Open Access Article
Open Access ArticleA hybrid nanoparticle/alkoxide ink for inkjet printing of TiO2: a templating effect to form anatase at 200 °C†
Josh Turnera,
Helen C. Aspinalla,
Simon Rushworthc and
Kate Black *b
*b
aDepartment of Chemistry, University of Liverpool, Liverpool L69 7ZD, UK
bSchool of Engineering, University of Liverpool, Liverpool L69 3GH, UK. E-mail: k.black@liverpool.ac.uk
cEpivalence Ltd., The Wilton Site, Wilton, Redcar TS10 4RF, UK
First published on 28th November 2019
Abstract
A reactive ink (Ink 1) containing Ti(OPri)4 in PriOH with dimethoxyethan as a kinetic stabiliser deposits TiO2 by inkjet printing. A hybrid ink (Ink 2) consists of Ink 1 with the addition of anatase NPs, which act as seeds for the formation of anatase TiO2 at 200 °C. Printing of anatase on PET is also reported.
TiO2, particularly in the form of anatase thin films, continues to attract significant interest, due to applications such as photocatalysis.1 Anatase thin films are typically prepared by sol–gel or chemical vapour deposition (MOCVD or ALD) techniques.2,3 As-deposited thin films from sol–gel synthesis are found to be amorphous and require annealing at >350 °C in order to form anatase. CVD techniques require sophisticated equipment, and although plasma enhanced CVD deposition of anatase at 100 °C has recently been reported,4 most CVD preparations of anatase require either a high substrate temperature (>300 °C) or high temperature annealing step.5
Inkjet printing is an alternative method for thin-film deposition and has the advantages of being both low-cost and low-waste, as well as enabling direct patterning.6,7 A major challenge in inkjet printing is the formulation of a stable ink with the correct chemical and rheological properties. To date, most published methods for inkjet printing of anatase have used colloidal TiO2 solutions.8 As-deposited films are amorphous and require annealing at >375 °C to convert the printed films to anatase.9 Suspensions of pre-formed anatase nanoparticles have also been used as inks; these inks often require the addition of high molecular weight stabilisers in order to keep the nanoparticles in suspension. A high-temperature (>350 °C) processing step is therefore required in order to remove the stabiliser and to sinter the nanoparticles to form a continuous film.10 In some cases a lengthy heat-treatment is required after each printing pass before a final high temperature annealing stage.11
As an alternative to inks containing pre-formed TiO2, we present a series of reactive inks that contain a metalorganic Ti precursor that reacts under ambient laboratory conditions with atmospheric moisture and surface OH groups to form TiO2. The aim of this work is to avoid the need for high temperature treatments and thus to develop a process for printing anatase TiO2 that is compatible with temperature-sensitive, flexible substrates such as polyethylene terephthalate (PET).12,13
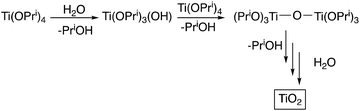
Ti(OPri)4 has been chosen as the reactive component of the ink: as it is readily available at relatively low cost, and has been extensively studied as a precursor for deposition of TiO2 by sol–gel techniques.14 Ti(OPri)4 reacts with H2O (and with Si–OH groups on the surface of a glass substrate) by a sequence of protonolysis/condensation reactions to form TiO2:
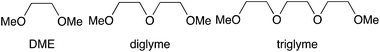
In addition to optimising rheological properties, an ink formulation that is stable enough to have an acceptable shelf-life and not to cause blockages in the printer is required. The ink formulation however needs to be reactive enough to produce TiO2 once printed under ambient conditions. PriOH was chosen as the carrier solvent as it is chemically compatible with Ti(OPri)4 and it also has compatible rheological properties for inkjet printing. However, a solution of Ti(OPri)4 in PriOH reacts within seconds with small traces of H2O to form insoluble TiO2, and this behaviour results in rapid blocking of the printer head. A variety of glycol ethers as kinetic stabilisers has therefore been investigated:
These glycol ethers are Lewis bases with the ability to coordinate to Ti(OPri)4, resulting in kinetic stabilisation by blocking access of H2O to vacant coordination sites at Ti.15 In optimising the ink formulation, we aimed to achieve a printable ink with: a reasonable level of Ti loading, a rate of reaction with ambient atmosphere that is compatible with printing multiple passes in rapid succession, and a convenient level of shelf-stability. Ti(OPri)4 concentrations from 0.05 M to 0.15 M, and glycol ether: Ti(OPri)4 ratios from 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 up to 10
1 up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 have been investigated.
