Open Access Article
Open Access ArticleCombining a ligand photogenerator and a Ru precatalyst: a photoinduced approach to cross-linked ROMP polymer films†
Thi Kim Hoang Trinhab,
Gautier Schrodjab,
Séverinne Rigoletab,
Julien Pinaud c,
Patrick Lacroix-Desmazes
c,
Patrick Lacroix-Desmazes c,
Loic Pichavant
c,
Loic Pichavant d,
Valérie Héroguez
d,
Valérie Héroguez d and
Abraham Chemtob
d and
Abraham Chemtob *ab
*ab
aInstitut de Science des Matériaux de Mulhouse, IS2M, UMR 7361 CNRS, Université de Haute-Alsace, Mulhouse, France. E-mail: abraham.chemtob@uha.fr
bUniversité de Strasbourg, Strasbourg, France
cICGM, Université de Montpellier, CNRS, ENSCM, Montpellier, France
dUniversité de Bordeaux, CNRS, Bordeaux INP, LCPO, UMR 5629, F-33600, Pessac, France
First published on 4th September 2019
Abstract
Although metathesis photoinduced catalysis is now well established, there is little development in thin film preparation using photochemically activated ring-opening metathesis polymerization (ROMP). Herein, a N-heterocyclic carbene (NHC) photogenerator (1,3-bis(mesityl)imidazolium tetraphenylborate) is combined with an inactive metathesis catalyst ([RuCl2(p-cymene)]2) to generate under UV irradiation an active catalyst (p-cymene)RuCl2 (NHC), that is capable of producing in a single step cross-linked copolymer films by ROMP of norbornene with dicyclopentadiene. The study shows that the photoinitiated catalytic system can be optimized by increasing the yield of photogenerated NHC through a sensitizer (2-isopropylthioxanthone), and by choosing [RuI2(p-cymene)]2 as precatalyst to provide a long-term photolatency. The cross-linked polymer structure is investigated by a range of techniques including gel content measurement, FT-IR and solid-state 13C NMR spectroscopy, TGA and DSC, which reveal a cross-linking mechanism proceeding through both metathesis and olefin coupling.
Introduction
As one of the most valuable tools for the generation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, olefin metathesis has been exploited in many useful ways in the field of polymers, including the synthesis of complex architectures,1 pharmaceutical products,2 or biodegradable copolymers.3 In all these cases, progress has been mainly driven by the development of advanced and well-defined metal alkylidene complexes, like the notorious Grubbs and Schrock catalysts.4,5 Ligand fine-tuning has resulted in a portfolio of catalysts featuring a broad range of reactivity, functional group tolerance and bench stability. In this respect, a major breakthrough took place in 1999 with the introduction of N-heterocyclic carbene (NHC) ligands in the place of the conventional phosphine compounds.6,7 In particular, sterically bulky and strong electron donating NHCs have proven to be highly effective, resulting in NHC-coordinated ruthenium complexes with longer life-time and superior catalytic activity.6,8 However, the main developments in olefin metathesis catalysis have responded more to the needs of synthetic chemistry (as the above mentioned examples show), but have been less useful for the preparation of polymer materials. Ring-opening metathesis polymerization (ROMP) of cyclic olefins is currently the main type of olefin metathesis polymerizations.6,9 Polymers with a broad spectrum of properties have been achieved through ROMP of norbornene (NB), cyclopentene, cyclooctene or dicyclopentadiene (DCPD). However, the use of ROMP polymers has been limited so far to the manufacturing of specific structural and engineering materials. For example, polycyclooctene (Vestenamer®) and polyNB (Norsorex®) elastomers are employed after vulcanization as shock absorbers and anti-grip materials. In addition, hydrogenated polyNB (Zeonex®) is a high performance optical thermoplastic, while polyDCPD (Metton® or Telene®) is known as a prime thermoset with mechanical properties similar to engineering thermoplastics.10 One reason for the limited applications of these polymers can be found in their restrictive processing methods. For example, polyDCPD is prepared essentially by reaction injection molding (RIM), which requires separate monomer and catalyst compartments, efficient mixing and titration heads, nitrogen inerting of the mold, etc.11–13
C double bonds, olefin metathesis has been exploited in many useful ways in the field of polymers, including the synthesis of complex architectures,1 pharmaceutical products,2 or biodegradable copolymers.3 In all these cases, progress has been mainly driven by the development of advanced and well-defined metal alkylidene complexes, like the notorious Grubbs and Schrock catalysts.4,5 Ligand fine-tuning has resulted in a portfolio of catalysts featuring a broad range of reactivity, functional group tolerance and bench stability. In this respect, a major breakthrough took place in 1999 with the introduction of N-heterocyclic carbene (NHC) ligands in the place of the conventional phosphine compounds.6,7 In particular, sterically bulky and strong electron donating NHCs have proven to be highly effective, resulting in NHC-coordinated ruthenium complexes with longer life-time and superior catalytic activity.6,8 However, the main developments in olefin metathesis catalysis have responded more to the needs of synthetic chemistry (as the above mentioned examples show), but have been less useful for the preparation of polymer materials. Ring-opening metathesis polymerization (ROMP) of cyclic olefins is currently the main type of olefin metathesis