 Open Access Article
Open Access ArticleA closed-loop electrogenerative recycling process for recovery of silver from a diluted cyanide solution†
Faiz Bukhari
Mohd Suah
 *ab,
Bee Ping
Teh
a,
Nadia
Mansor
a,
Hairul Hisham
Hamzah
*ab,
Bee Ping
Teh
a,
Nadia
Mansor
a,
Hairul Hisham
Hamzah
 a and
Norita
Mohamed
a
a and
Norita
Mohamed
a
aElectrogenerative Research Unit, School of Chemical Sciences, Universiti Sains Malaysia, 11800 Minden, Pulau Pinang, Malaysia. E-mail: fsuah@usm.my
bDepartment of Chemistry, Imperial College London, Exhibition Road, London SW7 2AZ, UK. E-mail: f.mohd-suah@imperial.ac.uk
First published on 7th October 2019
Abstract
A closed-loop process for the complete recovery of silver from a diluted silver cyanide solution has been constructed based on an electrogenerative process. It was shown that the reduction of silver was a mass transport controlled process. Under optimal experimental conditions, 100% of silver was recovered from 500 mg L−1 and 100 mg L−1 silver cyanide solutions by using a reticulated vitreous electrode (RVC) as the cathode. The cyanide solution was recycled and reused so that a closed-loop process was obtained. In addition, the RVC in this study can be used repeatedly up to 10 cycles with a calculated relative standard deviation of 1.90%.
Introduction
Silver is one of the precious metals and has been used as jewellery, coins, electrical goods and as catalysts in chemical reactions. In addition, its compounds are also used as disinfectants and in photographic films. Among hydrometallurgy processes, cyanide leaching is the most common treatments of silver ores.1 Later, the solutions undergo a separation process, which is removing the metallic species. Normally, this process uses activated carbon or ionic exchange resins. Finally, cementation and electrolysis processes are carried out to recover the metallic species.1In recent developments, interest in using porous electrodes in cementation and electrolysis processes has gained special attention.2–4 This is because porous electrodes have high specific area.4–8 In addition, three-dimensional (3D) electrodes serve as the ideal electrode materials for electrowinning and cementation processes. This is due to their special characteristics such as large surface area, chemical inertness, excellent electrical conductivity, high mechanical resistance and high area/volume ratios.9 These characteristics allow operations that produce relatively high current per unit of cell volume.10,11 Some authors have reported the use of 3D electrodes to improve the overall electrochemical performance in which the electrode overpotential is controlled by mass transport phenomena.11–13 However, all of these treatments (known as electrolytic cells) require power consumption and a high cost of the treatment process, especially for dilute sample solutions. In addition, dilute solutions of silver ion have low conductivities and side reactions tend to occur, thus reducing the efficiency of this system.14–16
To overcome this problem, an electrogenerative process can be introduced. In this process, an electrochemical reaction occurs spontaneously in a divided cell, in which the more electropositive catholyte ions are reduced and deposited at the cathode while the more electronegative metal anode is oxidized. This reaction then generates an external flow of current. Conventional treatment methods are eliminated in this electrogenerative process, thereby reducing the operational cost and waste. The electrogenerative process can be operated in numerous types of configurations such as a batch cell, flow cell or recycle batch cell.17
Here, an electrogenerative process has been utilized to recover silver from a simulated silver cyanide solution. A batch cell with an improved design which uses 3D cathodes is coupled with a two-dimensional (2D) anode, zinc, is applied. To emphasize here, two novel approaches are presented in this study. First, the full recovery of silver from a cyanide solution using an electrogenerative process. To the best of our knowledge, this approach has never been reported to date. This process also eliminates additional processes needed to recover silver from diluted concentrations such as preconcentration and precipitation. Second, this process eliminates the production of sludge or waste associated with the treatment of electronic waste because the process could fully recover the silver and cyanide solution and cathode electrodes utilized in this system are reusable. Used cyanide solution was determined according to American Public Health Association (APHA).18 The production of waste can be avoided by operating in a closed-loop process in which the cyanide solution is recycled.