1 have been investigated.
DME was identified as the optimum kinetic stabiliser: as its boiling point (85 °C) is close to that of PriOH (83 °C), resulting in evaporation of both carrier solvent and stabiliser at similar rates post-deposition. The complimentary evaporation rates minimises surface tension driven Marangoni effects due to compositional changes, and results in good uniformity of deposited material.16 The optimised formulation for the stabilised Ti(OPri)4 ink (designated as Ink 1) is given in Table 1. Ageing studies show a 1.34% increase in viscosity of Ink 1 after four weeks stored at room temperature (Fig. 1), indicating good ink stability.
| [Ti(OPri)4]/M | [DME]/M | Density/g cm−3 | Viscosity/mPa s | Surface tension/mN m−1 |
|---|---|---|---|---|
| 0.15 | 1.5 | 0.799 | 1.89 | 20.57 |
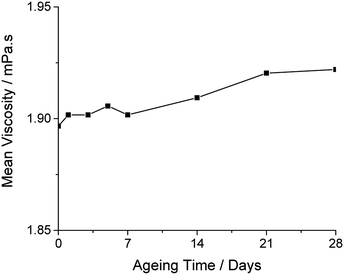 | ||
| Fig. 1 Ageing data of viscosity over time for Ink 1 over a 28 day maturation time held at room temperature. | ||
The waveform generated for pure PriOH is also suitable for Ink 1. The print speed, step width and substrate temperature has been optimised by printing a single pass track onto a glass substrate (see Fig. 2).
 | ||
| Fig. 2 Printed track using Ink 1 at ambient temperature with 10 mm s−1 print speed and 0.1 mm step size. | ||
The Ti loading in Ink 1 (0.15 M) is too low to print a film of viable thickness with a single pass (theoretically ca. 46 nm), and so a 1 cm × 1 cm square using 1 and 5 passes was printed (Fig. 3) along with more complex architectures such as “TiO2”.
Raman and XRD spectroscopy show that the as-deposited films are amorphous and require annealing at 450 °C in order to produce the desired anatase phase as shown in Fig. 4.
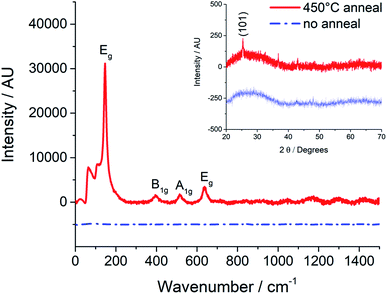 | ||
| Fig. 4 Raman and XRD (inset) spectra of an as deposited amorphous TiO2 film and an anatase film after annealing at 450 °C for 40 minutes (Ink 1 on a glass substrate). | ||
Ink 1 displays the desired shelf-stability (Fig. 1) combined with good printability and an appropriate rate of reaction to form TiO2 under ambient conditions. However, as seen with traditional sol–gel methods, annealing at 450 °C is required in order to form anatase17 (Fig. 4). Note that this processing temperature does not meet the stated aim of printing onto thermally-sensitive, flexible substrates.
Anatase can be deposited by inkjet printing with an ink containing pre-formed anatase nanoparticles (NPs); however in order to form a continuous film using a NP ink, a high temperature sintering step (>350 °C) is required. UV sintering of hybrid NP/titanium(IV) bis(ammonium lactate)dihydroxide ink can be achieved at 150 °C.18 It is reasoned that in a hybrid ink consisting of anatase NPs and reactive Ti(OPri)4, the NPs could act as templates for the formation of anatase on hydrolysis of Ti(OPri)4. This templating effect would obviate the need for a high temperature sintering step. Carboxylic acid functionalized anatase NPs have been chemically cross-linked by reaction with diamines to form thin films.19 The novel aspect of the work presented here, is that a reactive organometallic framework, such as Ti(OPri)4, and anatase NPs have not previously been combined and printed to enable the reduction of post processing temperatures.