polymerizations.6,9 Polymers with a broad spectrum of properties have been achieved through ROMP of norbornene (NB), cyclopentene, cyclooctene or dicyclopentadiene (DCPD). However, the use of ROMP polymers has been limited so far to the manufacturing of specific structural and engineering materials. For example, polycyclooctene (Vestenamer®) and polyNB (Norsorex®) elastomers are employed after vulcanization as shock absorbers and anti-grip materials. In addition, hydrogenated polyNB (Zeonex®) is a high performance optical thermoplastic, while polyDCPD (Metton® or Telene®) is known as a prime thermoset with mechanical properties similar to engineering thermoplastics.10 One reason for the limited applications of these polymers can be found in their restrictive processing methods. For example, polyDCPD is prepared essentially by reaction injection molding (RIM), which requires separate monomer and catalyst compartments, efficient mixing and titration heads, nitrogen inerting of the mold, etc.11–13
To unlock the potential of ROMP polymer materials, more efficient and “smarter” catalysts are crucial, especially to extend the shaping of ROMP polymers as film, particle or fiber, but also, to foster more versatile, energy-efficient and facile process conditions.14–20 To address those challenges, latent olefin metathesis catalysts have emerged that are able to release active species in situ and “on demand” by external stimulation. Heat,14–16 mechanical force,17 and radiation18–20 are the most common stimuli. A separate category concerns catalysts activated after the addition of an external chemical agent.21–23 Among these different options, the activation brought about by visible or ultraviolet irradiation is particularly attractive.24 Firstly, it allows activation of the catalyst at ambient temperature, and the use of storable and ready-to-use formulations combining together the catalyst and the monomer(s). Secondly, as a standard product of a photoactivated polymerization, thin films based on cross-linked ROMP polymer make possible the development of novel UV-curable coatings or adhesives. Thirdly, unlike the other triggers, light allows the reaction to be spatially-controlled, opening the way to applications in photolithography.23,25
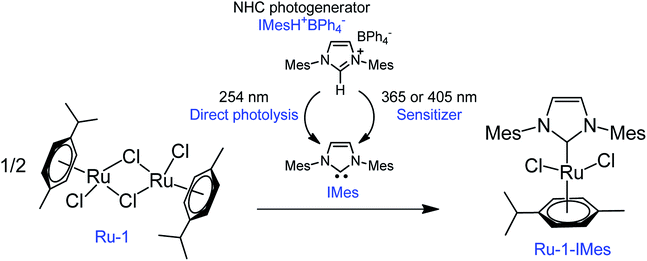
In the field of photochemically activated olefin metathesis, three main approaches have been proposed to promote activation of the catalyst that all involve the photochemical reaction of one ligand of a transition metal complex.13 The two most reported routes rely on ligand photodissociation26,27 and ligand cis-to-trans photoisomerization.18,20 The third route, which is the least investigated, is the focus of the present study. In the latter, the active olefin metathesis catalyst is produced from an inactive and non-absorbing precatalyst after reaction with a ligand generated in situ by a second photosensitive compound (ligand photogenerator). This methodology was first introduced by Grubbs et al. in 2009 by using a Ru–alkylidene complex ligated by acetylacetonate (acac) as precatalyst and a photoacid generator (PAG) based on triphenylsulfonium chloride.21 Upon irradiation at 254 nm, HCl was released, and the subsequent displacement of acac by Cl− resulted in an active metathesis catalyst. A similar method was also employed by Piers et al. using a ruthenium carbide and Ph3S triflate (OTf).22 Recently, we replaced the PAG with a NHC photogenerator and used a stable, commercially available dimeric Ru(II) complex as metathesis inactive catalyst (Scheme 1).28,29 Our tandem system made up of 1,3-bis(mesityl)imidazolium tetraphenylborate IMesH+BPh4− (1) as NHC progenitor and [RuCl2(p-cymene)]2 precatalyst (Ru-1) proved to be very efficient at ROMP of NB. In comparison with the examples discussed above, a safer UVA radiation (320–400 nm) was employed, and no ligand displacement occurred. The cleavage of the Ru-chlorido bridges of Ru-1 was rather enthalpically driven through the addition of 2 equivalents of free NHC IMes, that were photogenerated in situ from 1. As a result, the presumed active catalyst (Ru-1-IMes) features one p-cymene cycle, two chlorido ligands and one IMes ligand. First introduced by Hermann et al.,30 this class of RuCl2(p-cymene)NHC catalyst has been extensively studied by Noels et al. for the ROMP of NB and cyclooctene.31–33
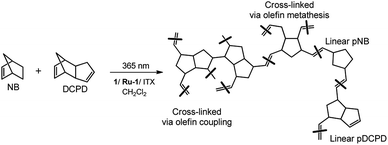
In this contribution, the main objective is to demonstrate the utility of this tandem approach for the photoinduced preparation of cross-linked ROMP polymer films. To this purpose, we report first our efforts to improve the photocatalytic system by addressing a number of issues: optimizing the yield of photogenerated IMes, ensuring a long-term photolatency, identifying the active metathesis catalyst and the initiating species, and finally, collecting some preliminary results on photoROMP in solution of common cyclic olefin monomers such as NB. In a second part, a range of copolymer films was prepared by photoinduced ROMP copolymerization between NB and DCPD using the photocatalytic mixture based on 1/ITX/Ru-1. The coatings were prepared in a single step from a one-pot formulation, which is close to the conditions of a conventional UV-curing process. The cross-linked copolymer structure was thoroughly investigated by gel content measurement, FT-IR and solid-state 13C NMR spectroscopy. The thermal properties of these polymer films were also studied through TGA and DSC.
