Open Access Article
Open Access ArticleWalnut protein isolates attenuate particulate matter-induced lung and cardiac injury in mice and zebra fish
Yuanyuan Zhang†
a,
Mingchuan Liu†b,
Ruiping Fana,
Qianliu Zhoua,
Jinping Yangb,
Shengjie Yangb,
Chaojih Wang*b and
Junping Kou *a
*a
aJiangsu Key Laboratory of TCM Evaluation and Translational Research, School of Traditional Chinese Pharmacy, China Pharmaceutical University, 639 Longmian Road, Nanjing 211198, China. E-mail: junpingkou@cpu.edu.cn; Fax: +86-25-86185158; Tel: +86-25-86185158
bR&D Center, Sinphar Tian-Li Pharmaceutical Co., Ltd., Yuhang Economic & Technological Development Zone, Hangzhou 311100, China. E-mail: zrwang@sinphar.com.tw; Fax: +86 571 8616 8991; Tel: +86 571 8616 8933
First published on 9th December 2019
Abstract
Air pollution is an increasingly serious problem, and the fine particles of air pollution can cause diseases of the respiratory, cardiovascular, and immune systems. Walnut protein isolates (WPIs) are peptides purified from walnut protein hydrolysates that have very high antioxidant and 1,1-diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) scavenging activities. In this study, mice and zebra fish were used to test the effect of WPIs on the acute lung injury (ALI) and heart injury induced by particulate matter (PM). The WPIs protected against ALI in the PM-induced ALI mouse model by inhibiting myeloperoxidase (MPO), nitric oxide (NO), interleukin 1β(IL-1β), and interleukin 6(IL-6) in ALI mouse bronchoalveolar lavage fluid (BALF) and pro-inflammatory cytokine production and acyl carrier protein (ACP) level. In the zebra fish model, the WPIs promoted the secretion of PM into the intestinal tract, protected against the heart injury caused by PM, and promoted the phagocytosis of zebra fish macrophages. Therefore, WPIs are potential candidates to be a health-promoting product with no toxicity.
1. Introduction
Air pollution is an increasingly serious problem, with a report indicating that 2.1 million people worldwide die every year from respiratory damage caused by fine particulate matter (PM).1 Particulate matter has many chemical components, including nitrate, sulfate, carbon in inorganic form, organic compounds (polycyclic aromatic hydrocarbons), biological compounds (endotoxins, cell debris), and metals (such as iron, copper, nickel, zinc, and vanadium).2 Particulate matter is much more harmful to human health than surface ozone. Because PM primarily enters the human body through the respiratory system, the organs of the respiratory system are directly affected.Based on the permeability of PM in the lungs, the EPA classifies PM into two categories: coarse particles (PM10) with an aerodynamic diameter of 10 μm and fine particles (PM2.5) with an aerodynamic diameter of 2.5 μm.3 Of note, recent studies show that the public health hazard from PM remains even when the exposure level of PM is very low (far below the national standard).4
Many epidemiological studies show that PM can cause diseases of the respiratory, cardiovascular, and immune systems. The lungs, as the initial site of fine particulate deposition, are the primary target of fine particulate toxicity. Fine particles can cause acute lung injury, airway inflammation, lung function decline, asthma, and chronic obstructive pulmonary disease occurrence and deterioration.5,6 These particles can induce an inflammatory response, inflammatory cell infiltration, and the release of inflammatory mediators. Inflammatory cytokines can further aggravate lung tissue damage, leading to alveolar collapse.7 The inflammatory response is a process in which the body reacts to stimuli after being harmfully stimulated. The response is activated when immune cells express pathogen-related molecular patterns such as LPS-binding pattern recognition receptors. In addition, other stimulants (such as cytokines) can bind to the plasma membrane or stress signals and activate the inflammatory response. Many studies show that inflammation is associated with PM exposure. According to one report, the binding of bacterial-derived endotoxins to the surface of PM is one of the pathogenic factors in lung injury mediated by PM.8
The components of PM2.5 are complex. After entering the respiratory system, the water-soluble components, some fine particles, and ultrafine particles enter the cardiovascular system through the lung-capillary barrier. On one hand, these components cause direct damage to the lungs by affecting the balance of the sympathetic–parasympathetic systems or by producing cytokines, free radicals, and so on. On the other hand, the cardiovascular system is directly damaged by many components that enter the blood circulation, such as metal ions and free radicals.9 The ultimate manifestation in the cardiovascular system is the result of the superposition of various functions. Currently, inflammation and oxidative stress are accepted as the main mechanisms of cardiovascular system damage caused by atmospheric fine particles.10
Walnut (Juglans regia L.), in the family Juglandaceae, is one of the nuts commonly found in diets.11 Walnuts have antiatherogenic, anti-inflammatory, and antimutagenic properties and antioxidant activities.12,13 Walnut protein isolates (WPIs) are peptides purified from walnut protein hydrolysates that exhibit very high antioxidant and DPPH scavenging activities.14
The hypothesis of this study was tested using the PM-induced ALI mouse model and the heart-injury zebra fish model. The aims of this study was detect whether WPIs could inhibited PM-induced proinflammatory responses in mouse lung tissue cells and improved the recovery of lung tissue damage and heart injury caused by PM.