In this study, the electrogenerative process was operated and the performance of the system using various concentrations of simulated silver cyanide solutions, cell conditions, different types of cathode materials and cathode conditions were evaluated. This process does not require an external supply of energy such as power supply or potentiostat due to the spontaneous redox reactions. Such a process is desirable due to its low cost, especially when dealing with diluted solutions. The selectivity and kinetics for particular reactions can be regulated by making suitable choices of cathode electrodes and also by controlling the potential. The overall cell potential is positive, and this confirms that the reaction is a spontaneous reaction. The electrochemical reactions in this process are presented below:
| Cathode: 2Ag(CN)2−(aq) + 2e− → 2Ag(s) + 4CN−(aq) E° = −0.31 V | (1) |
| Anode: Zn(s) + 4CN−(aq) → Zn(CN)42−(aq) + 2e−E° = +1.25 V | (2) |
| Overall: 2Ag(CN)2−(aq) + Zn(s) → 2Ag(s) + Zn(CN)42−(aq) E° = +0.94 V | (3) |
Silver recovery from the cyanide solution
It is known that cyanide ions are hydrolysed in water to produce molecular hydrogen cyanide (HCN), according to the equation below:| CN−(aq) + H2O(l) ⇌ HCN(g) + OH−(aq) | (4) |
The HCN is a weak acid with Ka = 4.89 × 10−10, which dissociates in aqueous solutions:
| HCN(aq) ⇌ H+(aq) + CN−(aq) | (5) |
There are three main cathodic reactions that possibly take place in the catholyte compartment containing basic silver cyanide solution. Besides the silver deposition process, oxygen reduction (eqn (6)) and water reduction (eqn (7)) reactions are also likely to occur:
| O2(g) + 2H2O(l) + 4e− ⇌ 4OH−(aq) E° = +0.40 V | (6) |
| 2H2O(l) + 2e− ⇌ H2(g) + 2OH−(aq) E° = −0.83 V | (7) |
According to Pletcher and Walsh,19 oxygen reduction is the most favourable reaction, followed by silver reduction and water reduction processes. This is because of the thermodynamic condition of the reactions. Therefore, dissolved O2 must be removed from the catholytes before and during experiments to increase the current efficiency and enhance the deposition of silver on the cathode.
As can be seen in eqn (1), both cyanide ion and silver ion complex concentrations influence the equilibrium potential for the silver deposition reaction. While at the anode, the reactions that occur are the formation of tetracyanozincate ion (eqn (2)), followed by cyanide oxidation (eqn (8)):
| CN− + 2OH− ⇌ CNO− + H2O + 2e− | (8) |
In this electrogenerative process, cell electromotive force provides the driving potential for the deposition process to occur at the cathode. In a controlled short-circuit operation, zinc ions are released at the anode to form zinc cyanide complex and electrons are transferred to the cathode via the external circuit. The electrons are utilized by the silver cyanide complex for deposition of silver to occur onto the surface of the cathode. All of the redox reactions are spontaneous and self-driven.
Optimum pH
The electrogenerative process was carried out to recover silver from the silver cyanide solution with different cathode materials (RVC and PG). The initial pH value of both catholyte and anolyte were between pH 10 and 11, but it then increased to pH 12 at the end of each experiment. Such increases were the results of side reactions (cyanide oxidation and water reduction) that occur in the cell throughout the experiment. Therefore, all experiments were performed at pH ≥ 10 to prevent HCN gas from being released.Results and discussion
Effect of cathode materials, initial concentrations and N2 gas purging