The starting point for a hybrid ink is Ink 1, to which anatase NPs (Sigma Aldrich; <25 nm) are added in various proportions (0.5–5 wt%). A 0.1 M Ti concentration of NPs produces an ink with good printability (eg viscosity and surface tension) and is here designated as Ink 2 (see Table 2). Prior to printing, Ink 2 was sonicated for 1 h to give a homogeneous dispersion of NPs (DLS studies show that sonication for > 1 h resulted in formation of aggregates of NPs) and the dispersion remained stable, without the addition of stabilisers, for up to 72 h. Ageing studies showed that after 4 weeks the ink viscosity increased by only 1.55%, indicating good ink stability (Fig. 5). As the inks were sonicated before each measurement, the initial decrease was likely caused by viscosity being measured before the solution had cooled to 20 °C.
| [Ti(OPri)4]/M | [Ti] from NPs/M | [DME]/M | Density/g cm−3 | Viscosity/mPa s | Surface tension/mN m−1 |
|---|---|---|---|---|---|
| 0.15 | 0.1 | 1.5 | 0.804 | 1.90 | 20.70 |
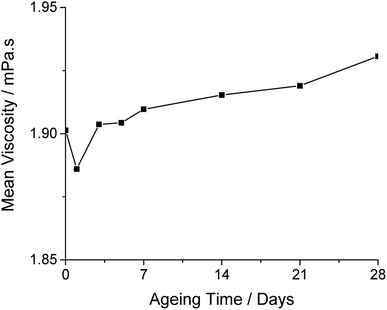 | ||
| Fig. 5 Ageing data of viscosity over time for Ink 2 over a 28 day maturation time held at room temperature. | ||
Ink 2 was printed onto a glass substrate using printing parameters similar to those used for Ink 1. Optical microscopy showed that Ink 2 produced a denser film than Ink 1, due to the increased Ti loading. Profilometry measurements showed that after annealing at 200 °C, a 5-pass print with Ink 2 had a mean thickness of ca. 1600 nm (see S1†).
The main objective for Ink 2 was to form a thin film of anatase without the requirement for high temperature post processing. Raman and XRD spectroscopy (Fig. 6) of a drop-tested film of Ink 2 shows that the as-deposited film contains a small amount of anatase, consistent with anatase NPs in a matrix of amorphous TiO2. However, on annealing at 200 °C for 160 min, there is a marked increase in the Raman and XRD intensities for peaks assigned to anatase (Fig. 6). This annealing temperature is 250 °C lower than that required to form anatase from Ink 1 (Fig. 4). The reduction in annealing temperature is consistent with the NPs acting as templates for the conversion of amorphous TiO2 to anatase.
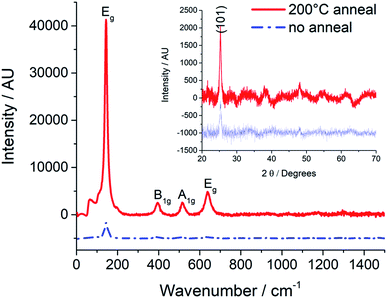 | ||
| Fig. 6 Raman and XRD (inset) spectra of drop-tested sample of Ink 2 (glass substrate) before and after annealing at 200 °C for 160 minutes. | ||
PET is a flexible material with a melting point of 250 °C.20 The annealing temperature of Ink 2 is substantially below the melting temperature of PET and as such represents a step closer to enabling inkjet printing of metal oxides on thermally sensitive, flexible substrates. Single and multiple-pass prints of 1 cm × 1 cm squares directly onto PET without the need for prior treatment of the substrate surface were achieved. A more complex architecture (3-pass print of “TiO2′′) as shown in Fig. 7 was also printed. The prints adhered well to the surface and survived over 10 cycles of bending. Raman spectroscopy of a drop-tested sample of Ink 2 on a PET substrate confirmed that conversion to anatase occurred after annealing at 200 °C for 160 minutes, as observed for Ink 2 on a glass substrate (see S2†). As this is above the glass transition temperature, Tg, of the PET substrate, minor deformation of the substrate was observed. Further reduction in processing temperature, minimising PET deformation, could be achieved by the optimisation of NP to organometallic ratio.
In conclusion, we have demonstrated for the first time that the combination of anatase NPs with a reactive organometallic component, (Ti(OPri)4) gives a novel hybrid ink that enables the inkjet printing of anatase TiO2 onto thermally sensitive flexible substrates. Ti(OPri)4 reacts with ambient atmospheric moisture to form a matrix of amorphous TiO2, and anatase NPs promote conversion of this matrix to anatase at the relatively low temperature of 200 °C.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Nakata and A. Fujishima, TiO2 photocatalysis: Design and applications, J. Photochem. Photobiol., C, 2012, 13(3), 169–189 CrossRef CAS.
- N. Rahimi, R. A. Pax and E. M. Gray, Review of functional titanium oxides. I: TiO2 and its modifications, Prog. Solid State Chem., 2016, 44(3), 86–105 CrossRef CAS.
- U. G. Akpan and B. H. Hameed, The advancements in sol–gel method of doped-TiO2 photocatalysts, Appl. Catal., A, 2010, 375(1), 1–11 CrossRef CAS.
- D. Li, et al., Nanostructure and photocatalytic properties of TiO2 films deposited at low temperature by pulsed PECVD, Appl. Surf. Sci., 2019, 466, 63–69 CrossRef CAS.
- G. Malandrino, Chemical Vapour Deposition. Precursors, Processes and Applications, Angew. Chem., Int. Ed., 2009, 48(41), 7478–7479 CrossRef CAS.
- G. Cummins and M. P. Y. Desmulliez, Inkjet printing of conductive materials: a review, Circuit World, 2012, 38(4), 193–213 CrossRef CAS.
- C. Gadea, D. Marani and V. Esposito, Nucleophilic stabilization of water-based reactive ink for titania-based thin film inkjet printing, J. Phys. Chem. Solids, 2017, 101, 10–17 CrossRef CAS.
- M. Yang, et al., Preparation, characterisation and sensing application of inkjet-printed nanostructured TiO2 photoanode, Sens. Actuators, B, 2010, 147(2), 622–628 CrossRef CAS.
- W. Y. Padron-Hernandez, et al., Stable inks for inkjet printing of TiO2 thin films, Mater. Sci. Semicond. Process., 2018, 81, 75–81 CrossRef CAS.
- M. Arin, et al., Deposition of photocatalytically active TiO2 films by inkjet printing of TiO2 nanoparticle suspensions obtained from microwave-assisted hydrothermal synthesis, Nanotechnology, 2012, 23(16) CrossRef CAS PubMed.
- M. Morozova, et al., Thin TiO2 films prepared by inkjet printing of the reverse micelles sol–gel composition, Sens. Actuators, B, 2011, 160(1), 371–378 CrossRef CAS.
- D. Cairns, et al., P-28: The Effect of Thermal Shrinkage on Indium Tin Oxide Coated Polyethylene Terephthalate for Flexible Display Applications, SID Int. Symp. Dig. Tech. Pap., 2001, 32 Search PubMed.
- X. F. Lu and J. N. Hay, Isothermal crystallization kinetics and melting behaviour of poly(ethylene terephthalate), Polymer, 2001, 42(23), 9423–9431 CrossRef CAS.
- H. Choi, E. Stathatos and D. D. Dionysiou, Sol–gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications, Appl. Catal., B, 2006, 63(1), 60–67 CrossRef CAS.
- B. Fleming and S. Rushworth, Investigation of glyme additives to metalorganic ink systems, J. Sol-Gel Sci. Technol., 2017, 82(1), 308–314 CrossRef CAS.
- C. Marangoni, Sul principio della viscosita’ superficiale dei liquidi stabilito dalsig. J. Plateau., Il Nuovo Cimento, 1871, 5(1), 239–273 CrossRef.
- Y. Djaoued, et al., Study of Anatase to Rutile Phase Transition in Nanocrystalline Titania Films, J. Sol-Gel Sci. Technol., 2002, 24(3), 255–264 CrossRef CAS.
- Y. Oh, et al., UV-Assisted Chemical Sintering of Inkjet-Printed TiO2 Photoelectrodes for Low-Temperature Flexible Dye-Sensitized Solar Cells, J. Electrochem. Soc., 2012, 159(10), H777–H781 CrossRef CAS.
- A. Salmatonidis, et al., Chemical Cross-Linking of Anatase Nanoparticle Thin Films for Enhanced Mechanical Properties, Langmuir, 2018, 34(21), 6109–6116 CrossRef CAS PubMed.
- M.-H. Chen, et al., Preparation of long-chain branched polyethylene terephthalates (PETs), and crystallization behaviors, thermal characteristics, and hydrolysis resistance of their biaxially stretching films, J. Phys. Chem. Solids, 2019, 129, 354–367 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06119c |
| This journal is © The Royal Society of Chemistry 2019 |