2. Materials and methods
2.1. Materials
The WPIs were obtained from walnut by using the CCCE process and included high quantities (99.10 and 99.37%) of peptides with molecular weights less than 1500 Da, with most in the range 200–1500 Da (60% at least, according to14). Dexamethasone (DEX) (Cat# 20180302) was purchased from Shenyang Guangda Pharmaceutical Co., Ltd. The MPO kit was obtained from the Jianceng Bioengineering Institute (Nanjing, Jiangsu, China). The enzyme-linked immunosorbent assay (ELISA) kits were purchased from SenBeiJia Biological Technology Co., Ltd. (Nanjing, China) for nitrous oxide (NO), IL-6, and IL-1β. An urban PM, SRM 1648a (PMa), that was collected in St. Louis, MO, USA, was obtained from the National Institute of Standards and Technology (Gaithersburg, MD, USA). The PM was suspended and ultra-sonicated for 30 min in phosphate-buffered saline (PBS) to a final concentration of 25 mg mL−1.152.2. Mice and treatment protocols
The mouse experiment was performed as the ref. 15. The C57 male mice (8 weeks old, 18–22 g) were purchased from the Experimental Animal Center of Yangzhou University (Yangzhou, Jiangsu, China). The welfare of all animals and the experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal Ethics Committee of China Pharmaceutical University approved the protocols. The mice were divided into the following four groups (n = 6): control, PMa, PM + WPIs (100 mg kg−1), and DEX (1 mg kg−1). DEX was used as the positive control because it is the most frequently used anti-inflammatory agent in ALI. A method of intratracheal instillation of PM was performed on the mice, which was modified from a previous report.15 The mice were intraperitoneally injected with 4% chloral hydrate, followed by shaving the hair on the neck and sterilizing the surgical area with 75% alcohol. A knife made a vertical 5 to 10 mm incision to expose the trachea. The anterior wall of the trachea was punctured using a 2 mL syringe needle at a 45° angle. A suspension containing 40 μL (50 mg kg−1) of PM in PBS was slowly instilled intratracheally in the PM groups. The mice received an equal volume of PBS in the control group. Thirty minutes after the PM intratracheal injection, intragastric administration was used for the WPI group, and the DEX group was injected via the tail vein. All mice were euthanized at 24 h post-PM-induction. The bronchoalveolar lavage fluid (BALF) and lung tissues were collected for subsequent experiments.2.3. Histological analyses of mouse lung tissues
The lung tissues were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). Then, an optical microscope (Olympus, Japan) was used to observe the pathological changes in the lung tissues.2.4. Lung wet/dry weight ratio and lung wet/body weight ratio in mice
The right lung was collected, and the wet weight was obtained. Then, the lung was oven-dried at 120 °C for 24 h, and the dry weight was obtained. Lung edema was determined by calculating the lung wet/dry (W/D) weight ratio and lung wet weight/body weight ratio (LW/BW).162.5. MPO assay of mouse lung tissue
The lung tissues were homogenized after collection, and the MPO activity in lung tissues was measured according to the manufacturer's instructions. The enzymatic activity was detected at 460 nm using a microplate spectrophotometer (Tecan, Switzerland).2.6. ELISA assays for IL-1β, IL-6, and NO in mouse lung tissue
The levels of IL-1β, IL-6, and NO were measured using ELISA kits according to the manufacturer's instructions. All measurements were performed in duplicate, and the optical density of each well was read at 450 nm using a microplate spectrophotometer (Tecan, Switzerland).2.7. Evaluation of WPIs in improving cardiovascular injury induced by PM in zebra fish