Experimental
Materials
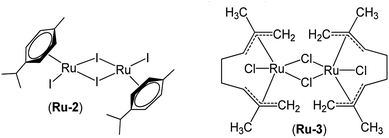
Dicyclopentadiene (DCPD, >97%, TCI), 2-norbornene (NB, >99%, TCI), 5-ethylidene-2-norbornene (ENB, >98%, TCI), vinyl ethyl ether (>98%, TCI), 2-isopropylthioxanthone (ITX, analytical standard, Aldrich), 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene (IMes, 97%, Aldrich) and 1,3-di-p-tolylcarbodiimide (CDI, 96%, Aldrich) were used as received without purification. 1,3-bis(2,4,6-trimethylphenyl)imidazolium tetraphenylborate (IMesH+BPh4−, 1) was prepared following a procedure published elsewhere.28 Dichloro(p-cymene)ruthenium(II) dimer (Ru-1, 98%), diiodo(p-cymene)ruthenium(II) dimer (Ru-2, 98%) and dichlorodi-μ-chlorobis[1,2,3,6,7,8-η-2,7-dimethyl-2,6-octadiene-1,8-diyl] diruthenium(IV) (Ru-3, 98%) were bought from Strem Chemicals. Dichloromethane-d2 (CD2Cl2, 99.5% D, Eurisotop), tetrahydrofuran-d8 (THF-d8, 99.5% D, Eurisotop) and other HPLC grade solvents such as ethanol, acetonitrile and tetrahydrofuran (THF) received from VWR were distilled and stored over molecular sieve (4 Å) under nitrogen atmosphere before use.Syntheses and procedures
Characterization methods
All 1H-NMR spectra were recorded in appropriate deuterated solvents with tetramethylsilane (TMS) as the internal reference on a Varian 300. All chemical resonances were documented in parts per million (ppm) relative to the residual THF-d8 (δ 1.72 ppm and 3.58 ppm), and CD2Cl2 (δ 5.32 ppm). Peaks multiplicities in 1H-NMR spectra were abbreviated as s (single), d (double), t (triplet), m (multiplet), br (broad).Molecular weights of polyNB and polyENB were determined using a size exclusion chromatography (SEC) instrument from a Varian apparatus equipped with TOSOHAAS TSK gel columns and a refractive index detector. The mobile phase was THF at a flow rate of 1 mL min−1; the injected volume was 100 μL. The MALS instrument was normalized using a THF solution of polystyrene standard.
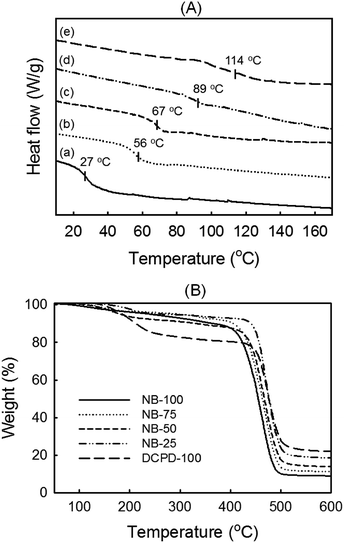
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) experiments were performed using, respectively, a TGA/DSC3+ (Metter Toledo) and a DSC1 (Metter Toledo) under nitrogen atmosphere. For TGA analysis, the samples (8–15 mg) were heated from 30 °C to 600 °C at a heating rate of 10 °C min−1. For DSC analysis, the samples (3 mg) were scanned from – 80 °C to 250 °C at a heating rate of 10 °C min−1. The glass transition temperatures were determined in the second run.
To determine gel content, a polymer specimen (∼50 mg) was placed into a 5 mL vial containing 4 mL of CH2Cl2. After magnetic stirring for 24 h at room temperature, the solvent was removed and insoluble component was dried under vacuum at 40 °C until weight constant. The gel content was finally measured.
13C CP MAS NMR spectra were recorded by using an Advance II 400 MHz Bruker Spectrometer (Ascend™ magnet) (B0 = 9.4 T) operating at 100.64 MHz and controlled by TOPSPIN 3.2 software. The solid films were grounded into fine powders prior to measurement. Experiments were performed at ambient temperature with a 4 mm double resonance MAS Bruker probe at a speed of 12 kHz, a 13C π/2 pulse duration of 5.6 μs, a contact time of 1 ms and a recycle delay of 2 s. Chemical shifts reported thereafter are relative to tetramethylsilane and the deconvolution of the experimental spectra were carried out with the DMfit software.34 The fraction of pendant cyclopentene rings involved in olefin coupling reaction can be determined semi-quantitatively from the 13C CP MAS NMR spectrum. DCPD contains initially 4sp2 and 6sp3 carbons. The ratio of sp2-C to sp3-C does not change when cyclopentene reacts by ROMP. Conversely, olefin coupling leads to the replacement of 2sp2-C by 2sp3-C. This change can be used to estimate the fraction of cyclopentene reacting via olefin coupling (x) in polyDCPD film (eqn (1)):35
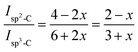 | (1) |
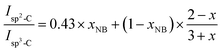 | (2) |
 in polyNB (calculated from sample NB-100), and xNB is the molar fraction of NB in the NB/DCPD monomer mixture.
in polyNB (calculated from sample NB-100), and xNB is the molar fraction of NB in the NB/DCPD monomer mixture.
FTIR spectra were recorded with a Nicolet 8700 spectrophotometer (Thermo Scientific) using a mercury cadmium telluride detector. Two modes were used, transmission for monitoring the real-time kinetics of photoROMP in solution and attenuated total reflectance (ATR) for characterization of polymer films. All the spectra were recorded in the range 4000–600 cm−1. The real-time measurements used a scanning velocity of 11.45 cm s−1 corresponding to a resolution of 0.964 cm−1. Monomer conversion (Conv.) was determined grounded on the changing of absorption area At at 1566 cm−1 (NB, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch), 3047 cm−1 (DCPD, sp2 = CH) (eqn (3)):
C stretch), 3047 cm−1 (DCPD, sp2 = CH) (eqn (3)):
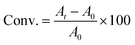 | (3) |
Results and discussion
Study and optimization of the tandem photocatalytic system
| Entry | NHC photogenerator | Irradiation wavelengtha (nm) | IMes yieldb (%) |
|---|---|---|---|
| a Irradiances values: 65 mW cm−2 (365 nm), 100 mW cm−2 (405 nm) and 75 mW cm−2 (254 nm).b NHC yield was obtained indirectly after addition of 1,3-di-p-tolylcarbodiimide (CDI) ([CDI] = 0.03 M, 1 equiv.) to the as-irradiated medium. The stable IMes-CDI adduct is formed, which can be precisely quantified by 1H-NMR.29 | |||
| a | 1 | 254 | 11 |
| b | 1/ITX | 365 | 67 |
| c | 1/ITX | 405 | 29 |
Having proved the aptitude of 1 to act as NHC precursor under irradiation (in combination with a sensitizer), we studied the subsequent reaction of IMes with [RuCl2(p-cymene)]2 dimer Ru-1 to form an active ROMP catalyst.28,29 For this, we performed a control experiment in which Ru-1 was reacted with free IMes in THF-d8 at room temperature. An orange solution formed spontaneously, and subsequent 1H-NMR analysis (performed without prior isolation) established that the expected catalyst structure (p-cymene)RuCl2(IMes) was formed in high yield (see Fig. S1 in ESI†). The spectrum found was indeed in full agreement with previous literature data.40 This suggests that Ru-1-IMes could be actually the active catalytic species when the tandem system NHC photogenerator/Ru precatalyst is irradiated. However, all attempts to characterize by 1H-NMR the photogenerated active catalyst have failed, preventing to confirm our hypothesis. The spectra of the catalytic reaction mixture 1/Ru-1/ITX show after irradiation too many signals, preventing the unequivocal identification of the chelated NHC–Ru complex (see Fig. S2 in ESI†).
