell line was from human colorectal cancer cells, and cells grown on a porous permeable membrane after inoculation at a specific density can fuse and differentiate to form a continuous monolayer. Because of its similarity to differentiated intestinal epithelial cells (microvilli structure, enzymes associated with the intestinal brush border epithelium, cell polarity and tight junctions), it is commonly used to simulate the human intestinal epithelium after a Caco-2 monolayer was established.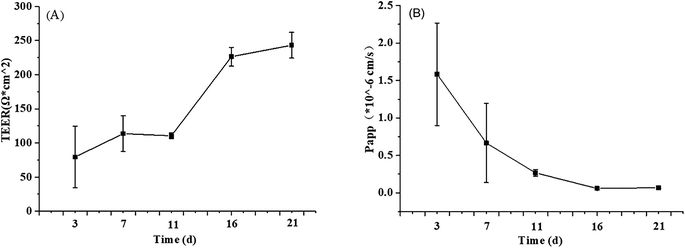 | ||
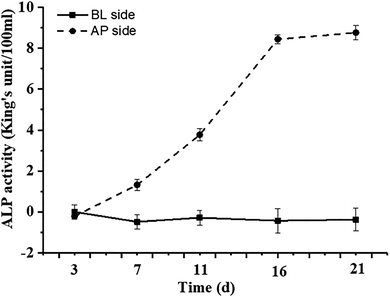
| Fig. 1 The TEER value (A) and FD-4 permeability (B) at different stages of Caco-2 cell proliferation and differentiation. The data were presented as mean ± SD of at least three replicates. | ||
Another risk in the process of Caco-2 cell modeling is the possibility of forming multilayer cells rather than the monolayer model required by the research. Propranolol, as a marker of intracellular transport, was used to verify the integrity of Caco-2 cell monolayer according to its Papp value (>10 × 10−6 cm s−1). In the current study, the average Papp value of propranolol determined by HPLC was 13.69 × 10−6 cm s−1 (ESI Fig. A and B†), which is greater than the standard, demonstrating that the permeability of the Caco-2 cells model meets the experimental standard.32
3.2. Determination of DON and kaempferol (KAM) treatment concentration
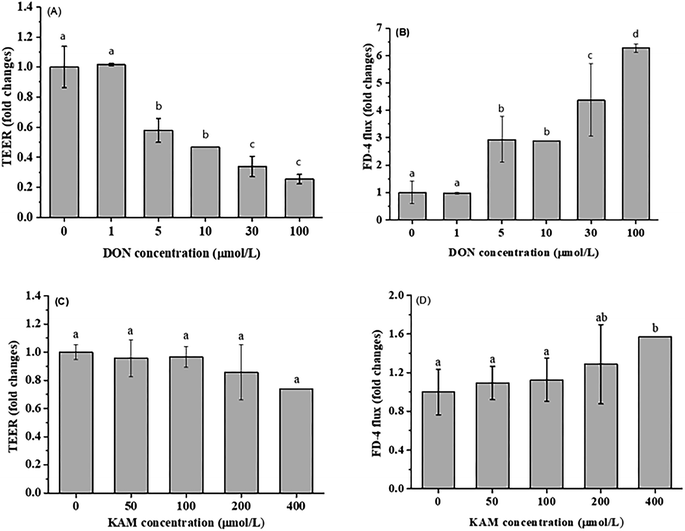
The differentiation of Caco-2 cells is a continuous process involving cell proliferation and differentiation. Thus, the Caco-2 cell differentiation affected by DON and KAM was studied according the selected time scheme to determine the dosage for later TJ protein expression studies. When the cell viability (proliferation stage) was measured after incubation in a complete culture medium containing different concentrations of DON or kaempferol for 24 hours, different profiles were shown for DON and KAM. The inhibition rate of DON was tightly dependent on the concentration of DON (Fig. 3A), and polynomial fit analysis demonstrated that the half-maximal inhibitory concentration (IC50) of DON for Caco-2 cell proliferation was 8.50 μM. For kaempferol, the minimal concentration to inhibit Caco-2 cell growth was 100 μM (Fig. 3B). Thus, DON is more toxic than KAM concerning the cell viability, and the negative inhibition rate at lower concentrations of KAM (<100 μM) suggests it may even be beneficial to the cell growth.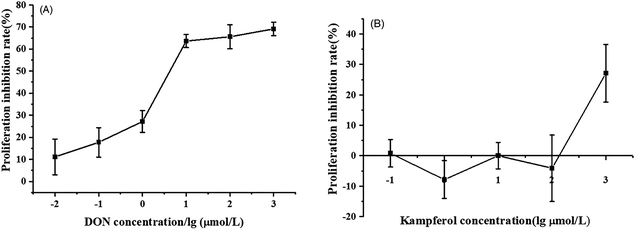 | ||
| Fig. 3 Effects of DON (A) and kaempferol (B) on proliferation inhibition rate of Caco-2 cells. The data were presented as mean ± SD of at least three replicates. | ||