Ninety wild-type AB strains of zebra fish were randomly selected two days after fertilization (2 dpf) and placed in six-well plates. Thirty zebra fish were treated in each well (experimental groups). Water-soluble PM2.5 was given to evaluate the cardiovascular toxicity of zebra fish. WPIs at the concentration of 100 μg mL−1 were added to the water-soluble solution, and this treatment was compared with the normal control group (zebra fish treated with water for fish farming) and the model control group (PM2.5). The capacity of each well was 3 mL. After 24 h of treatment with PM2.5, 10 zebra fish in each experimental group were randomly selected to count the heart rate under the microscope. Another 10 zebra fish were randomly selected to record a blood flow video under the heartbeat and blood flow analysis system, which was used to analyze and quantify the blood flow velocity. After recording the blood flow video, photographs were taken and data were collected under an anatomical microscope. The cardiac area, which is statistically related to the heart rate and blood flow velocity, was used to evaluate whether the WPIs led to improvement in the cardiovascular toxicity induced by PM2.5.2.8. Evaluation of WPIs in promoting PM secretion in zebra fish
One hundred and fifty zebra fish of the translucent Albino strain with melanin allele mutation were randomly selected two days after fertilization. Thirty zebra fish were treated in each well (experimental groups). A model for evaluating the intestinal function of zebra fish was established by intramuscular injection of PM. WPIs at the concentration of 250 μg mL−1 were added to the water-soluble solution, and the treatment was compared with the normal control group (zebra fish treated with water for fish farming) and the model control group (PM2.5). The capacity of each well was 3 mL. After six days of treatment, the zebra fish were counted that secreted PM into the intestine. The effect of the WPIs on the secretion of PM into the intestine was evaluated according to the statistical significance of the incidence of secretion.2.9. Evaluation of WPIs in promoting macrophage phagocytosis in zebra fish
The method for measuring macrophage phagocytosis in zebra fish was modified as previous report.17 One hundred and fifty zebra fish of the translucent Albino strain with melanin allele mutation were randomly selected two days after fertilization. Thirty zebra fish were treated in each well (experimental groups). The model of zebra fish phagocytosis was established by intravenous injection of PM. WPIs at the concentration of 250 μg mL−1 were added to the water-soluble solution, and the treatment was compared with the normal control group (zebra fish treated with water for fish farming) and the model control group (PM2.5). The capacity of each well was 3 mL. After two days of treatment, zebra fish were stained in vivo with neutral red solution for 16 h, and the number of macrophages that engulfed PM was counted under an anatomical microscope. The effect of WPIs on macrophage phagocytosis was evaluated according to the statistical significance of the number of macrophages that engulfed PM.2.10. Statistical analyses
The data were analyzed by GraphPad Prism (version 5.0) and are expressed as the mean ± standard deviation (SD). Statistical significance was analyzed using the one way ANOVA followed by Tukey post-hoc test. Values of p < 0.05 were considered statistically significant.3. Results
3.1. WPIs improve the lung damage and decrease the ACP activity of macrophages induced by PM in mice
In a pre-experiment, 30, 100, and 300 mg kg−1 WPIs affected injury to the lung. Because no significant difference was detected between the 100 and 300 mg kg−1 groups, the concentration of 100 mg kg−1 was chosen for the study (data not shown). The lung tissue of the control group was pink and moist, and no abnormality was apparent (Fig. 1A), whereas the model group had dark lung tissue with obvious bleeding points, and some areas were black and deteriorated. After the administration of WPIs, the black area of the whole lung decreased, with lungs showing significant improvement. As shown in Fig. 1B, the lung tissue of the control group was composed of alveoli, bronchial branches, blood vessels, and interstitium. The structure was clear, and no congestion in the alveolar wall and no infiltration of inflammatory cells were observed. The model group show the infiltration of inflammatory cells around bronchiole branches in the lung. Neutrophils were the primary type of inflammatory cells, and mononuclear macrophages were observed. A small amount of fibroblast proliferation was observed in the lung tissue, primarily around the bronchi and blood vessels. In the group with WPIs, the degree of infiltration of peribronchitis cells in the group was reduced, differing significantly from that in the model group. As shown in Fig. 1D and E, the ratio of wet to dry