From Fig. 1, it can be observed that the recovery rates depend on the type of cathode materials used. For both concentrations of silver cyanide (500 mg L−1 and 100 mg L−1), 100% of silver was recovered within 2 h of experiment using RVC as the cathode electrode. In contrast, 98.0% (±0.2) of silver (for 500 mg L−1 catholyte) and 96.9% (±0.3) of silver (for 100 mg L−1 catholyte) were recovered using the PG electrode. It is noticed that the recovery rate from 500 mg L−1 silver solution is always greater than from 100 mg L−1 silver solution. The 10 mg L−1 and 50 mg L−1 silver cyanide as the initial concentrations for the electrogenerative process were also investigated. The experiment produced a low recovery rate of 39.2% (±0.8) and 55.6% (±0.8) respectively after 2 h of experiment with similar experimental conditions. However, this result is not presented in this paper because the silver deposition process at a very low concentration (≤50 mg L−1) was not controlled by mass transport phenomena and reproducibility of the data is low.19,20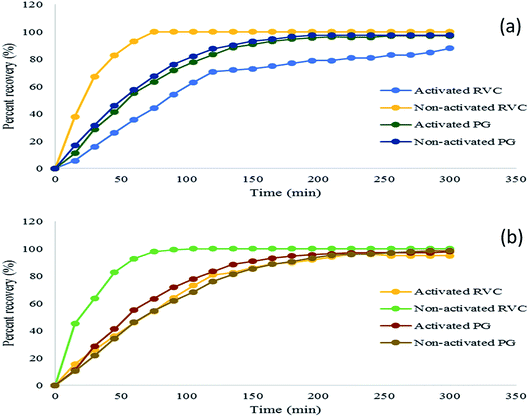 | ||
| Fig. 1 Percent of silver recovery with different cathodes from (a) 500 mg L−1 and (b) 100 mg L−1 within 5 h of experiment. | ||
From this analysis, it is observed that RVC is the most suitable cathode electrode for the recovery of silver from the cyanide solution. This feature is achieved due to the advantages of RVC such as having a high volumetric surface area (specific surface area of the cathode), ≈53 cm2 cm−3 that provides homogeneous and porous structure, low density and high capacity of metal loading.21,22Table 1 confirms that the RVC is superior to PG in terms of porosity, specific area and Brunauer–Emmett–Teller (BET surface area), thus proving its suitability in this study.23
| Cathode material | Porosity (%) | Specific area (m2 m−3) | BET surface area (m2 g−1) |
|---|---|---|---|
| RVC (80 ppi) | 97 | 5300 | 1 |
| PG (SG 132) | 50 | — | 0.3 |
The purpose of purging N2 gas before and during the experiment is to eliminate the presence of dissolved O2. From the experiment, it was observed that recovery rates of silver for all cathodes are slower in the presence of dissolved O2. Therefore, the recovery of silver is conducted in the absence of O2 which can be achieved by purging with N2 gas.
Effect of cathode activation
As shown in Fig. 1(a), the use of activated cathodes resulted in lower silver recovery from 500 mg L−1 of silver concentration than with non-activated cathodes. After 2 h of the experiment, the percent of recovery using non-activated PG is 87.7% (±0.6) while the percent recovery for activated PG is only 83.5% (±0.9). On the other hand, the percent recovery of silver with non-activated RVC is 100% whereas, for activated RVC, the silver recovery is only 70.9% (±0.6). This result shows that no extra treatment (activation) of cathode material is needed to achieve full recovery of silver. A similar pattern is also observed for an experiment with an initial concentration of 100 mg L−1 (Fig. 1(b)).Mass transport coefficient
A model of the concentration–time relationship for 3D electrodes has been proposed and is shown in eqn (9):| ln[Ct/Co] = −(VekmAet)/VR, | (9) |
Fig. 2 shows the linearization of normalized silver for 500 mg L−1 of the initial concentration of silver from cyanide ion media for both non-activated RVC and PG cathodes. The behaviour of each reactor system is in accordance with eqn (9) for operating under mass transport control.27 Their values are shown in Table 2. The values of t70% represent the recovery rate of silver. On the other hand, the kmAe values represent the rate constant for silver deposition on the cathode. The nonconformity of data from the trend lines may be due to the expansion of surface area during the deposition process and increase of turbulence in the fluid flow due to roughening effects of deposition.28 It is also noted that after 1 h of experiment, linearization of silver for the RVC cathode cannot be determined because the quotient of concentration at that time over initial concentration (Ct/C0) was negative.25
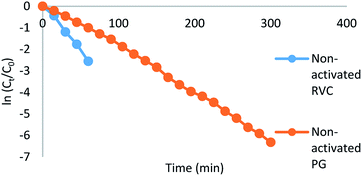 | ||
| Fig. 2 Linearization of normalized silver (500 mg L−1) from cyanide media for both non-activated RVC and PG cathodes. | ||
| Cathode | C 0 (mg L−1) | t 70% (min) | Slope (min−1) | R 2 | k m A e (s−1) |
|---|---|---|---|---|---|
| RVC | 100 | 17 | −0.063 | 0.993 | 1.8 × 10−2 |
| RVC | 500 | 30 | −0.041 | 0.996 | 9.9 × 10−3 |
| PG | 100 | 105 | −0.014 | 0.952 | 2.7 × 10−3 |
| PG | 500 | 75 | −0.017 | 0.993 | 4.6 × 10−3 |
Morphology study
Silver deposited from 500 mg L−1 silver cyanide with non-activated RVC was analysed using SEM-EDX. The morphology of the silver deposits is shown in Fig. 3. The white spherical shape particles on the cathode's surfaces correspond to silver deposits. The EDX analysis shows the presence of silver as the dominant element within the deposits (Fig. 3). This observation confirms that silver was deposited at the RVC.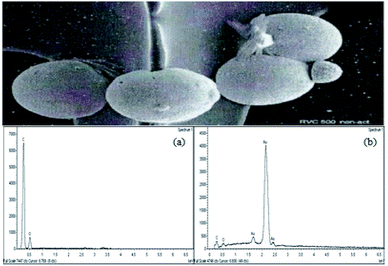 | ||
| Fig. 3 SEM micrograph of deposited silver at RVC at ×1000 magnification. Inset shows the EDX spectra (a) before and (b) after the silver recovery process. | ||
Reusing the cyanide residue and RVC cathode
As mentioned before, one of the advantages of this electrogenerative process is that the cyanide solution is reusable. Therefore, in order to establish the viability of a closed-loop process, the recovery of cyanide solution was also investigated. After the recovery process is complete, concentrations of the leftover catholyte (KCN and NaCN) and anolyte (only NaCN) were determined using the proposed method by the APHA.18 It was found that KCN and NaCN are reduced from 0.2% (w/v) to 0.1% (w/v), which is a 50% reduction. This is because catholyte was withdrawn periodically during the experiment in order to determine the silver concentration. In contrast, in the anode compartment, only a slight decrease of NaCN has been observed (0.5% (w/v) to 0.47% (w/v)). These cyanide solutions were consumed again for the subsequent silver recovery experiment without any additional treatment. However, these cyanide solutions were adjusted to their initial concentrations prior to the new cycle of analysis.In addition, the RVC used in this study could be used repeatedly. Essentially, after the silver recovery experiment, RVC was treated with HNO3 overnight and washed with deionized water. Then the RVC was soaked in 95% ethanol solution for 4 h and was rinsed. The RVC was kept in distilled water prior to use. It was found that the RVC was able to be used repeatedly up to 10 cycles without affecting the performance of silver recovery process. Interestingly, the calculated relative standard deviation (RSD) for the 10 cycles of RVC usage was found to be 1.90%.
Experimental
Details of the experimental procedures are provided in the ESI.†Conclusions
The uses of 3D cathode materials for the electrochemical recovery of silver from silver cyanide solution were demonstrated by using an electrogenerative process. This process promises an alternative in the treatment of diluted and low concentration of silver cyanide solutions. It was confirmed that the pretreatment of the cathode materials did not influence the silver recovery process. Therefore, this electrogenerative process has a massive potential to be applied in real-life applications due to the fact it could fully recover the silver from the silver cyanide solution. In addition, the leftover cyanide solutions could also be recovered and reused, showing the viability of a closed-loop process. Finally, an electrogenerative process can be considered as a green and sustainable process owing to its capabilities of fully recovering the metal (silver) and generating zero waste (zero sludge disposal and the cyanide solution and cathode electrode are reusable).Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors would like to thank Universiti Sains Malaysia for sponsoring this research via Research Grants of (304/PKIMIA/6315197) and (304/PKIMIA/6316020). Finally, F. B. Mohd Suah would like to thank Universiti Sains Malaysia and Ministry of Education of Malaysia for his research leave to Imperial College London.References
- V. Reyes-Cruz, M. T. Oropeza, I. Gonzalez and C. Ponce-de Leon, J. Appl. Electrochem., 2002, 32, 473–479 CrossRef CAS.
- V. Reyes-Cruz, I. Gonzalez and M. T. Oropeza, Electrochim. Acta, 2004, 49, 4417–4423 CrossRef CAS.
- M. Fabián, P. Baláž and J. Briančin, Hydrometallurgy, 2009, 97, 15–20 CrossRef.
- A. Stavart, C. Leroy and A. Van Lierde, Miner. Eng., 1999, 12, 545–588 CrossRef CAS.
- C. Y. Yap and N. Mohamed, Chemosphere, 2007, 67, 1502–1510 CrossRef CAS.
- L. F. Arenas, R. P. Boardman, C. Ponce-de-León and F. C. Walsh, Carbon, 2018, 135, 85–94 CrossRef CAS.
- C. Ponce-de-León and F. C. Walsh, Electrochim. Acta, 1996, 41, 533–541 CrossRef.
- R. C. Widner, M. F. B. Sousa and R. Bertazzoli, J. Appl. Electrochem., 1998, 28, 201–207 CrossRef CAS.
- M. Friedrich, C. Ponce-de-León, G. W. Reade and F. C. Walsh, J. Electroanal. Chem., 2004, 561, 203–217 CrossRef.
- F. C. Walsh, L. F. Arenas and C. Ponce-de-León, J. Chem. Technol. Biotechnol., 2018, 12, 529–570 Search PubMed.
- S. S. Oishi, A. B. Couto, E. C. Botelho and N. G. Ferreira, ECS Trans., 2017, 80, 1073–1080 CrossRef CAS.
- L. F. Castañeda, F. C. Walsh, J. L. Nava and C. Ponce-de-León, Electrochim. Acta, 2017, 258, 1115–1139 CrossRef.
- A. A. Abahussain, C. Ponce-de-León and F. C. Walsh, J. Electrochem. Soc., 2018, 165, F198–F206 CrossRef CAS.
- M. R. V. Lanza and R. Bartazzoli, J. Appl. Electrochem., 2000, 30, 61–70 CrossRef CAS.
- M. Spritzer and R. Bartazzoli, Hydrometallurgy, 2004, 74, 233–242 CrossRef.
- V. G. Gamboa, N. M. Medina and V. A. Lopez, Hydrometallurgy, 2005, 76, 193–205 CrossRef.
- C. Y. Yap and N. Mohamed, Clean: Soil, Air, Water, 2008, 36, 443–452 CAS.
- American Public Health Association (APHA), Standard methods for the examination of water and wastewater, Washington, D. C., 20th edn, 1999 Search PubMed.
- D. Pletcher and F. C. Walsh, Industrial electrochemistry, Chapman & Hall, London, 2nd edn, 1990 Search PubMed.
- Q. De Radigues, P. Y. Sevar, F. V. Wonterghem, R. Santoro and J. Proost, Ind. Eng. Chem. Res., 2012, 51, 14229–14235 CrossRef CAS.
- F. C. Walsh, L. F. Arenas, C. Ponce-de-León, G. W. Reade, I. Whyte and B. G. Mellor, Electrochim. Acta, 2016, 215, 566–591 CrossRef CAS.
- B. K. Fereirra, Miner. Process. Extr. Metall. Rev., 2008, 29, 330–371 CrossRef.
- Electrochemistry for a Cleaner Environment, ed. D. Pletcher, F. C. Walsh, D. Gernders and N. Weinberg, The Electrosynthesis Company, New York, 1992 Search PubMed.
- G. W. Reade, C. Ponce-de-León and F. C. Walsh, Electrochim. Acta, 2006, 51, 2728–2736 CrossRef CAS.
- N. A. Roslan, F. B. M. Suah and N. Mohamed, Sep. Purif. Technol., 2017, 182, 1–8 CrossRef CAS.
- L. F. Arenas, C. Ponce-de-León and F. C. Walsh, Electrochem. Commun., 2017, 77, 133–137 CrossRef CAS.
- G. W. Reade, C. Ponce-de-León and F. C. Walsh, Electrochim. Acta, 2006, 51, 2728–2736 CrossRef CAS.
- F. J. Recio, P. Herrasti Gonzalez, L. Vazquez, C. Ponce-de-León and F. C. Walsh, Electrochim. Acta, 2013, 90, 507–513 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05557f |
| This journal is © The Royal Society of Chemistry 2019 |