For differentiated Caco-2 cells, the parameter of cell permeability was applied to evaluate the impact of DON and KAM on the integrity of cell-to-cell adhesion or tight junction. Obviously, the DON treatment substantially reduced the transepithelial electrical resistance (TEER) values in a concentration-dependent manner (≥ 5 μM), which is consistent with the conversely increased FD-4 flux rate (Fig. 4A and B). However, in the KAM treatment (up to 200 μM) groups, no significant impact on cell permeability was observed, implying that KAM would not adversely affect the Caco-2 cell monolayer in the used dosage (Fig. 4C and D).
In summary, by considering the above effects of DON and KAM on the proliferation and the permeability of differentiated monolayer Caco-2 cells as well as the existed literature reports,29,33 a dosage of 5 μM DON and 100 μM KAM were used to study the effects of DON and KAM on the tight junction of Caco-2 cells at different stages of proliferation and differentiation.
3.3. Tight junction protein expression in Caco-2 cells at different stages of proliferation and differentiation
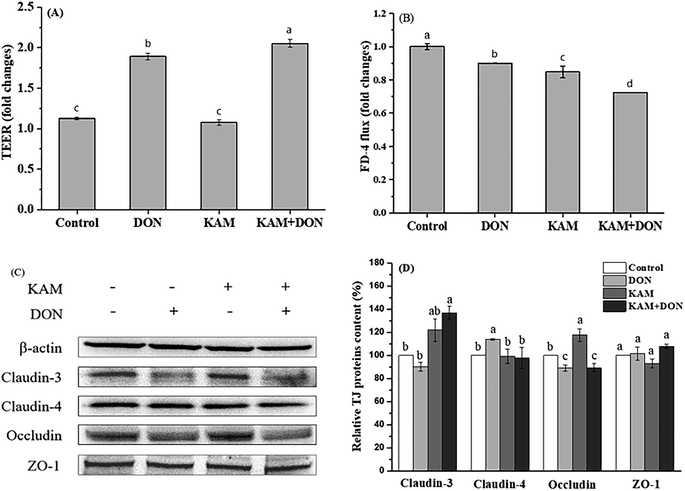
The permeability of monolayer Caco-2 cell is intimately associated to the cell adhesion, particularly the tight junction which is a network formed by many different proteins. In order to gain a complete understanding, occludin and claudins (3 and 4) representing the transmembrane protein, and peripheral adaptor protein of zonula occludens-1 (ZO-1) were selected as the targets to investigate the impact of DON and KAM on the integrity of TJ at different stages (proliferative stage at 3rd day, turning point at 11th day, and differentiated stage at 21st day).When the TJ protein expression was examined (Fig. 5C and D), KAM treatment alone significantly reduced the protein expression of occludin, and DON significantly reduced the expression of claudin-4 and occludin, which is consistent with the decreased TEER and high flux rate of FD-4. KAM pretreatment did not improve the expression of claudin-4 adversely affected by DON, but the protein expression of claudin-3 was increased significantly. When the correlation between TEER and protein expression was analyzed, the expression level of claudin-4 is significantly correlated with the cell permeability, indicating the TJ protein of claudin-4 is associated with the cell permeability at the proliferation stage. DON treatment not only reduced claudin-4, but also reduce the cell proliferation (Fig. 3), and both can cause a high permeability. Although KAM is better than DON (probably due to no inhibition for cell proliferation), KAM pretreatment worsened the TEER (maybe related to ZO-1) with an increase of claudin-3, indicating claudin-3 is not related to the permeability at the proliferation stage of the Caco-2 cells. Thus, both inhibiting cell proliferation and impairing tight junctions are likely the main reasons for the adverse effect of DON and KAM on the cell permeability in the early stage of Caco-2 cell differentiation.