weight and the wet to body weight in the model group was significantly higher than that in the control group. As shown in Fig. 1F, the ACP activity in the model group was significantly higher than that in the control group, indicating that fine particulate matter stimulated macrophage phagocytosis and inflammation in lung tissue of mice. The ACP activity of macrophages in the WPI-treated group was significantly lower than that in the model group, suggesting that the activation of macrophages was reduced and that the WPIs could improve the PM-caused injury. The difference between the two groups was significant. With the addition of WPIs, the ratio of wet to dry weight of lung tissue decreased significantly in mice with ALI induced by PM. These results suggest that WPIs can significantly improve the pulmonary edema induced by fine particles in ALI mice.3.2. WPIs ameliorate the lung inflammation factors induced by PM in mice
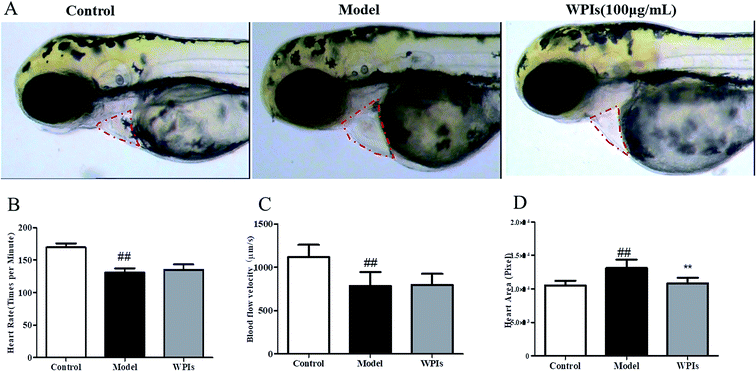
The production of NO and proinflammatory cytokines such as IL-6 and IL-1β is a crucial indicator reflecting the inflammatory process, and an increase in MPO activity in the lung indicates neutrophil tissue infiltration. As shown in Fig. 2, compared with the control group, PM greatly increased the MPO activity in lung tissue and the production of NO, IL-6, and IL-1β in BALF in the model group. However, these increases were significantly inhibited by treatment with WPIs or DEX. As shown in Fig. 2, the levels of MPO, IL-6, IL-1β, and NO in the WPI and DEX groups were significantly lower than those in the model group.3.3. WPIs reduce the cardiac enlargement induced by PM in zebra fish
As shown in Fig. 3B, the heart rate of zebra fish in the model control group (131 beats per min) was significantly lower that of the normal control group (170 beats per min), indicating that the model was successfully established. The heart rate of zebra fish in the 100 μg mL−1 WPI-concentration group was 135 beats per minute, which was an increase in the heart rate of 10%, compared with that in the model control group, suggesting that WPIs could improve the heart rate slowdown caused by PM under the experimental concentration. The blood flow velocity of zebra fish in the model control group (786 m s−1) was compared with that of the normal control group (1120 m s−1) (Fig. 3C), indicating that the model was successfully established. The blood flow velocity of zebra fish in the 100 μg mL−1 WPI-concentration group was 798 m s−1, which was an increase in the blood flow velocity of 4%, compared with that in the model control group, suggesting that WPIs reduced the decrease in the blood flow velocity caused by PM under the experimental concentration. However, the improvement in blood velocity with WPIs was not significant. The heart area of zebra fish in the model control group (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 pixels) was significantly enlarged compared with that of the normal control group (10
121 pixels) was significantly enlarged compared with that of the normal control group (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 pixels), indicating that the model was successfully established (Fig. 3D). The heart area of zebra fish in the 100 μg mL−1 WPI-concentration group was 10
526 pixels), indicating that the model was successfully established (Fig. 3D). The heart area of zebra fish in the 100 μg mL−1 WPI-concentration group was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 849 pixels. Compared with the model control group, the WPIs significantly reduced heart enlargement, an improvement of 88%. Thus, WPIs could decrease the enlargement of the heart caused by PM2.5 under the experimental concentration. Based on these experimental results, the WPIs reduced the cardiac enlargement induced by PM but had no significant effect on the slowdown in heart rate and blood flow induced by PM in zebra fish.
849 pixels. Compared with the model control group, the WPIs significantly reduced heart enlargement, an improvement of 88%. Thus, WPIs could decrease the enlargement of the heart caused by PM2.5 under the experimental concentration. Based on these experimental results, the WPIs reduced the cardiac enlargement induced by PM but had no significant effect on the slowdown in heart rate and blood flow induced by PM in zebra fish.