| Entry | Precatalyst | Pre-catalyst/1/ITX (equiv.) | Conv.a (%) | σcisb | Mn-thc (kg mol−1) | Mn-SECd (kg mol−1) | Đd |
|---|---|---|---|---|---|---|---|
a Determined by 1H-NMR.b Calculated from 1H-NMR spectrum.c Theoretical number average molar mass 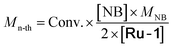 .d Experimental number average molar mass Mn-th obtained by SEC.e Irradiation at 405 nm, 4.7 mW cm−2, 1 min. n.d. means not determined due to the absence of reaction or the formation of an insoluble polymer. .d Experimental number average molar mass Mn-th obtained by SEC.e Irradiation at 405 nm, 4.7 mW cm−2, 1 min. n.d. means not determined due to the absence of reaction or the formation of an insoluble polymer. |
|||||||
| a | Ru-1 | 0/5/2.5 | 0 | n.d | n.d | n.d | n.d |
| b | Ru-1 | 1/0/0 | 1 | 9 | 0.36 | n.d. | n.d. |
| c | Ru-1 | 1/0/2.5 | 7 | 23 | 1.60 | n.d. | n.d. |
| d | Ru-1 | 1/5/2.5 | 78 | 52 | 18.69 | 146.8 | 3.02 |
| e | Ru-1 | 1/5/5 | 94 | 52 | 22.57 | 108.4 | 1.96 |
| fe | Ru-1 | 1/5/2.5 | 22 | 65 | 5.27 | 63.4 | 2.05 |
| g | Ru-2 | 1/5/2.5 | 69 | 52 | 16.43 | 447.2 | 1.59 |
| h | Ru-3 | 1/5/2.5 | 67 | 48 | 16.10 | n.d. | n.d. |
A much higher catalytic activity was achieved in presence of 1 (entry d) with 78% conversion being reached after 1 min. This suggests the successful formation of a highly active Ru-arene complex, which is likely to be Ru-1-IMes. The content of cis double bond (σcis = 52%) was similar to that obtained with previous systems and supports this assumption.41,47 Upon increasing the proportion of ITX from 2.5 equiv. to 5 equiv., a significant increase of conversion was noted (94%, entry e) which correlates with a higher amount of NHC released and thus of generated Ru-1-IMes. This result is also confirmed by the lower molecular weight (Mn-SEC) of the resulting polymer using 5 equiv. of ITX as compared to the one obtained with 2.5 equiv. In contrast, irradiation at 405 nm instead of 365 nm led to a much lower conversion (22%, entry f). This can be rationalized on the basis of a lower IMes yield at higher excitation wavelength (vide supra). For the polymer produced with the tandem approach precatalyst/NHC (entries d–f), the molecular weight distributions were monomodal (SEC data), suggesting that only one mechanism is involved in the catalysis. However, high molecular weights (Mn-SEC) and rather broad polydispersities (Đ) were obtained, which reflect a poor control of the initiation step. The source of the problem is the ill-defined initiation mechanism and above all, the poor initiation efficiency resulting in only a small number of propagating centers produced in solution. This issue has been widely reported with such class of NHC-coordinated Ru complex.31,32,48,49 The effect of the precatalyst structure was also examined. Upon replacing Ru-1 by Ru-2, the rate of polymerization slightly decreased (69%, entry g). By the same reasoning used above to explain latency, it was expected that catalyst activity would decrease from Cl to I. In this case, there are consequently less backbiting reactions, resulting in polymer with a higher molecular weight and a fairly narrow dispersity index. Ru-3 precatalyst (entry h) led to similar conversions, but the polymer was not soluble in THF, indicating a very high molecular weight polymer and an even lower initiation efficiency. It can be concluded that Ru-1 achieves the best results in terms of NB conversion, but lacks of latency in presence of this monomer. Conversely, Ru-2 exhibits a much better photolatency but at the expense of decreasing reactivity. Our photocatalytic system was also active in the ROMP of ENB. The results are summarized in Table S1.†
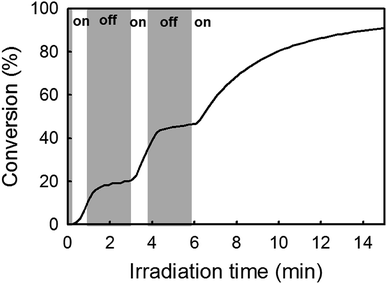 | ||
| Fig. 1 Conversion–time curve for the ROMP of NB in solution in CH2Cl2: light off (grey area), light on (white area). NB/Ru-1/1/ITX = 510/1/5/0.5 equiv. Irradiation conditions: LED@365 nm, 65 mW cm−2. | ||
Based on this observation, we propose a putative reaction mechanism (Scheme 3) for the ROMP photoinitiation. First, the active complex RuCl2(arene)(NHC) Ru-1-IMes is subjected to the rapid loss of p-cymene ring upon photochemical activation to form an unsaturated Ru species A.33,41,50 As a subsequent step, the insertion of NB into the metal center occurs to form the Ru complex RuCl2(IMes)(NB) B. Then a sigmatropic [1,2-H] rearrangement leads to the formation of a ruthenium–alkylidene complex C (initiating species).51 The next steps follow the typical chain-growth polymerization mechanism involving the metallacyclization and cycloreversion steps, with the particularity that the insertion of new cyclic olefin molecules could be facilitated by light.
Preparation of cross-linked ROMP polymer films
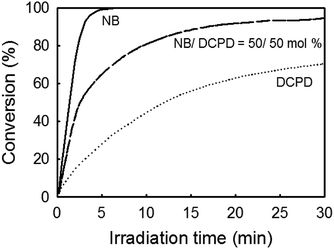 | ||
| Fig. 2 Photopolymerization kinetic of NB, DCPD and NB/DCPD (50/50 mol%) using photocatalyst system Ru-1/1/ITX in CH2Cl2 (Monomer/Ru-1/1/ITX = 510/1/5/2.5 equiv.). | ||
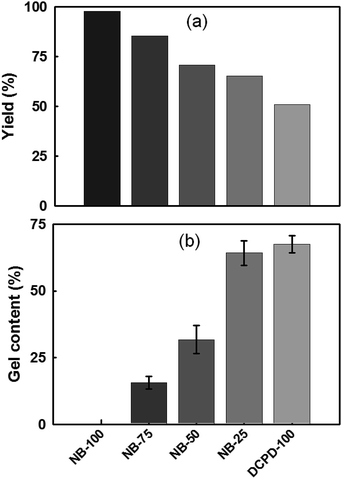
Given the potential of this approach, a range of different (co)polymer films were prepared from a mixture of NB and DCPD (NB-25: 25/75 mol%; NB-50: 50/50 mol% and NB-75: 75/25 mol%). For comparison, two control experiments using NB (NB-100) and DCPD (DCPD-100) alone were also performed under similar conditions. As can be seen in Fig. 3, a mixture of monomer and photocatalyst dissolved in CH2Cl2 was placed in a Teflon mold, covered with a glass slide to avoid solvent or monomer evaporation, and irradiated for 1 h under a LED panel (365 nm, 5.5 mW cm−2) in order to reach a high monomer conversion. The swollen polymer films were then dried at ambient conditions, placed under vacuum, then characterized using a range of spectroscopic techniques (FTIR-ATR, 13C CP MAS), gravimetry, gel content, DSC and TGA. All these techniques are intended to give insight into the structure of the polymer, and in particular the possibility of cross-linking. With regard to this last point, Scheme 4 shows the various possible structures arising from the copolymerization of DCPD with NB. Because the cyclopentene-derived double bond is much less reactive than the norbornenyl double-bond, we assume that the two monomers DCPD and NB could copolymerize first, resulting in the formation of a linear backbone. The cyclopentene-derived double bonds can react subsequently to form a cross-linked network. It has been established that the pendant cyclopentene ring from DCPD monomer may react via two possible pathways: ROMP and olefin addition through radical coupling.35,56,57
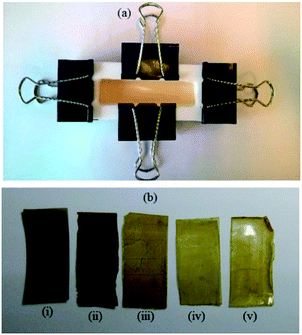 | ||
| Fig. 3 Photographs of: (a) general set up for the preparation of cross-linked films and (b) polymeric films (i) NB-100, (ii) NB-75, (iii) NB-50, (iv) NB25 and (v) DCPD-100. | ||
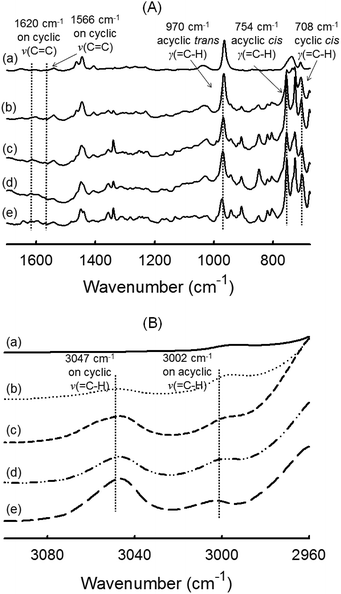
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of norbornenyl groups (present indistinctly in NB or DCPD).28,60 This suggests that norbornenyl rings were either completely reacted during the ROMP or removed upon drying (unreacted monomer). Because cyclopentene has a lower ring strain than NB, the frequency of the C
C stretching vibration of norbornenyl groups (present indistinctly in NB or DCPD).28,60 This suggests that norbornenyl rings were either completely reacted during the ROMP or removed upon drying (unreacted monomer). Because cyclopentene has a lower ring strain than NB, the frequency of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration is decreased and generally arises at 1610–1650 cm−1. The ν(C
C stretching vibration is decreased and generally arises at 1610–1650 cm−1. The ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of the internal double bond of cyclopentene (which is anyway a weakly intense band) is not visible in our system regardless of DCPD content. To estimate the amount of residual cyclopentene rings, the specific out-of-plane deformation vibration at ∼710 cm−1 was considered. Clearly visible at 708 cm−1, its presence supports an incomplete cross-linking process.57 In addition, the signals at 970 cm−1 and 754 cm−1 were attributed to the deformation vibrations of
C) of the internal double bond of cyclopentene (which is anyway a weakly intense band) is not visible in our system regardless of DCPD content. To estimate the amount of residual cyclopentene rings, the specific out-of-plane deformation vibration at ∼710 cm−1 was considered. Clearly visible at 708 cm−1, its presence supports an incomplete cross-linking process.57 In addition, the signals at 970 cm−1 and 754 cm−1 were attributed to the deformation vibrations of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bonds on trans and cis acyclic double bonds, respectively.57,61 They indicate that the chain backbone is made up of a mixture of both trans- and cis-disubstituted alkene –(CH
C–H bonds on trans and cis acyclic double bonds, respectively.57,61 They indicate that the chain backbone is made up of a mixture of both trans- and cis-disubstituted alkene –(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)– in accordance with a ROMP mechanism. The
CH)– in accordance with a ROMP mechanism. The ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching region at 3000–3100 cm−1 is also informative because it is not overlapped by alkane C–H stretching absorption occurring below 3000 cm−1. As shown in Fig. 5B, two broad signals at 3047 cm−1 and 3002 cm−1 were found in the spectra of polymer films containing DCPD, that are readily ascribed to the
C–H stretching region at 3000–3100 cm−1 is also informative because it is not overlapped by alkane C–H stretching absorption occurring below 3000 cm−1. As shown in Fig. 5B, two broad signals at 3047 cm−1 and 3002 cm−1 were found in the spectra of polymer films containing DCPD, that are readily ascribed to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching of –CH
C–H stretching of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– vinylene groups. The band at 3047 cm−1 can be associated to cyclic olefins (residual cyclopentene) while the second signal at 3002 cm−1 reflects acyclic olefin (cyclopentene reacted by ROMP).35,60,62 In conclusion, the FTIR data of DCPD-based films show a partial cross-linking by reaction of the cyclopentene ring, which occurs through a metathesis mechanism. The possibility of cross-linking via olefin coupling of the cyclopentene ring is examined in the next section that deals with the use of 13C solid state NMR to characterize the polymer films.
CH– vinylene groups. The band at 3047 cm−1 can be associated to cyclic olefins (residual cyclopentene) while the second signal at 3002 cm−1 reflects acyclic olefin (cyclopentene reacted by ROMP).35,60,62 In conclusion, the FTIR data of DCPD-based films show a partial cross-linking by reaction of the cyclopentene ring, which occurs through a metathesis mechanism. The possibility of cross-linking via olefin coupling of the cyclopentene ring is examined in the next section that deals with the use of 13C solid state NMR to characterize the polymer films.
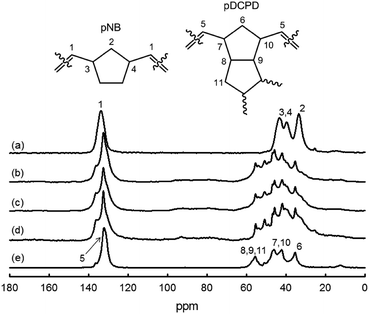
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of cyclopentene ring can undergo either metathesis or olefin coupling reactions. Of interest is that 13C CP MAS NMR can be used to estimate the contribution of olefin coupling. Indeed, occurrence of olefin coupling is manifested by the conversion of two sp2 carbons (from cyclopentene) into two sp3 carbons. Consequently, the ratio of integrated area of sp2-C and sp3-C can give access to the fraction of cyclopentene involved in olefin coupling (see Experimental section for calculation details).35,63 Our calculations reveal a significant contribution of olefin coupling which ranges from 11 to 34%. Olefin coupling of cyclopentene ring tends to increase with a higher concentration of NB: 11% (DCPD-100) <22% (NB-25) <26% (NB-50) <34% (NB-75). This trend can be rationalized on the basis that ROMP of NB is known to be highly exothermic and that olefin coupling is activated by heat.56
C double bonds of cyclopentene ring can undergo either metathesis or olefin coupling reactions. Of interest is that 13C CP MAS NMR can be used to estimate the contribution of olefin coupling. Indeed, occurrence of olefin coupling is manifested by the conversion of two sp2 carbons (from cyclopentene) into two sp3 carbons. Consequently, the ratio of integrated area of sp2-C and sp3-C can give access to the fraction of cyclopentene involved in olefin coupling (see Experimental section for calculation details).35,63 Our calculations reveal a significant contribution of olefin coupling which ranges from 11 to 34%. Olefin coupling of cyclopentene ring tends to increase with a higher concentration of NB: 11% (DCPD-100) <22% (NB-25) <26% (NB-50) <34% (NB-75). This trend can be rationalized on the basis that ROMP of NB is known to be highly exothermic and that olefin coupling is activated by heat.56
Conclusions
We have demonstrated that cross-linked ROMP polymer films can be produced under UV irradiation through the copolymerization of NB with DCPD. The approach for photoactivated olefin metathesis used a NHC photogenerator (1/ITX) combined with an inactive Ru precatalyst (Ru-1, Ru-2 and Ru-3). An active NHC-coordinated Ru-arene complex was presumed to form by reaction between the ruthenium precatalyst and the photogenerated NHC IMes. Irradiation at 365 nm was also proved to have a critical role in the formation of the active metathesis species by promoting the decoordination of the p-cymene ligand and the multiple insertions of new NB units into the propagating centre. The films were homogeneous, and the possibility of cross-linking was attested by the measurement of gel contents (10–70%) and the rising of Tg value when increasing DCPD loading. FTIR data showed a complete conversion of norbornenyl group in both monomers, but an incomplete ring-opening of the cyclopentene pendant group from DCPD. This lower ring-strained cyclic olefin is subjected to metathesis but also to olefin coupling reactions as evidenced by 13C solid-state NMR. We anticipate that photoROMP of NB and DCPD derivatives will enable the development of new cross-linked coatings with chemical, thermal and mechanical properties currently not attainable with conventional radical photopolymerization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by French National Research Agency (ANR program: DS0304 2016, contract number: ANR-16-CE07-0016) is gratefully acknowledged. We are grateful to Dr Olivier Soppera, Dr Julien Poly, Dr Aissam Airoudj and Mr Stephan Knopf for their support in analysis of results.Notes and references
- J. B. Matson and R. H. Grubbs, J. Am. Chem. Soc., 2008, 130, 6731–6733 CrossRef CAS PubMed
.
- X. Bantreil and S. P. Nolan, Nat. Protoc., 2011, 6, 69–77 CrossRef CAS PubMed
.
- K. J. Arrington, J. B. Waugh, S. C. Radzinski and J. B. Matson, Macromolecules, 2017, 50, 4180–4187 CrossRef CAS
.
- P. Schwab, M. B. France, J. W. Ziller and R. H. Grubbs, Angew. Chem., Int. Ed., 1995, 34, 2039–2041 CrossRef CAS
.
- R. R. Schrock, J. S. Murdzek, G. C. Bazan, J. Robbins, M. DiMare and M. O'Regan, J. Am. Chem. Soc., 1990, 112, 3875–3886 CrossRef CAS
.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953–956 CrossRef CAS PubMed
.
- J. Huang, E. D. Stevens, S. P. Nolan and J. L. Petersen, J. Am. Chem. Soc., 1999, 121, 2674–2678 CrossRef CAS
.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS
.
- C. W. Bielawski and R. H. Grubbs, Angew. Chem., Int. Ed., 2000, 39, 2903–2906 CrossRef CAS
.
- W. A. Green, J. Mol. Catal. A: Chem., 2004, 213, 39–45 CrossRef
.
- Z. Yao, L. Zhou, B. Dai and K. Cao, J. Appl. Polym. Sci., 2012, 125, 2489–2493 CrossRef CAS
.
- J. Asrar, J. Appl. Polym. Sci., 1993, 47, 289–293 CrossRef CAS
.
- A. E. Goetz and A. J. Boydston, J. Am. Chem. Soc., 2015, 137, 7572–7575 CrossRef CAS PubMed
.
- A. Ben-Asuly, E. Tzur, C. E. Diesendruck, M. Sigalov, I. Goldberg and N. G. Lemcoff, Organometallics, 2008, 27, 811–813 CrossRef CAS
.
- C. Slugovc, D. Burtscher, F. Stelzer and K. Mereiter, Organometallics, 2005, 24, 2255–2258 CrossRef CAS
.
- M. Barbasiewicz, A. Szadkowska, R. Bujok and K. Grela, Organometallics, 2006, 25, 3599–3604 CrossRef CAS
.
- A. Piermattei, S. Karthikeyan and R. P. Sijbesma, Nat. Chem., 2009, 1, 133–137 CrossRef CAS PubMed
.
- A. Ben-Asuly, A. Aharoni, C. E. Diesendruck, Y. Vidavsky, I. Goldberg, B. F. Straub and N. G. Lemcoff, Organometallics, 2009, 28, 4652–4655 CrossRef CAS
.
- A. Aharoni, Y. Vidavsky, C. E. Diesendruck, A. Ben-Asuly, I. Goldberg and N. G. Lemcoff, Organometallics, 2011, 30, 1607–1615 CrossRef CAS
.
- Y. Ginzburg, A. Anaby, Y. Vidavsky, C. E. Diesendruck, A. Ben-Asuly, I. Goldberg and N. G. Lemcoff, Organometallics, 2011, 30, 3430–3437 CrossRef CAS
.
- B. K. Keitz and R. H. Grubbs, J. Am. Chem. Soc., 2009, 131, 2038–2039 CrossRef CAS PubMed
.
- A. Y. Khalimon, E. M. Leitao and W. E. Piers, Organometallics, 2012, 31, 5634–5637 CrossRef CAS
.
- C. Theunissen, M. A. Ashley and T. Rovis, J. Am. Chem. Soc., 2019, 141, 6791–6796 CrossRef CAS PubMed
.
- O. Eivgi and N. Lemcoff, Synthesis, 2018, 50, 49–63 CrossRef CAS
.
- R. A. Weitekamp, H.
A. Atwater and R. H. Grubbs, J. Am. Chem. Soc., 2013, 135, 16817–16820 CrossRef CAS PubMed
.
- D. Wang, K. Wurst, W. Knolle, U. Decker, L. Prager, S. Naumov and M. R. Buchmeiser, Angew. Chem., Int. Ed., 2008, 47, 3267–3270 CrossRef CAS PubMed
.
- D. Wang, K. Wurst and M. R. Buchmeiser, Chem.–Eur. J., 2010, 16, 12928–12934 CrossRef CAS PubMed
.
- J. Pinaud, T. K. H. Trinh, D. Sauvanier, E. Placet, S. Songsee, P. Lacroix-Desmazes, J.-M. Becht, B. Tarablsi, J. Lalevée, L. Pichavant, V. Héroguez and A. Chemtob, Chem.–Eur. J., 2018, 24, 337–341 CrossRef CAS PubMed
.
- T. K. H. Trinh, J.-P. Malval, F. Morlet-Savary, J. Pinaud, P. Lacroix-Desmazes, C. Reibel, V. Héroguez and A. Chemtob, Chem.–Eur. J., 2019, 25, 9242–9252 CAS
.
- W. A. Herrmann, M. Elison, J. Fischer, C. Köcher and G. R. J. Artus, Chem.–Eur. J., 1996, 2, 772–780 CrossRef CAS
.
- A. Tudose, A. Demonceau and L. Delaude, J. Organomet. Chem., 2006, 691, 5356–5365 CrossRef CAS
.
- L. Delaude, M. Szypa, A. Demonceau and A. F. Noels, Adv. Synth. Catal., 2002, 344, 749 CrossRef CAS
.
- L. Delaude, A. Demonceau and A. F. Noels, Chem. Commun., 2001, 986–987 RSC
.
- D. Massiot, F. Fayon, M. Capron, I. King, S. L. Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS
.
- D. P. Mohite, S. Mahadik-Khanolkar, H. Luo, H. Lu, C. Sotiriou-Leventis and N. Leventis, Soft Matter, 2013, 9, 1516–1530 RSC
.
- J. D. Wilkey and G. B. Schuster, J. Org. Chem., 1987, 52, 2117–2122 CrossRef CAS
.
- X. Sun, J. P. Gao and Z. Y. Wang, J. Am. Chem. Soc., 2008, 130, 8130–8131 CrossRef CAS PubMed
.
- X. Sun, Development of tetraphenylborate-based photobase generators and sacrificial polycarbonates for radiation curing and photoresist applications, PhD thesis, Carleton University, 2009
.
- A. V. Zhukhovitskiy, J. Geng and J. A. Johnson, Chem.–Eur. J., 2015, 21, 5685–5688 CrossRef CAS PubMed
.
- L. Jafarpour, J. Huang, E. D. Stevens and S. P. Nolan, Organometallics, 1999, 18, 3760–3763 CrossRef CAS
.
- Y. Zhang, D. Wang, P. Lönnecke, T. Scherzer and M. R. Buchmeiser, Macromol. Symp., 2006, 236, 30–37 CrossRef CAS
.
- A. Hafner, A. Mühlebach and P. A. van der Schaaf, Angew. Chem., Int. Ed., 1997, 36, 2121–2124 CrossRef CAS
.
- E. L. Dias, S. T. Nguyen and R. H. Grubbs, J. Am. Chem. Soc., 1997, 119, 3887–3897 CrossRef CAS
.
- M. S. Sanford, J. A. Love and R. H. Grubbs, J. Am. Chem. Soc., 2001, 123, 6543–6554 CrossRef CAS PubMed
.
- L. Delaude, A. Demonceau and A. F. Noels, Macromolecules, 2003, 36, 1446–1456 CrossRef CAS
.
- A. Demonceau, A. F. Noels, E. Saive and A. J. Hubert, J. Mol. Catal., 1992, 76, 123–132 CrossRef CAS
.
- L. Delaude, A. Demonceau and A. F. Noels, Curr. Org. Chem., 2006, 10, 203–215 CAS
.
- M. Ahr, C. Thieuleux, C. Copéret, B. Fenet and J.-M. Basset, Adv. Synth. Catal., 2007, 349, 1587–1591 CrossRef CAS
.
- P. Alvarez, J. Gimeno and E. Lastra, Organometallics, 2002, 21, 5678–5680 CrossRef CAS
.
- C. S. Day and D. E. Fogg, Organometallics, 2018, 37, 4551–4555 CrossRef CAS
.
- D. Wang, J. Unold, M. Bubrin, W. Frey, W. Kaim and M. R. Buchmeiser, ChemCatChem, 2012, 4, 1808–1812 CrossRef CAS
.
- S. Saha, Y. Ginzburg, I. Rozenberg, O. Iliashevsky, A. Ben-Asuly and N. Gabriel Lemcoff, Polym. Chem., 2016, 7, 3071–3075 RSC
.
- J. Chen, F. P. Burns, M. G. Moffitt and J. E. Wulff, ACS Omega, 2016, 1, 532–540 CrossRef CAS PubMed
.
- G. Yang, T. C. Mauldin and J. K. Lee, RSC Adv., 2015, 5, 59120–59130 RSC
.
- K. Nomura and X. Hou, Dalton Trans., 2016, 46, 12–24 RSC
.
- T. A. Davidson, K. B. Wagener and D. B. Priddy, Macromolecules, 1996, 29, 786–788 CrossRef CAS
.
- G. Raptopoulos, G. C. Anyfantis, D. Chriti and P. Paraskevopoulou, Inorg. Chim. Acta, 2017, 460, 69–76 CrossRef CAS
.
- S. J. Cantrill, R. H. Grubbs, D. Lanari, K. C.-F. Leung, A. Nelson, K. G. Poulin-Kerstien, S. P. Smidt, J. F. Stoddart and D. A. Tirrell, Org. Lett., 2005, 7, 4213–4216 CrossRef CAS PubMed
.
- L. Gong, K. Liu, E. Ou, F. Xu, Y. Lu, Z. Wang, T. Gao, Z. Yang and W. Xu, RSC Adv., 2015, 5, 26185–26188 RSC
.
- Q. Sun, S. Ma, Z. Ge and Y. Luo, High Perform. Polym., 2017, 29, 931–936 CrossRef CAS
.
- M. J. Abadie, M. Dimonie, C. Couve and V. Dragutan, Eur. Polym. J., 2000, 36, 1213–1219 CrossRef CAS
.
- S. Saha, Y. Ginzburg, I. Rozenberg, O. Iliashevsky, A. Ben-Asuly and N. Gabriel Lemcoff, Polym. Chem., 2016, 7, 3071–3075 RSC
.
- A. Bang, D. Mohite, A. M. Saeed, N. Leventis and C. Sotiriou-Leventis, J. Sol-Gel Sci. Technol., 2015, 75, 460–474 CrossRef CAS
.
- R. F. Ohm and T. M. Vial, J. Elastomers Plast., 1978, 10, 150–162 CrossRef CAS
.
- A. M. Spring, D. Maeda, M. Ozawa, K. Odoi, F. Qiu, K. Yamamoto and S. Yokoyama, Polym. Bull., 2015, 72, 503–521 CrossRef CAS
.
- M. R. Kessler and S. R. White, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2373–2383 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05831a |
| This journal is © The Royal Society of Chemistry 2019 |