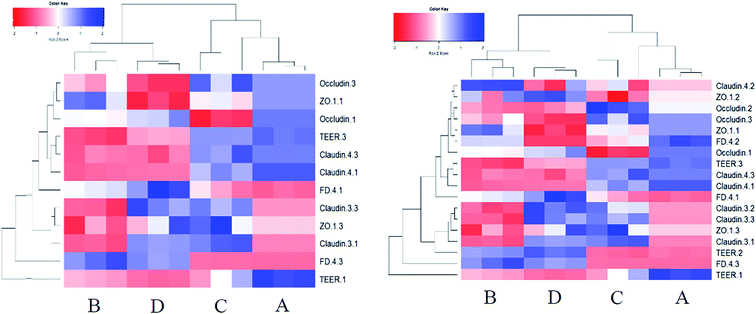
The TJ protein expression pattern after treatments also showed a substantial difference from the proliferative Caco-2 cells (Fig. 6C and D). In DON alone treatment group, except a slight reduced occludin expression, the expression of claudin-4 was significantly increased. For KAM group, the occludin expression was significantly increased, and a higher level of claudin-3 was also observed (compared to the control). After kaempferol pretreatment, no changes were found for the expression levels of claudin-4, occludin and ZO-1, but the expression of claudin-3 was significantly increased, which was consistent with the permeability of KAM pretreatment group. Altogether, no reduction of TJ protein expression was found in the treatment group compared to the control, which is substantially different from the proliferative Caco-2 cells. When the relationship between TEER and TJ protein was analyzed, it seems both claudin-4 (high TEER in DON alone) and claudin-3 (high TEER in KAM pretreatment group) contribute to the reduced permeability of Caco-2 cells in the proliferative/differentiated stage.
The disappearance of inverse relationship between TEER and FD-4 flux rate and positive effect of both DON and KAM on TJ protein expression signify the uniqueness of the Caco-2 cell model at the 11th day, which might represent the separation point of cell proliferation and differentiation. Although we do not have a good explanation for the observed phenomenon, a higher tolerance to environments or the basic biological mechanism of compensating changes for adaption and survive might played a role in this special stage of Caco-2 cell differentiation.
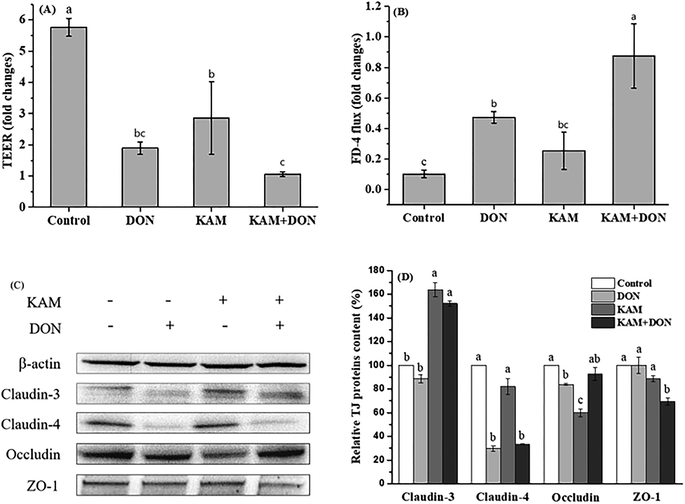
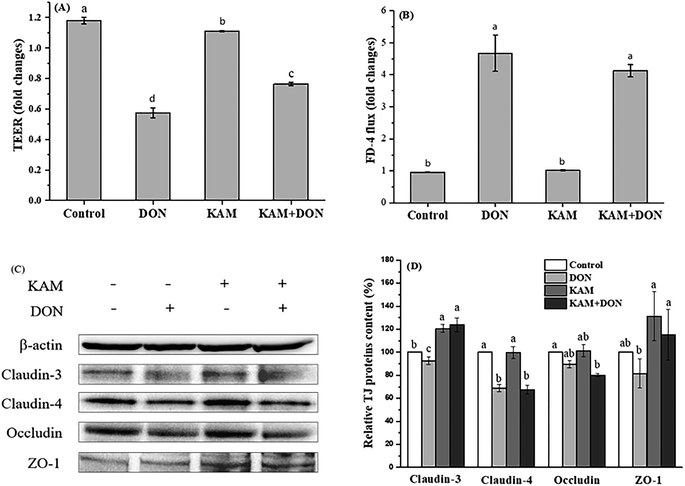
From the results of protein expression (Fig. 7C and D), DON alone caused a noteworthy decrease in claudin-4, while KAM alone and its pretreatment significantly increased the protein expression of claudin-3 and ZO-1 without affecting the expression of claudin-4.
4. Discussion
The intestinal epithelium, which is composed of a monolayer of cells, acts not only as the primary site for nutrient uptake but also as the barrier to the external environment. The rapid turnover of the monolayer cells needs a continuous dividing of intestinal stem cells resided at the bottom of crypts. The divided stem cells then enter the transit-amplifying zone where they proliferate and differentiate until they migrate to the villus tip as matured cells. Certainly, the microenvironment formed by luminal materials, particularly food materials in the lumen, will have a great impact on cell proliferation and differentiation. The phenolic compounds of KAM rich in fruit and vegetable is a common food component with a variety of health benefits. Mycotoxin of DON, which is toxic to human health, is becoming a food hazard problem due to its wide contamination in many crops and food products. Thus, the antagonistic effect of KAM on the cytotoxicity DON affecting the epithelium barrier function, as the first step to enter into the body, was investigated by mimicking the process of intestinal cell proliferation and differentiation as they moved from the base of the crypt to the villus tip.The toxic effect of DON on the Caco-2 cell monolayer was evidenced by a dose-dependent decrease of the TEER values at a concentration of ≥5 μM (Fig. 4). Further study demonstrated the TJ protein expression of claudin-4 and claudin-3 were significantly reduced in differentiated monolayer stage (Fig. 7). This result is consistent with literature reports using Caco-2 cell monolayer model5 and porcine intestinal epithelial cell line (IPEC-1).13 Regarding the mechanism, the inhibition of protein synthesis and activation of a mitogen-activated protein kinases (MAPK) signaling pathway, specifically the reduced TJ protein claudin-4 all contribute to the toxic effect of DON to the epithelium barrier function.5,13,34 However, we also observed that the impact of DON one Caco-2 cell permeability and the expression on claudin-4 is dependent on the stages of Caco-2 cell proliferation and differentiation. DON inhibited the expression of claudin-4 at its proliferation and differentiated (monolayer) stages, but increased the claudin-4 expression at 11th day, which is an intermediate stage of Caco-2 cell differentiation. Although we do not know the exact reason, it does strengthen the relationship between permeability and the expression of claudin-4.
In contrast to the development stage-dependent toxicity of DON, KAM did not affect the expression of claudin-4, but always promotes the expression of TJ protein of claudin-3. However, KAM only induced a leveled permeability to the control, which is not correlated to the expression of claudin-3 (Fig. 5–7). However, for the differentiated Caco-2 cell monolayer, KAM pretreatment did significantly reduced the permeability after DON exposure (Fig. 7 KAM + DON) without influencing the level of claudin-4. Actually, there have been reports on the beneficial effects on epithelium barrier of many polyphenol compounds including berberine, grape seeds extract, quercetin, green tea polyphenols through MAPK activation and inhibiting inflammation induced by NF-κB as well as scavenging ROS.35 Similarly, KAM also regulates MAPKs and NF-κB signaling pathways to induce many health benefits,36 and it should be beneficial to the barrier function of epithelium. Indeed, there have been numerous studies indicating that claudin-3 is a barrier-forming TJ protein.37 The permeability of Caco-2 cells grown on transwell filters was reduced by mycotoxin of ochratoxin A treatment, which is due to reduced expression of claudin-3 and 4.38 An increase of gastric epithelium permeability by oxidative stress is also associated with a reduction of claudin-3 protein.39 Thus, pretreatment by KAM, which specifically upregulates the expression of claudin-3, may have the potential to reduce the damage to epithelium permeability caused by DON. In the meantime, KAM treatment also upregulate the expression of ZO-1 that may also contribute the reduced permeability of Caco-2 cell monolayers.
5. Conclusion
In this study, the response of tight junctions of Caco-2 cells at different stages of proliferation and differentiation to mycotoxin DON and phenolic compound kaempferol was studied. The results showed that DON and kaempferol affect the cell proliferation in a dose-dependent manner, and the permeability of the Caco-2 TJ affected by DON and KAM is highly dependent on the stage of cell proliferation and differentiation. Further research found that claudin-4 is the target protein of DON while claudin-3 is specifically upregulated by KAM, and KAM pretreatment significantly increase the integrity of differentiated Caco-2 cell monolayer. Thus, KAM, through upregulating claudin-3 and ZO-1, may have a high potential to improve the epithelium integrity damaged by DON exposure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was funded by National Natural Science Foundation of China No: 31471585.References
- E. F. S. Authority, EFSA J., 2013, 11 DOI:10.2903/j.efsa.2013.3379.
- J. J. Pestka and A. T. Smolinski, J. Toxicol. Environ. Health, Part B, 2005, 8, 39–69 CAS.
- B. Grenier and T. J. Applegate, Toxins, 2013, 5, 396–430 CrossRef CAS.
- J. J. Pestka, Arch. Toxicol., 2010, 84, 663–679 CrossRef CAS.
- J. V. De Walle, T. Sergent, N. Piront, O. Toussaint, Y. J. Schneider and Y. Larondelle, Toxicol. Appl. Pharmacol., 2010, 245, 291–298 CrossRef.
- P. Pinton, J. P. Nougayrede, J. C. Del Rio, C. Moreno, D. E. Marin, L. Ferrier, A. P. Bracarense, M. Kolf-Clauw and I. P. Oswald, Toxicol. Appl. Pharmacol., 2009, 237, 41–48 CrossRef CAS.
- P. Pinton, D. Tsybulskyy, J. Lucioli, J. Laffitte, P. Callu, F. Lyazhri, F. Grosjean, A. P. Bracarense, M. Kolf-Clauw and I. P. Oswald, Toxicol. Sci., 2012, 130, 180–190 CrossRef CAS.
- A. Springler, S. Hessenberger, G. Schatzmayr and E. Mayer, Toxins, 2016, 8 DOI:10.3390/toxins8090264.
- S. Tsukita, H. Tanaka and A. Tamura, Trends Biochem. Sci., 2019, 44, 141–152 CrossRef CAS.
- P. Artursson and S. Knight, Science, 2015, 716–717 CrossRef CAS.
- M. S. Balda and K. Matter, Curr. Opin. Cell Biol., 2016, 42, 94–101 CrossRef CAS.
- P. Rowart, J. Wu and M. J. Caplan, Int. J. Mol. Sci., 2018, 19 DOI:10.3390/ijms19072040.
- P. Pinton, C. Braicu, J. P. Nougayrede, J. Laffitte, I. Taranu and I. P. Oswald, J. Nutr., 2010, 140, 1956–1962 CrossRef CAS.
- T. Suzuki and H. Hara, J. Nutr., 2009, 139, 965–974 CrossRef CAS.
- T. Suzuki and H. Hara, J. Nutr. Biochem., 2011, 22, 401–408 CrossRef CAS.
- M. C. Valenzano, K. DiGuilio, J. Mercado, M. Teter, J. To, B. Ferraro, B. Mixson, I. Manley, V. Baker, B. A. Moore, J. Wertheimer and J. M. Mullin, PLoS One, 2015, 10, e0133926 CrossRef.
- B. Lee, K. M. Moon and C. Y. Kim, J. Immunol. Res., 2018, 2018, 2645465 Search PubMed.
- M. B. G. Kiewiet, M. I. Gonzalez Rodriguez, R. Dekkers, M. Gros, L. H. Ulfman, A. Groeneveld, P. de Vos and M. M. Faas, Food Funct., 2018, 9, 4164–4172 RSC.
- P. Akbari, S. Braber, A. Alizadeh, K. A. Verheijden, M. H. Schoterman, A. D. Kraneveld, J. Garssen and J. Fink-Gremmels, J. Nutr., 2015, 145, 1604–1613 CrossRef CAS.
- S. Noda, S. Tanabe and T. Suzuki, J. Funct. Foods, 2014, 10, 112–116 CrossRef CAS.
- S. Noda, S. Tanabe and T. Suzuki, Mol. Nutr. Food Res., 2013, 57, 2019–2028 CrossRef CAS.
- L. Gu, N. Li, Q. Li, Q. Zhang, C. Wang, W. Zhu and J. Li, Fitoterapia, 2009, 80, 241–248 CrossRef CAS.
- M. Shigeshiro, S. Tanabe and T. Suzuki, J. Funct. Foods, 2013, 5, 949–955 CrossRef CAS.
- T. Chen, C. Y. Kim, A. Kaur, L. Lamothe, M. Shaikh, A. Keshavarzian and B. R. Hamaker, Food Funct., 2017, 8, 1166–1173 RSC.
- J. M. Calderón-Montaño, E. Burgos-Morón, C. Pérez-Guerrero and M. López-Lázaro, Mini-Rev. Med. Chem., 2011, 11, 298–344 CrossRef.
- A. Sekiguchi, S. I. Motegi, C. Fujiwara, S. Yamazaki, Y. Inoue, A. Uchiyama, R. Akai, T. Iwawaki and O. Ishikawa, J. Dermatol. Sci., 2019, 96, 8–17 CrossRef CAS.
- S. Cui, J. Tang, S. Wang and L. Li, Biomed. Pharmacother., 2019, 115, 108888 CrossRef CAS PubMed.
- R. M. Hussein, W. R. Mohamed and H. A. Omar, Pestic. Biochem. Physiol., 2018, 152, 29–37 CrossRef CAS.
- T. Suzuki, S. Tanabe and H. Hara, J. Nutr., 2011, 141, 87–94 CrossRef CAS.
- N. Li, L. Gu, L. Qu, J. Gong, Q. Li, W. Zhu and J. Li, Eur. J. Pharm. Sci., 2010, 40, 1–8 CrossRef CAS.
- Y. Sang, W. Li and G. Zhang, Food Funct., 2016, 7, 3703–3715 RSC.
- S. Yee, Pharm. Res., 1997, 763–766 CrossRef CAS.
- P. Akbari, S. Braber, H. Gremmels, P. J. Koelink, K. A. Verheijden, J. Garssen and J. Fink-Gremmels, FASEB J., 2014, 28, 2414–2429 CrossRef CAS.
- P. Pinton, J. P. Nougayrède, J. C. Del Rio, C. Moreno, D. E. Marin, L. Ferrier, A. P. Bracarense, M. Kolf-Clauw and I. P. Oswald, Toxicol. Appl. Pharmacol., 2009, 237, 41–48 CrossRef CAS.
- G. Yang, S. Bibi, M. Du, T. Suzuki and M. J. Zhu, Crit. Rev. Food Sci. Nutr., 2017, 57, 3830–3839 CrossRef CAS.
- X. Chen, X. Yang, T. Liu, M. Guan, X. Feng, W. Dong, X. Chu, J. Liu, X. Tian, X. Ci, H. Li, J. Wei, Y. Deng, X. Deng, G. Chi and Z. Sun, Int. Immunopharmacol., 2012, 14, 209–216 CrossRef CAS.
- D. Gunzel and A. S. Yu, Physiol. Rev., 2013, 93, 525–569 CrossRef.
- J. McLaughlin, P. J. Padfield, J. P. Burt and C. A. O'Neill, Am. J. Physiol. Cell Physiol., 2004, 287, C1412–C1417 CrossRef CAS.
- K. Hashimoto, T. Oshima, T. Tomita, Y. Kim, T. Matsumoto, T. Joh and H. Miwa, Biochem. Biophys. Res. Commun., 2008, 376, 154–157 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06222j |
| This journal is © The Royal Society of Chemistry 2019 |