3.4. WPIs promote the secretion of PM into zebra fish intestines
As shown in Fig. 4B, in the model control group of zebra fish, the tail number of PM secretions was 15/30, and the rate of PM secretion was 50%. The tail number of PM secreted by zebra fish in the 250 μg mL−1 WPI group was 23/30, and the incidence of PM secreted by zebra fish was 76.7%. Compared with the model control group, the tail number of PM secreted by zebra fish in the 250 μg mL−1 WPI group was significantly lower (p < 0.05). This result suggested that WPIs promoted the secretion of PM into the intestinal tract of zebra fish at the experimental concentration. Based on the above experimental results, the WPIs can promote the secretion of PM into zebrafish intestine at the experimental concentration.3.5. WPIs promote macrophage phagocytosis in zebra fish
The number of macrophages in the model control group was 12, as shown in Fig. 5B. By contrast, 20 macrophages were in the 250 μg mL−1 WPI group, an increase of 61% compared with the number in the model control group. This result suggested that WPIs, at the experimental concentration, promoted the phagocytosis of zebra fish macrophages. Under the experimental concentration, the WPIs could promote the phagocytosis of zebrafish macrophages shown in Fig. 5.4. Discussion
In this study, WPIs inhibited the production of pro-inflammatory cytokines in the PM-induced ALI mouse model. DEX was used as the positive control because it is the most frequently used anti-inflammatory agent to treat ALI. In zebra fish, WPIs promoted the secretion of PM into the intestine and protected against heart injury without cardiotoxicity.The impact of PM exposure on human health depends not only on weather, season, topography, source of particulate matter, emission concentration, and microenvironment but also on human physical characteristics (e.g., human respiratory pattern, rate, and volume).18 To date, fine particles with diameters less than 10 μm have been shown to have the greatest impact on human health. These particles can enter deep alveoli from the nasal cavity. Particles between approximately 5 and 10 μm are likely to be deposited in bronchial trees, whereas particles between 1 and 5 μm are deposited in bronchioles and alveoli during gas exchange. Metals may be mediators of PM-induced airway injury and inflammation through the Fenton reaction.19 In addition, the endotoxins in PM related to Gram-negative bacterial contamination are thought to cause airway inflammation and pulmonary dysfunction.20 These endotoxins can be deposited in the trachea, bronchus, and alveoli during breathing, causing an inflammatory reaction of the immune system through mechanical injury and oxidative stress reaction and eventually leading to respiratory diseases. When fine particles enter the blood through the respiratory system, they can also cause hypertension, stroke, and systemic diseases.21 In vivo, fine particles also cause local and systemic inflammation. The number of total cells, macrophages, neutrophils, and lymphocytes, protein content, and inflammatory mediators such as TNF-α and IL-6 in bronchoalveolar lavage fluid increase.22 Simultaneously, the expression of pro-inflammatory cytokines and transcription factors (NF-κB) is upregulated in lung tissue, and macrophages with phagocytic granules are observed.23 The release of chemokines, such as monocyte/macrophage attractant intercellular adhesion molecule-1 and monocyte chemoattractant protein-1, in the blood can promote the infiltration of inflammatory cells into lung tissue. In addition, PM can induce asthmatic airway inflammation by increasing the number of total cells and IL-5, IL-22, eosinophil-activated chemokine 2, and metal lothionein 3 in mouse alveolar lavage fluid.24
Intratracheal instillation is a convenient and effective method used with PM to induce ALI in mice.25 Compared with the models using whole-body inhalation chambers and nasal drip, this model requires one single dose of intratracheal instillation of PM with the features of accuracy and efficiency. Particulate matter is a term used to describe the mixture of solid particles and liquid droplets in the air. The constituents of PM include ammonium, sulfates, nitrates, elemental carbon, other inorganic ions, polycyclic aromatic hydrocarbons, and biological components.3 Many components of PM that enter the blood circulation can cause direct damage to the cardiovascular system, such as metal ions and free radicals.9 Additionally, PM can decrease lung function and increase morbidity and mortality.26 The primary organ that PM attacks is the lung, where PM can be deposited on the surface of alveoli and internalized into lung epithelial cells and alveolar macrophages.27 Particulate matter can also stimulate inflammatory responses, resulting in neutrophil infiltration. The excessive inflammatory responses of PM include autophagy, which cleans up the dead cells but also induces overproduction of inflammatory mediators.28 Acidic phosphatase, ACP, is primarily in lysosomes and is considered as a marker of macrophages. The activity of acid phosphatase in macrophages reflects the degree of macrophage activation.29 In this study, WPIs protected against the ALI induced by PM in mice (Fig. 1).
Pulmonary edema is measured using the lung W/D weight ratio.30 The results indicated that WPIs decreased the PM-induced lung W/D weight ratio, which suggests that WPIs can alleviate pulmonary edema. The MPO level can be used to determine polymorphonuclear leukocyte activation and oxidation stress as NO production.31 An essential character of PM-induced ALI is the release of inflammatory mediators, including IL-6 and IL-1β oxidation molecules, which can expand the inflammatory cascade and increase neutrophil transfer into the alveoli and damage the lung.32 In this study, WPIs were used to treat PM-stimulated mice, and the levels of MPO, NO, and the pro-inflammatory cytokines IL-6 and IL-1β in BALF significantly decreased compared with those in the untreated mice samples (Fig. 2).
The zebra fish has been widely used in developmental biology, toxicology, genetics, and pharmacology because the fish is small, easy to cultivate and manage, and has a short reproductive cycle; in vitro fertilization and development are possible; and the embryos are easily observed and treated.33,34 The components of PM2.5 are complex and enter the cardiovascular system through the lung-capillary barrier. Currently, the toxicity screening research and application of drugs resulting from this research primarily focus on neurotoxicity, cardiotoxicity, embryonic developmental toxicity, and so on. The cardiotoxicity of many traditional Chinese medicines and their active components has not been clearly defined, which limits the research and development of new drugs. The zebra fish, as a reproducible model for screening drug toxicity in vivo, is of great value in the research and development of new drugs. In this study, WPIs reduced the cardiac enlargement induced by PM2.5 but did not alleviate the slowdown in heart rate and blood flow velocity induced by PM2.5. Compared with the model control group, WPIs protected against the heart injury caused by PM2.5 under the experimental concentration (Fig. 3). In addition, WPIs promoted the secretion of PM into the intestinal tract of zebra fish at the concentration of 250 μg mL−1 (Fig. 4).
Macrophages are a major component of the innate immune system, responding efficiently to tissue damage and infection. During infection, macrophages have diverse roles, including phagocytosis of foreign bodies, release of cytotoxic factors, and coordination of the inflammatory response via the secretion of chemokines and cytokines.35 In addition, the interactions between immune cells and alveolar epithelial cells and the release of epidermal growth factor receptor (EGFR) ligands are involved in pro-inflammatory and repair responses. The interaction between these cytokines and their receptors will lead to bronchial remodeling, bronchial wall thickening, and pulmonary fibrosis.36 Particulate matter can induce inflammation and oxidative stress in macrophages in a variety of ways, including increasing the expression levels of inflammatory interleukin (IL)-1, cyclooxygenase-2, and heme oxygenase-1 genes in mouse macrophages.37,38 Zebra fish should be a suitable predictive screening model to assess macrophages in vivo.39 In this study, WPIs were confirmed to promote the phagocytosis of zebra fish macrophages at the experimental concentration (Fig. 5).
Walnut (Juglans regia L.) is the most widespread tree nut worldwide. The biological activities of walnut include antiatherogenic and antioxidant activity and anti-inflammatory and anti-mutagenic properties.40,41 The health benefits of walnut are usually attributed to their chemical composition, which includes polyphenols, flavones, polysaccharides, aminophenols, minerals, and so on.42–44 The peptides from walnut protein hydrolysates exhibited very high antioxidant activities and show angiotensin-converting enzyme inhibitor activity.45,46 The WPIs show antioxidant activity.14
Overall, in this study, the antioxidant activity of WPIs inhibited pro-inflammatory cytokine production in the PM-induced ALI mouse model. In addition, WPIs promoted the secretion of PM into the zebra fish intestine without cardiotoxicity.
5. Conclusions
Overall, the findings of this study provide pharmacological evidence supporting the protective effect of WPIs in treating PM-induced ALI in mice and heart injury in zebra fish. However, further studies are needed to explore additional aspects of WPI use, including the mechanism of the protective activity of WPIs on PM-induced ALI in the mouse model and the toxicity of WPIs. First, the safety of WPIs in other animal needed to be investigated in future studies. Second, the mechanism of WPIs on PM-induced ALI mouse model requires further study. This study supports new prospects for WPI development, and therefore, WPIs may be potential candidates for healthy products.Abbreviations
| WPIs | Walnut protein isolates |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl |
| ALI | Acute lung injury |
| PM | Particulate matter |
| MPO | Myeloperoxidase |
| NO | Nitric oxide |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| BALF | Bronchoalveolar lavage fluid |
| ACP | Acyl carrier protein |
| CCCE | Continuous countercurrent extraction |
| ELISA | Enzyme-linked immunosorbent assay |
| Dex | Dexamethasone |
Availability of data and material
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.Conflicts of interest
None.References
- S. Feng, D. Gao, F. Liao, F. Zhou and X. Wang, Ecotoxicol. Environ. Saf., 2016, 128, 67–74 CrossRef CAS.
- C. Song, L. Wu, Y. Xie, J. He, X. Chen, T. Wang, Y. Lin, T. Jin, A. Wang, Y. Liu, Q. Dai, B. Liu, Y. N. Wang and H. Mao, Environ. Pollut., 2017, 227, 334–347 CrossRef CAS.
- Z. Li, Q. Wen and R. Zhang, Sci. Total Environ., 2017, 586, 610–622 CrossRef CAS.
- K. H. Kim, E. Kabir and S. Kabir, Environ. Int., 2015, 74, 136–143 CrossRef CAS PubMed.
- Y. Shi, T. Zhao, X. Yang, B. Sun, Y. Li, J. Duan and Z. Sun, Sci. Total Environ., 2019, 650, 908–921 CrossRef CAS PubMed.
- X. Y. Gu, X. Chu, X. L. Zeng, H. R. Bao and X. J. Liu, Environ. Pollut., 2017, 226, 163–173 CrossRef CAS.
- J. Shi, R. Chen, C. Yang, Z. Lin, J. Cai, Y. Xia, C. Wang, H. Li, N. Johnson, X. Xu, Z. Zhao and H. Kan, Environ. Res., 2016, 150, 264–268 CrossRef CAS.
- W. Yue, L. Tong, X. Liu, X. Weng, X. Chen, D. Wang, S. C. Dudley, E. K. Weir, W. Ding, Z. Lu, Y. Xu and Y. Chen, Redox Biol., 2019, 22, 101161 CrossRef CAS.
- J. Duan, H. Hu, Y. Zhang, L. Feng, Y. Shi, M. R. Miller and Z. Sun, Chemosphere, 2017, 180, 24–32 CrossRef CAS PubMed.
- R. Li, X. Kou, H. Geng, J. Xie, J. Tian, Z. Cai and C. Dong, J. Hazard. Mater., 2015, 287, 392–401 CrossRef CAS PubMed.
- C. Calcabrini, R. De Bellis, U. Mancini, L. Cucchiarini, V. Stocchi and L. Potenza, Plant Foods Hum. Nutr., 2017, 72, 192–197 CrossRef CAS PubMed.
- K. J. Anderson, S. S. Teuber, A. Gobeille, P. Cremin, A. L. Waterhouse and F. M. Steinberg, J. Nutr., 2001, 131, 2837–2842 CrossRef CAS PubMed.
- I. Oliveira, A. Sousa, I. C. Ferreira, A. Bento, L. Estevinho and J. A. Pereira, Food Chem. Toxicol., 2008, 46, 2326–2331 CrossRef CAS PubMed.
- M. C. Liu, S. J. Yang, D. Hong, J. P. Yang, M. Liu, Y. Lin, C. H. Huang and C. J. Wang, Chem. Cent. J., 2016, 10, 39 CrossRef PubMed.
- Y. Xia, S. Dolgor, S. Jiang, R. Fan, Y. Wang, Y. Wang, J. Tang, Y. Zhang, R. L. He, B. Yu and J. Kou, Biomed. Pharmacother., 2018, 108, 906–913 CrossRef CAS PubMed.
- J. C. Parker and M. I. Townsley, Am. J. Physiol., 2004, 286, L231–L246 CAS.
- J. Wei, T. Zhou, Z. Hu, Y. Li, H. Yuan, K. Zhao, H. Zhang and C. Liu, Chemosphere, 2018, 210, 93–101 CrossRef CAS PubMed.
- J. S. Brown, T. Gordon, O. Price and B. Asgharian, Part. Fibre Toxicol., 2013, 10, 12 CrossRef.
- S. Pattanaik, F. E. Huggins and G. P. Huffman, Sci. Total Environ., 2016, 562, 898–905 CrossRef CAS.
- Y. Yoda, K. Tamura and M. Shima, Indoor Air, 2017, 27, 955–964 CrossRef CAS PubMed.
- A. S. Shah, J. P. Langrish, H. Nair, D. A. McAllister, A. L. Hunter, K. Donaldson, D. E. Newby and N. L. Mills, Lancet, 2013, 382, 1039–1048 CrossRef CAS.
- B. Altemose, M. G. Robson, H. M. Kipen, P. Ohman Strickland, Q. Meng, J. Gong, W. Huang, G. Wang, D. Q. Rich, T. Zhu and J. Zhang, J. Exposure Sci. Environ. Epidemiol., 2017, 27, 244–250 CrossRef CAS.
- C. Zhao, J. Liao, W. Chu, S. Wang, T. Yang, Y. Tao and G. Wang, Inhalation Toxicol., 2012, 24, 918–927 CrossRef CAS PubMed.
- K. Ogino, K. Nagaoka, T. Okuda, A. Oka, M. Kubo, E. Eguchi and Y. Fujikura, Environ. Toxicol., 2017, 32, 1047–1054 CrossRef CAS PubMed.
- X. C. Xu, Y. F. Wu, J. S. Zhou, H. P. Chen, Y. Wang, Z. Y. Li, Y. Zhao, H. H. Shen and Z. H. Chen, Toxicol. Lett., 2017, 280, 206–212 CrossRef CAS PubMed.
- A. Faustini, M. Stafoggia, G. Cappai and F. Forastiere, Epidemiology, 2012, 23, 861–879 CrossRef PubMed.
- Q. Zhao, H. Chen, T. Yang, W. Rui, F. Liu, F. Zhang, Y. Zhao and W. Ding, Biochim. Biophys. Acta, Gen. Subj., 2016, 1860, 2835–2843 CrossRef CAS PubMed.
- Z. H. Chen, Y. F. Wu, P. L. Wang, Y. P. Wu, Z. Y. Li, Y. Zhao, J. S. Zhou, C. Zhu, C. Cao, Y. Y. Mao, F. Xu, B. B. Wang, S. A. Cormier, S. M. Ying, W. Li and H. H. Shen, Autophagy, 2016, 12, 297–311 CrossRef CAS PubMed.
- K. Song, Y. Yang, L. Xu, J. Tian, J. Fan, Z. Jiao, S. Feng, H. Wang, Y. Wang, L. Wang and T. Liu, Mater. Sci. Eng., C, 2016, 62, 787–794 CrossRef CAS PubMed.
- W. Jiang, F. Luo, Q. Lu, J. Liu, P. Li, X. Wang, Y. Fu, K. Hao, T. Yan and X. Ding, Chem.-Biol. Interact., 2016, 243, 127–134 CrossRef CAS PubMed.
- R. Blondonnet, J. M. Constantin, V. Sapin and M. Jabaudon, Dis. Markers, 2016, 2016, 3501373 Search PubMed.
- L. Z. Gu, H. Sun and J. H. Chen, Biomed. Pharmacother., 2017, 85, 756–762 CrossRef CAS PubMed.
- L. H. J. Richter, J. Herrmann, A. Andreas, Y. M. Park, L. Wagmann, V. Flockerzi, R. Muller and M. R. Meyer, Toxicol. Lett., 2019, 305, 73–80 CrossRef CAS.
- F. Cao, C. L. Souders 2nd, P. Li, O. Adamovsky, S. Pang, L. Qiu and C. J. Martyniuk, Chemosphere, 2019, 214, 303–313 CrossRef CAS PubMed.
- N. Yoshida, E. M. Frickel and S. Mostowy, Front. Immunol., 2017, 8, 1703 CrossRef PubMed.
- M. He, T. Ichinose, S. Yoshida, T. Ito, C. He, Y. Yoshida, K. Arashidani, H. Takano, G. Sun and T. Shibamoto, J. Appl. Toxicol., 2017, 37, 1203–1218 CrossRef CAS PubMed.
- K. Bekki, T. Ito, Y. Yoshida, C. He, K. Arashidani, M. He, G. Sun, Y. Zeng, H. Sone, N. Kunugita and T. Ichinose, Environ. Toxicol. Pharmacol., 2016, 45, 362–369 CrossRef CAS.
- S. Liu, W. Zhang, F. Zhang, P. Roepstorff, F. Yang, Z. Lu and W. Ding, Int. J. Environ. Res. Public Health, 2018, 16(1), E98 CrossRef PubMed.
- S. Sieber, P. Grossen, P. Uhl, P. Detampel, W. Mier, D. Witzigmann and J. Huwyler, Nanomedicine, 2019, 17, 82–93 CrossRef CAS PubMed.
- R. Jahanbani, S. M. Ghaffari, M. Salami, K. Vahdati, H. Sepehri, N. N. Sarvestani, N. Sheibani and A. A. Moosavi-Movahedi, Plant Foods Hum. Nutr., 2016, 71, 402–409 CrossRef CAS.
- D. Ren, F. Zhao, C. Liu, J. Wang, Y. Guo, J. Liu and W. Min, J. Sci. Food Agric., 2018, 98, 5142–5152 CrossRef CAS.
- D. Labuckas, D. Maestri and A. Lamarque, Nat. Prod. Commun., 2016, 11, 637–640 CrossRef.
- M. H. Zhao, Z. T. Jiang, T. Liu and R. Li, Sci. World J., 2014, 2014, 303878 Search PubMed.
- W. Ruijun, W. Shi, X. Yijun, T. Mengwuliji, Z. Lijuan and W. Yumin, Int. J. Biol. Macromol., 2015, 72, 771–775 CrossRef CAS PubMed.
- N. Chen, H. Yang, Y. Sun, J. Niu and S. Liu, Peptides, 2012, 38, 344–349 CrossRef CAS PubMed.
- N. Aissaoui, F. Abidi, J. Hardouin, Z. Abdelkafi, N. Marrakchi, T. Jouenne and M. N. Marzouki, Biotechnol. Appl. Biochem., 2017, 64, 201–210 CrossRef CAS PubMed.
Footnote |
| † Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |