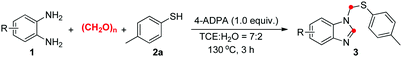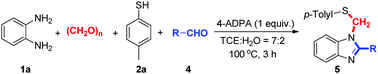Open Access Article
Open Access ArticleConcise synthesis of N-thiomethyl benzoimidazoles through base-promoted sequential multicomponent assembly†
Jingxin
Tian‡
,
Shanshan
Yuan‡
,
Fuhong
Xiao
 *,
Huawen
Huang
*,
Huawen
Huang
 and
Guo-Jun
Deng
and
Guo-Jun
Deng
 *
*
Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, Key Laboratory for Green Organic Synthesis and Application of Hunan Province, College of Chemistry, Xiangtan University, Xiangtan 411105, China. E-mail: fhxiao@xtu.edu.cn; gjdeng@xtu.edu.cn
First published on 26th September 2019
Abstract
An efficient method for the synthesis of N-thiomethyl benzimidazoles from o-phenylenediamines, thiophenols, and aldehydes via C–N/C–S bond formation under metal-free conditions is described. A broad range of functional groups attached to the substrates were well tolerated in this reaction system. Stable and low-toxicity paraformaldehyde was used as the carbon source.
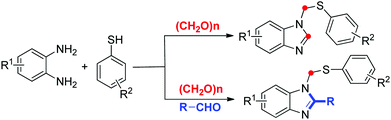
Benzimidazole and its derivatives are extremely important in pharmaceuticals and bioactive natural products because they have found applications in diverse therapeutic areas, such as anticancer agents, antimicrobials, antivirals, antifungals and so on.1 Furthermore, benzimidazoles are very important intermediates in organic synthesis and widely used as organocatalysts, ionic liquids and N-heterocyclic carbenes.2 In particular, the broad spectrum of biological activities of compounds with the 1,2-disubstituted benzimidazole moiety make them highly sought as synthetic targets.3 Therefore, several efficient protocols have been developed to synthesize substituted benzimidazoles over the past several decades.4 Three general methods for the construction of 1,2-disubstituted benzimidazole are as follows: (1) oxidative cyclocondensation,5 (2) C-1 or N-2 alkylation/arylation,6 and (3) inter/intramolecular N-arylation.7 To date, most of these methods are reported for 1-phenyl-2-benzyl benzimidazoles synthesis. However, few examples of multicomponent reactions led to the development of N-thiomethyl benzimidazoles from simple starting materials under mild conditions.8
Paraformaldehyde has been receiving more and more attention among molecule synthesis because of the advantages of low-cost, stability and low toxicity.9 It has been used as one-carbon homologation reagents for the synthesis of an incredible variety of complicated skeletons.10 In recent years, various methods have been developed to use paraformaldehyde to install a methylene,11 carbonyl12 and hydroxymethyl group13 into desired products. With respect to our recent studies on paraformaldehyde insertion to construct heterocycles, we reported synthetic methods for phthalazinones, 2-aroylbenzofurans and quinazolines formation.14 Very recently, we reported on an ethylenediamine-promoted three-component synthesis of 3-(thiomethyl)indoles from indoles, thiophenols, and paraformaldehyde.15 As part of our continuing efforts on using paraformaldehyde as the carbon source, herein, we describe a new and efficient multicomponent reaction for N-thiomethyl benzimidazoles formation from readily available starting materials. The reaction is successfully achieved in one-pot fashion under simple and facile reaction conditions (Scheme 1).
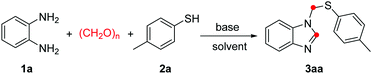
For the optimization of reaction conditions, o-phenylenediamine (1a), p-toluenethiol (2a) and paraformaldehyde were chosen as model substrates (Table 1). To our delight, the desired product 3aa was obtained in 38% yield when the reaction was performed with K2CO3 in TCE/H2O (v/v = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 130 °C for 3 h (entry 1). Afterwards, a variety of bases were investigated (Table 1, entries 2–8), and among them 4-ADPA (4-aminodiphenylamine) showed the best efficiency to give the corresponding product 3aa in 75% yield (Table 1, entry 8). As a control experiment, the desired product 3aa was obtained in 30% yield when the reaction was carried out in absence of 4-aminodiphenylamine (entry 9). The ratio of TCE/H2O slightly affected the reaction yield (entries 10–11). Other mixture solvents such as DMSO/H2O, anisole/H2O and PhCl/H2O were less effective for this kind of reaction (entries 12–14). Decreasing the reaction temperature or the amounts of 4-aminodiphenylamine all reduced the reaction yields (entries 15–16).
2) at 130 °C for 3 h (entry 1). Afterwards, a variety of bases were investigated (Table 1, entries 2–8), and among them 4-ADPA (4-aminodiphenylamine) showed the best efficiency to give the corresponding product 3aa in 75% yield (Table 1, entry 8). As a control experiment, the desired product 3aa was obtained in 30% yield when the reaction was carried out in absence of 4-aminodiphenylamine (entry 9). The ratio of TCE/H2O slightly affected the reaction yield (entries 10–11). Other mixture solvents such as DMSO/H2O, anisole/H2O and PhCl/H2O were less effective for this kind of reaction (entries 12–14). Decreasing the reaction temperature or the amounts of 4-aminodiphenylamine all reduced the reaction yields (entries 15–16).
| Entry | Base | Solvent (v/v) | Yieldb (%) |
|---|---|---|---|
| a Conditions: 1a (0.2 mmol), 2a (0.5 mmol), base (0.2 mmol), paraformaldehyde (0.8 mmol), solvent (0.9 mL), 130 °C, 3 h, air. PDA = 1,4-benzenediamine, 4-ADPA = 4-aminodiphenylamine, TCE = 1,1,1,2-tetrachloroethane. b GC yields. c 110 °C. d 4-ADPA (0.1 mmol). | |||
| 1 | K2CO3 | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
38 |
| 2 | NaOH | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
37 |
| 3 | KOtBu | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
31 |
| 4 | Piperidine | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
57 |
| 5 | DMAP | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
40 |
| 6 | Morpholine | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
35 |
| 7 | PDA | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
58 |
| 8 | 4-ADPA | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
75 |
| 9 | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
30 | |
| 10 | 4-ADPA | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 5 H2O = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
48 |
| 11 | 4-ADPA | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
35 |
| 12 | 4-ADPA | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
22 |
| 13 | 4-ADPA | Anisole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
43 |
| 14 | 4-ADPA | PhCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
29 |
| 15c | 4-ADPA | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
61 |
| 16d | 4-ADPA | TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 7 H2O = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
52 |
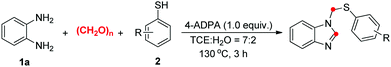
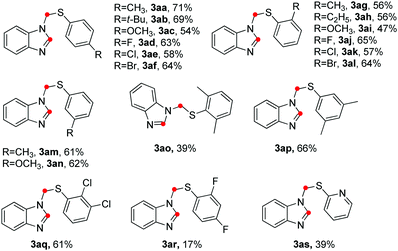
With the optimized reaction conditions established, the scope of thiophenols was probed for the three-component reaction (Table 2). The model reaction of o-phenylenediamine (1a) with p-toluenethiol (2a) gave the desired product 3aa in 71% isolated yield. Other para-substituted thiophenols reacted with 1a to give N-thiomethyl benzoimidazoles (3ab–3af) in moderate to good yields. The position of the substituents on the benzene ring (ortho or meta) did not significantly affect the reaction yields (3ag–3an). A large range of functional groups attached to the benzene ring such as t-Bu (2b), methoxyl (2c) and halo (F, Cl, Br, 2d–2f) were well tolerated under the optimized reaction conditions. Notably, the C-halo bond cleavage of the substrates was not observed in the present base system. The reaction yields decreased dramatically when 2,6-dimethylbenzenethiol (2o) and 2,4-difluorobenzenethiol (2r) were used as the substrate. Modest yields were obtained when 3,5-dimethylbenzenethiol (2p) and 2,3-dichlorobenzenethiol (2q) were used as the substrates. To our delight, heteroaromatic thiols such as pyridine-2-thiol (2s) also participated in the reaction, albeit in low yield. Unfortunately, aliphatic thiols were much less effective coupling partners under the current reaction conditions.
| a Reaction conditions: 1a (0.2 mmol), 2 (0.5 mmol), 4-ADPA (0.2 mmol), paraformaldehyde (0.8 mmol), TCE (0.7 mL), H2O (0.2 mL), 130 °C, 3 h, air. Isolated yield based on 1a. |
|---|
|
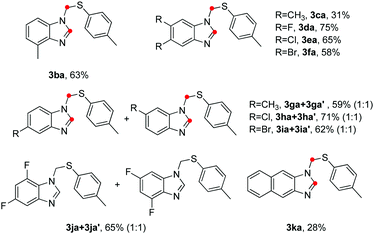
To further evaluate the scope and limitations of the reaction, various o-phenylenediamines were treated with p-toluenethiol (2a) and the results are presented in Table 3. When 3-methylbenzene-1,2-diamine (1b) was used as the substrate, the product 3ba was formed in 63% isolated yield with high levels of regioselectivity. Lower yield was obtained when 4,5-dimethylbenzene-1,2-diamine (1c) was used. Halogen substituents on the benzene ring, including F, Cl and Br, were compatible for this type of cyclocondensation (3da–3fa). As for non-symmetrical o-phenylenediamines (1g–1j), a mixture of two isomers (near 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) were obtained and difficult to be separated. In addition, the reaction of 2a with naphthalene-2,3-diamine 1k afforded the desired product 3ka in 28% yield.
1 ratio) were obtained and difficult to be separated. In addition, the reaction of 2a with naphthalene-2,3-diamine 1k afforded the desired product 3ka in 28% yield.
| a Reaction conditions: 1 (0.2 mmol), 2a (0.5 mmol), 4-ADPA (0.2 mmol), paraformaldehyde (0.8 mmol), TCE (0.7 mL), H2O (0.2 mL), 130 °C, 3 h, air. Isolated yield. |
|---|
|
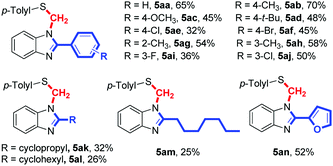
The reaction of o-phenylenediamine (1a), p-toluenethiol (2a) and paraformaldehyde with other different aldehydes was investigated (Table 4). We slightly modified the reaction conditions to find that the reactions could be smoothly performed at 100 °C. When benzaldehyde (4a) was used as the fourth component, the corresponding product 5aa was obtained in 65% yield. In general, other aromatic aldehyde derivatives bearing electron-donating groups such as CH3 or OCH3, or halogens such as Cl or Br on the aromatic ring all gave the desired products. The position of the substituent on the benzene ring (ortho or meta) affected slightly the reaction yields (5ag–5aj). In addition, aliphatic aldehydes such as cyclopropanecarbaldehyde (4k), cyclohexanecarbaldehyde (4l) and octanal (4m) were also suitable substrates for this kind of reaction, giving the corresponding products in moderate yields.
| a Reaction conditions: 1a (0.2 mmol), 2a (0.5 mmol), 4 (0.4 mmol), 4-ADPA (0.2 mmol), paraformaldehyde (0.5 mmol), TCE (0.7 mL), H2O (0.2 mL), 100 °C, 3 h, air. Isolated yield. |
|---|
|
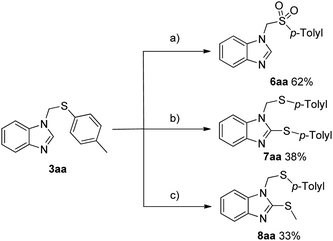
We evaluated the late-stage transformation of 3aa (Scheme 2). When 3aa was treated with m-chlorope-roxybenzoic acid (m-CPBA) in CH2Cl2 at 50 °C, the oxidized sulfone product 6aa was obtained in 62% yield. The disulfonyl product 7aa and 8aa were obtained when p-toluenethiol (2a) or DMSO were used as the substrate with 3aa, although giving the corresponding products in lower yields.
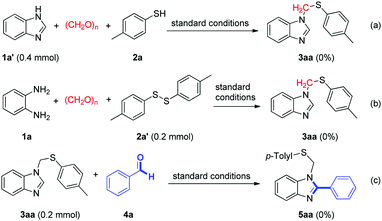
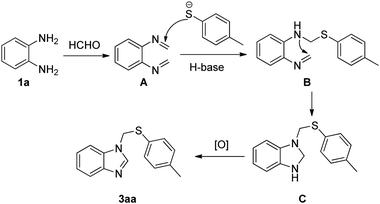
To understand the mechanism of the reaction, we performed some control experiments under modified conditions. No desired product was observed when benzimidazole (1a′) was treated with p-toluenethiol (2a) and paraformaldehyde under standard conditions (Scheme 3a). No reaction occurred upon the treatment of o-phenylenediamine (1a) with 1,2-di-p-tolyldisulfane (2a′) and paraformaldehyde under the optimized reaction conditions (Scheme 3b). The reaction of 3aa with benzaldehyde (4a) could not give the corresponding product 5aa under standard conditions (Scheme 3c). According to above control experiments, a possible mechanism is illustrated in Scheme 4. Initially, condensation of o-phenylenediamine (1a) with formaldehyde generates an imine intermediate A. Then, intermediate A reacts with thiophenol anion to afford an intermediate B, which proceeds through intramolecular nucleophilic annulation to form intermediate C. Finally, dehydrogenative aromatization of intermediate C occurs to give desired product (3aa). On the other hand, the reaction mechanism of compound 5aa is similar to that of compound 3aa. Condensation of o-phenylenediamine (1a) with formaldehyde and benzaldehyde (4a) generates a non-symmetrical imine intermediate, which proceeds through nucleophilic addition/annulation/dehydrogenative aromatization sequence to give desired product (5aa).
In summary, we have developed a simple and efficient method for the synthesis of N-thiomethyl benzoimidazoles from o-phenylenediamines, thiophenols, and aldehydes under metal-free conditions. In this system, three carbon–nitrogen bonds and one carbon–sulfur bond were formed with high levels of chemo-selectivity. Various functional groups were well tolerated under the optimized reaction conditions. This method affords an efficient and general approach for the synthesis of biologically important 1,2-disubstituted benzimidazole from readily available starting materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Supported by the National Natural Science Foundation of China (21572194, 21502160, and 21871226), the Collaborative Innovation Center of New Chemical Technologies for Environmental Benignity and Efficient Resource Utilization.References
- (a) S. Bhattacharya and P. Chaudhuri, Curr. Med. Chem., 2008, 15, 1762 CrossRef CAS; (b) J. E. Saxton, Chemistry of Heterocyclic Compounds: Benzimidazoles and Cogeneric Tricyclic Compounds, Part 1, Wiley, Hoboken, 2008 Search PubMed; (c) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS; (d) S. Vasiliou, Drugs Today, 2011, 47, 647 CrossRef CAS; (e) I. Tamm, Science, 1957, 126, 1235 Search PubMed; (f) E. Marqués-López and R. P. Herrera, Multicomponent Reactions: Concepts and Applications for Design and Synthesis, Wiley, Hoboken, 2015 Search PubMed.
- For selected recent examples see: (a) M. Lee, L. Zhang, Y. Park and H. Park, Tetrahedron, 2012, 68, 1452 CrossRef CAS; (b) C. Nájera and M. Yus, Tetrahedron Lett., 2015, 56, 2623 CrossRef; (c) D. R. Ñíguez, G. Guillena and D. A. Alonso, ACS Sustainable Chem. Eng., 2017, 5, 10649 CrossRef; (d) Mayank, B. K. Billing, P. K. Agnihotri, N. Kaur, N. Singh and D. O. Jang, ACS Sustainable Chem. Eng., 2018, 6, 3714 CrossRef CAS; (e) W. Raimondi, D. Bonne and J. Rodriguez, Angew. Chem., Int. Ed., 2012, 51, 40 CrossRef CAS PubMed.
- For selected examples see: (a) K. Seth, P. Purohit and A. K. Chakraborti, Curr. Med. Chem., 2017, 24, 4638 CAS; (b) P. Singla, V. Luxami and K. Paul, RSC Adv., 2014, 4, 12422 RSC; (c) Z. Wang and C. J. Rizzo, Org. Lett., 2001, 3, 565 CrossRef CAS PubMed; (d) X. Wang, P. A. Bhatia, J. F. Daanen, S. P. Latsaw, J. Rohde, T. Kolasa, A. A. Hakeem, M. A. Matulenko, M. Nakane, M. E. Uchic, L. N. Miller, R. Chang, R. B. Moreland, J. D. Brioni and A. O. Stewart, Bioorg. Med. Chem., 2005, 13, 4667 CrossRef CAS; (e) M. P. Windisch, S. Jo, H. Y. Kim, S. H. Kim, K. Kim, S. Kong, H. Jeong, S. Ahn, Z. No and J. Y. H wang, Eur. J. Med. Chem., 2014, 78, 35 CrossRef CAS.
- For selected reviews see: (a) M. Largeron and K. M. H. Nguyen, Synthesis, 2018, 50, 241 CrossRef CAS; (b) S. Rajasekhar, B. Maiti, M. M. Balamurali and K. Chanda, Curr. Org. Synth., 2017, 14, 40 CrossRef CAS; (c) N. A. Keiko and N. V. Vchislo, Asian J. Org. Chem., 2016, 5, 1169 CrossRef CAS; (d) L. C. R. Carvalho, E. Fernandes and M. M. B. Marques, Chem.–Eur. J., 2011, 17, 12544 CrossRef CAS.
- For selected recent examples see: (a) H. Sharma, N. Kaur, N. Singh and D. O. Jang, Green Chem., 2015, 17, 4263 RSC; (b) K. Bahrami, M. M. Khodaei and A. Nejati, Green Chem., 2010, 12, 1237 RSC; (c) K. M. H. Nguyen and M. Largeron, Eur. J. Org. Chem., 2016, 1025 CrossRef CAS; (d) H. Hikawa, R. Ichinose, S. Kikkawa and I. Azumaya, Asian J. Org. Chem., 2018, 7, 416 CrossRef CAS.
- (a) R. Lv, Y. Wang, C. Zhou, L. Li and R. Wang, ChemCatChem, 2013, 5, 2978 CrossRef CAS; (b) R. Jeyachandran, H. K. Potukuchi and L. Ackermann, Beilstein J. Org. Chem., 2012, 8, 1771 CrossRef CAS; (c) P. Sharma, S. Rohilla and N. Jain, J. Org. Chem., 2015, 80, 4116 CrossRef CAS; (d) G. Tran, D. Confair, K. D. Hesp, V. Mascitti and J. A. Ellman, J. Org. Chem., 2017, 82, 9243 CrossRef CAS; (e) G. L. Turner, J. A. Morris and M. F. Greaney, Angew. Chem., Int. Ed., 2007, 46, 7996 CrossRef CAS; (f) H. T. T. Nguyen, D. N. A. Doan and T. Truong, J. Mol. Catal. A: Chem., 2017, 426, 141 CrossRef CAS; (g) T. Truong, V. T. Nguyen, H. T. X. Le and N. T. S. Phan, RSC Adv., 2014, 4, 52307 RSC; (h) W. Zhang, Y. Tian, N. Zhao, Y. Wang, J. Li and Z. Wang, Tetrahedron, 2014, 70, 6120 CrossRef CAS.
- For selected recent examples see: (a) C. Xie, X. Han, J. Gong, D. Lia and C. Ma, Org. Biomol. Chem., 2017, 15, 5811 RSC; (b) N. Zheng, K. W. Anderson, X. Huang, H. N. Nguyen and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 7509 CrossRef CAS; (c) N. Zheng and S. L. Buchwald, Org. Lett., 2007, 9, 4749 CrossRef CAS; (d) C. Deldaele and G. Evano, ChemCatChem, 2016, 8, 1319 CrossRef CAS; (e) A. R. Hajipour, Z. Khorsandi, M. Mortazavicd and H. Farrokhpour, RSC Adv., 2015, 5, 107822 RSC; (f) Z. Gu, Y. Liu, F. Wang, X. Bao, S. Y. Wang and S. J. Ji, ACS Catal., 2017, 7, 3893 CrossRef CAS; (g) A. R. Hajipour and Z. Khorsandi, New J. Chem., 2016, 40, 10474 RSC.
- (a) A. R. Katritzky, W. H. Ramer and J. N. Lam, J. Chem. Soc., Perkin Trans. 1, 1987, 775 RSC; (b) M. S. Rosen, C. L. Stern and C. A. Mirkin, Chem. Sci., 2013, 4, 4193 RSC.
- For a review, see: W. F. Li and X. F. Wu, Adv. Synth. Catal., 2015, 357, 3393 CrossRef CAS.
- R. T. Taylor and T. J. O'ullivan, in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, Chichester, 2001 Search PubMed.
- (a) M. H. Li, C. He, F. Chen and Y. L. Gu, Adv. Synth. Catal., 2010, 352, 519 CrossRef CAS; (b) K. Park, Y. Heo and S. Lee, Org. Lett., 2013, 15, 3322 CrossRef CAS; (c) Y. Ohta, Y. Kubota, T. Watabe, H. Chiba, S. Oishi, N. Fujii and H. Ohno, J. Org. Chem., 2009, 74, 6299 CrossRef CAS PubMed; (d) Y. Ohta, H. Chiba, S. Oishi, N. Fujii and H. Ohno, J. Org. Chem., 2009, 74, 7052 CrossRef CAS; (e) K. Natte, W. Li, S. Zhou, H. Neumann and X. F. Wu, Tetrahedron Lett., 2015, 56, 1118 CrossRef CAS; (f) N. Y. T. Mana, W. Lib, S. G. Stewarta and X. F. Wu, Chimia, 2015, 69, 345 CrossRef.
- (a) J. M. Garcia, G. O. Jones and K. Virwani, Science, 2014, 6185, 732 CrossRef; (b) K. Natte, A. Dumrath, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2014, 53, 10090 CrossRef CAS; (c) W. F. Li and X. F. Wu, J. Org. Chem., 2014, 79, 10410 CrossRef CAS.
- (a) M. Y. Ngai, E. Skucas and M. J. Krische, Org. Lett., 2008, 10, 2705 CrossRef CAS; (b) T. Okachi, K. Fujimoto and M. Onaka, Org. Lett., 2002, 4, 1667 CrossRef CAS; (c) K. Köpfer, B. Sam, B. Breit and M. J. Krische, Chem. Sci., 2013, 4, 1876 RSC; (d) W. Li and X. F. Wu, Eur. J. Org. Chem., 2015, 331 CrossRef CAS.
- (a) H. M. Wang, J. H. Cai, H. W. Huang and G. J. Deng, Org. Lett., 2014, 16, 5324 CrossRef CAS; (b) F. Cheng, Y. Peng, J. Wu and G. J. Deng, Org. Biomol. Chem., 2016, 14, 2819 RSC; (c) X. Cheng, H. Wang, X. F. Xiao and G. J. Deng, Green Chem., 2016, 18, 5773 RSC.
- F. Xiao, S. Yuan, H. Huang and G. J. Deng, Synlett, 2018, 29, 2693 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06144d |
| ‡ J. Tian and S. Yuan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |