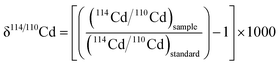Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cadmium isotope fractionation in the soil – cacao systems of Ecuador: a pilot field study†
Fiorella Barraza‡§
 a,
Rebekah E. T. Moore§
a,
Rebekah E. T. Moore§ b,
Mark Rehkämper
b,
Mark Rehkämper *b,
Eva Schrecka,
Grégoire Lefeuvrea,
Katharina Kreissigb,
Barry J. Colesb and
Laurence Maurice
*b,
Eva Schrecka,
Grégoire Lefeuvrea,
Katharina Kreissigb,
Barry J. Colesb and
Laurence Maurice ac
ac
aGéosciences Environnement Toulouse (GET), Observatoire Midi-Pyrénées, CNRS, IRD, Université de Toulouse, 14 avenue E. Belin, F-31400 Toulouse, France. E-mail: fiorella.barraza@hotmail.com
bDepartment of Earth Science & Engineering, Imperial College London, London SW7 2AZ, UK. E-mail: markrehk@imperial.ac.uk; r.moore13@imperial.ac.uk
cUniversidad Andina Simón Bolívar, Área de Salud, P.O. Box 17-12-569, Quito, Ecuador
First published on 23rd October 2019
Abstract
The often high Cd concentrations of cacao beans are a serious concern for producers in Latin America due to the implementation of stricter Cd limits for cocoa products by the European Union in 2019. This is the first investigation to employ coupled Cd isotope and concentration measurements to study soil – cacao systems. Analyses were carried out for 29 samples of soils, soil amendments and cacao tree organs from organic farms in Ecuador that harvest three distinct cacao cultivars. The majority of soils from 0–80 cm depth have very similar δ114/110Cd of about −0.1‰ to 0‰. Two 0–5 cm topsoils, however, have high Cd concentrations coupled with heavy Cd isotope compositions of δ114/110Cd ≈ 0.2%, possibly indicating Cd additions from the tree litter used as organic fertilizer. Whilst cacao leaves, pods and beans are ubiquitously enriched in Cd relative to soils there are distinct Cd isotope signatures. The leaves and pods are isotopically heavier than the soils, with similar Δ114/110Cdleaf–soil values of 0.22 ± 0.07‰ to 0.41 ± 0.09‰. In contrast, the data reveal differences in Δ114/110Cdbean–leaf that may be linked to distinct cacao cultivars. In detail, Δ114/110Cdbean–leaf values of −0.34‰ to −0.40‰ were obtained for Nacional cacao from two farms, whilst CCN-51 hybrid cacao from a third farm showed no fractionation within error (−0.08 ± 0.13‰). As such, further work to investigate whether Cd isotopes are indeed useful for tracing sources of Cd enrichments in soils and to inform genetic efforts to reduce the Cd burden of cocoa is indicated.
1. Introduction
The toxicity of Cd to animals and plants is well documented. For humans, Cd is highly toxic even at relatively low concentrations as it accumulates in the body over decades and is carcinogenic and damaging to kidneys and bones following chronic exposure.1,2 In response to concerns that cocoa often features relatively high Cd concentrations, the European Union implemented strict new upper limits on the Cd concentrations of cocoa products in 2019.3 This recent development has spurred research into the extent of Cd enrichment in cacao beans from different growing regions and the causes of the observed enrichments.Such work has shown that there are large regional variations of Cd levels in cacao beans, whereby problematically high concentrations are particularly prominent in Latin America and some Asian countries.4–13 In these regions, the Cd concentrations of beans commonly exceed 0.6 to 0.8 mg kg−1,14 which is the approximate upper range for manufacturing cocoa products that are in accord with the new EU regulations.8,15 For example, a recent study of more than 500 farms in Ecuador revealed that nearly 50% of the cacao beans had Cd concentrations that exceeded 0.6 mg kg−1.15 For Ecuador and other affected countries, these thresholds may have grave economic consequences, whereby the numerous small-hold farmers and their families, which grow and harvest the cacao beans, might be most severely affected.
As Cd is a non-essential but toxic element, plants have developed specific sequestration strategies to cope with uptake of unwanted Cd from soils.16,17 In comparison to other food crops, however, cacao plants commonly take up significantly more Cd, such that they have been termed a Cd ‘accumulator’.6,15 Whilst the ultimate causes of Cd enrichment in beans remain unclear, the issue is of particular relevance, as the development of feasible mitigation strategies requires a better understanding of both natural and anthropogenic Cd sources and of the mechanisms that govern Cd uptake by cacao as well as transfer and accumulation of the element within the plants.
Most recent investigations of soil – cacao systems concur that high Cd levels in beans are correlated with high concentrations of mobile, and hence readily plant-available, Cd in soils.5,6 The interpretation of this observation differs, however. For example, particularly high Cd levels in the uppermost soil layers (<20 cm depth) were interpreted by some workers as a consequence of agricultural practices, such as the application of mineral P fertilizers,6,8 which often feature high Cd concentrations, and/or decomposition of cacao leaves and discarded pods, which are commonly left in plantations as an organic fertilizer.8,12 Other studies linked the high concentrations of plant-available Cd in soils to soil characteristics, such as pH and contents of Mn, Fe and Al oxyhydroxides, which affect the fraction of Cd that is readily mobile rather than tightly adsorbed to soil particles.15,18
Only limited understanding is available on the mechanisms that control Cd uptake by cacao from soils and the processes that take place within cacao, which are responsible for translocation of Cd from the roots to above-ground parts and the internal sequestration of the element. In particular, processes such as uptake into and retention in root cells, loading into the xylem, transfer from the xylem to the phloem and transport through phloem appear to play a key role in the Cd accumulation and distribution within cacao.16,19–24 It has also been proposed that Cd can be directly loaded from stems and branches into cacao beans without passing through leaves and that Cd is relocated into developing beans from pod husks.25
Previous studies on annual crops such as barley, potatoes and rice revealed clear cultivar-specific differences in Cd uptake and particularly root to shoot translocation, whereby the latter appears to be governed by the ability of plants to sequester Cd in roots with the aid of transporter proteins.26–29 Two recent investigations on cacao also revealed cultivar-specific differences in Cd handling but with contrasting results. One study found that cultivars primarily differ in the efficiency of Cd translocation from shoots to beans, due to differences in the efficiency of Cd transfer from xylem to phloem.25 In contrast, a second study revealed clear cultivar-related differences in soil to shoot translocation of Cd, that were attributed to differential Cd uptake and/or retention in the roots.30
There are only few previous Cd isotope investigations of plants and soil – plant systems. Such studies have already shown, however, that coupled Cd concentration and isotope data can be used to trace the origin of isotopically distinct Cd inputs, such as mineral P fertilizers, into soils.31–35 In addition, isotope data can provide constraints on the distinct cellular and molecular processes that are employed by plants for the uptake, internal transport and sequestration of Cd. In particular, recent publications demonstrated that wheat and barley display similar trends of Cd isotope fractionation33 and these differ significantly from the isotope systematics observed for Cd-tolerant and accumulator cultivars.36 As various genotypes of cacao differ in the extent to which Cd is enriched in the plants relative to soil and in how Cd is partitioned within the plants,7,25,30 such genotypes might also exhibit distinct Cd isotope signatures as a consequence of distinct pathways for Cd uptake, transport and sequestration.
In the following, results are presented from the first investigation that employed Cd isotope data for the study of soil – cacao systems. In detail, the study encompassed coupled Cd concentration and isotope analyses for 29 samples of soils, organic soil amendments and cacao plants from three farms in Ecuador. The data are applied to evaluate (i) whether they might provide constraints on the origin of any Cd enrichments in soils; and (ii) if different cacao cultivars exhibit distinct Cd isotope distributions, which may point to genetic differences in how the plants take up Cd and distribute the element between different organs.
2. Materials and methods
2.1. Study sites and samples
The samples for this study are from three small-scale cacao farms in Ecuador. Farm A is in the North Amazonian province of Sucumbíos, whilst Farms B and C are in the Pacific Coast province of Esmeraldas (Table 1 and ESI Fig. S1†). Although both provinces have similar average annual temperatures (∼25 °C) and total annual rainfall (>3000 mm), precipitation is evenly-distributed in the North Amazon region but seasonal near the Pacific Ocean.37,38 All three farms do not apply irrigation water and they follow organic practice, with no use of mineral P fertilizers, chemical pesticides or herbicides. Whilst assurances were provided by the farmers that organic practices had been followed for several years at all sites, independent verification of this information is not possible. Farm A is a cacao monoculture plantation and one of the two sites investigated at this farm (Site A-1) is located on an island in the Aguarico River. At Farm B, the cacao grows alongside timber trees, whilst cacao, timber, banana and avocado trees are grown together at Farm C (Table 1).| Sampling site | A-1 | A-2 | B | C-a | C-b |
|---|---|---|---|---|---|
| a The pH and CEC values for 0–20 cm depth were calculated for Sites A-1 and A-2 (from individual data for 0–5 cm and 5–20 cm layers) and directly measured for soils from Farms B and C. The detailed soil data for Sites A-1 and A-2 are available in Table S4 of the ESI.b Organic soil amendments in parentheses are used in the farms but were not sampled and analyzed in this study. | |||||
| GPS coordinates | 0°1′41.08′′ N | 0°1′41.08′′ N | 0°29′50.04′′ N | 0°45′55.48′′ N | 0°45′55.48′′ N |
| 76°36′36.82′′ E | 76°36′36.82′′ E | 79°51′8.41′′ E | 79°56′5.06′′ E | 79°56′5.06′′ E | |
| Province | Sucumbíos | Sucumbíos | Esmeraldas | Esmeraldas | Esmeraldas |
| Cacao cultivar | Nacional hybrid | CCN-51 hybrid | Nacional | Nacional | Nacional |
| Age of trees | Unknown | Unknown | 6 years | 10 years | 20 years |
| Culture system | Cacao monoculture | Cacao monoculture | Cacao & timber trees | Cacao, banana, avocado, timber trees | Cacao, banana, avocado, timber trees |
| Soil type | Andisol | Ultisol | Inceptisol | Mollisol | Mollisol |
| Soil pHa | 6.78 | 6.52 | 6.30 | 7.14 | 7.08 |
| Soil CEC (cmol kg−1)a | 37.0 | 39.8 | 7.7 | 2.9 | 1.7 |
| Organic soil amendmentsb | Chicken manure, (tree litter) | Chicken manure (tree litter) | Tree litter (cattle manure) | Tree litter (cattle manure) | Tree litter (cattle manure) |
There are two main Ecuadorian cacao varieties. The Nacional cultivar produces fine-flavour specialty cocoa whilst CCN-51 is a high-yield cultivar that is resistant to fungal diseases but provides cocoa of lower aromatic quality.39,40 In most farms, various hybrids of these two varieties are cultivated. In the current study, the cacao samples were assigned to three distinct cultivars, based on phenotype and information supplied by the farmers. The two sites at Farm A are used to cultivate different cacao phenotypes, which were termed Nacional hybrid and CCN-51 hybrid cacao plants, respectively, by the farmers. The samples from Farm B and two sites at Farm C were provided by the French Cooperative ‘Ethiquable’ as part of a larger study, which investigates the effects of agricultural practices and cacao processing on Cd concentrations in beans. The cacao from these sites is phenotypically similar and classified as a relatively pure Nacional cultivar by the cooperative. Similar sampling methods were employed at all sites, which followed established practices8 and are further detailed in the ESI.†
From Farm A, mature cacao leaves as well as the husks and beans of mature cacao pods were sampled: Nacional hybrid cacao at Site A-1 and a CCN-51 hybrid cacao at Site A-2 (Table 1). Cocoa liquor produced from these two varieties of cacao was also analysed (see ESI for details†). The soil samples of Farm A were collected at depths of 0–5 cm, 5–20 cm, 20–60 cm, and 60–80 cm. Farm-produced chicken manure that was used as an organic soil amendment at Sites A-1 and A-2 was collected at both locations (Table 1).
The samples from Farm B and two proximal sites at Farm C encompass cacao beans and leaves from a 6 year old Nacional tree at Farm B and two Nacional trees with ages of 10 years (Site C-a) and 20 years (Site C-b) at Farm C (Table 1). The beans from the C-a and C-b sites were combined by the cooperative to prepare a single mixed cacao bean sample for a quality assay, and this was subsequently made available for this investigation. In addition, 0–20 cm soil samples were taken in the vicinity of the sampled trees at Farms B and C (Table 1), as most of the cacao roots are located at this depth.41,42 The natural tree litter, which was used as organic fertilizer in addition to cattle manure at Farms B and C, was collected from underneath the sampled cacao trees (see ESI for details†).
2.2. Initial sample preparation and physicochemical analyses of soils
At the GET Laboratory in Toulouse, all samples were first air and then oven-dried at 45 °C. The samples were subsequently homogenized using a vibrating cup mill with a steel grinding set and agate discs (soils, chicken manure, litter, cacao leaves, pod husks) or in an agate mortar with liquid nitrogen (cacao beans with shells, cocoa liquor).The physicochemical characterisation of the soils in Toulouse included (i) measurement of soil pH with a Metrohm 744 pH meter on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mixture of ground soil in water and (ii) determination of cation exchange capacity (CEC) by spectrocolorimetry using cobalt hexamine trichloride.43
5 mixture of ground soil in water and (ii) determination of cation exchange capacity (CEC) by spectrocolorimetry using cobalt hexamine trichloride.43
2.3. Determination of Cd concentrations and isotope compositions
The isotopic analyses were performed with a Nu Instruments Nu Plasma HR MC-ICP-MS (multiple collector inductively coupled mass spectrometer), using an Aridus II desolvation system with a PFA nebulizer (CETAC Technologies). The sample analyses were interspersed between runs of spiked solutions of the NIST SRM 3108 Cd isotope reference material,45 which were measured at dilutions and S/N values comparable to the samples. The isotopic compositions of the samples are reported relative to the NIST SRM 3108 Cd standard:
The quoted analytical precision of the δ114/110Cd data (Table 2) was estimated from the repeatability of the bracketing analyses of matching NIST 3108 Cd–Cd DS mixtures, which was typically between 0.05‰ and 0.10‰ (2sd). The isotopic data obtained in the sample runs were also processed using the isotope dilution technique to determine the final Cd concentrations of the samples.
| Sampling site | A-1, Nacional hybrid cacao | A-2, CCN-51 hybrid cacao | B, Nacional cacao | C-a, Nacional cacao | C-b, Nacional cacao | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd (mg kg−1) | δ114/110Cd (‰) | Cd (mg kg−1) | δ114/110Cd (‰) | Cd (mg kg−1) | δ114/110Cd (‰) | Cd (mg kg−1) | δ114/110Cd (‰) | Cd (mg kg−1) | δ114/110Cd (‰) | |
| a The quoted errors for the δ114/110Cd data denote the 2sd mass spectrometric reproducibility of the isotopic measurements.b Weighted mean calculated from measured results for 0–5 cm and 5–20 cm soil layers.c The cacao beans from Sites C-a and C-b were mixed and analyzed together; hence only a single result is given (see ESI for details). | ||||||||||
| Soil samples and organic soil amendments | ||||||||||
| 0–5 cm | 0.515 | 0.19 ± 0.04 | 1.10 | 0.24 ± 0.06 | — | — | — | — | — | — |
| 5–20 cm | 0.260 | −0.07 ± 0.09 | 0.345 | −0.09 ± 0.06 | — | — | — | — | — | — |
| 0–20 cm | 0.324b | 0.01 ± 0.08b | 0.533b | 0.04 ± 0.06b | 0.384 | 0.10 ± 0.05 | 1.60 | −0.09 ± 0.05 | 0.809 | −0.03 ± 0.05 |
| 20–60 cm | 0.395 | −0.09 ± 0.06 | 0.253 | −0.04 ± 0.08 | — | — | — | — | — | — |
| 60–80 cm | 0.167 | −0.27 ± 0.10 | 0.734 | 0.01 ± 0.07 | — | — | — | — | — | — |
| Chicken manure | 0.271 | −0.09 ± 0.05 | 0.273 | 0.11 ± 0.07 | — | — | — | — | — | — |
| Tree litter | — | — | — | — | 3.84 | 0.28 ± 0.08 | 6.23 | 0.19 ± 0.04 | 1.15 | 0.29 ± 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| Cacao trees and cocoa products | ||||||||||
| Leaves | 2.12 | 0.42 ± 0.04 | 2.38 | 0.42 ± 0.11 | 3.63 | 0.31 ± 0.05 | 3.06 | 0.30 ± 0.05 | 1.95 | 0.28 ± 0.06 |
| Pod husks | 0.99 | 0.53 ± 0.05 | 2.14 | 0.50 ± 0.05 | — | — | — | — | — | — |
| Beans | 1.26 | 0.18 ± 0.05 | 2.17 | 0.34 ± 0.07 | 3.38 | −0.02 ± 0.05 | 3.92c | −0.11 ± 0.05c | 3.92c | −0.11 ± 0.05c |
| Cocoa liquor | 1.44 | 0.10 ± 0.04 | 3.95 | 0.33 ± 0.05 | — | — | — | — | — | — |
Procedural blanks processed alongside the samples were about 50 pg Cd during the study. This corresponds to a contribution of less than 0.1% to the total Cd present in the samples. The blank was subtracted from the measured Cd concentrations but no corrections were applied to δ114/110Cd data. Quality control and standard reference materials were analyzed to validate the accuracy and precision of the measurements. These results are in good agreement with independent reference values (Table S3 of ESI†).
For the comparison of Cd isotope compositions, the apparent isotope fractionation between two samples or reservoirs was calculated as:
| Δ114/110CdA–B = δ114/110CdA − δ114/110CdB |
3. Results
3.1. Soil taxonomy and properties
At Farm A, the young volcanic andisols of Site A-1 differ from the older, more intensely weathered ultisols at Site A-2. Both, however, display a narrow range of pH values from about 6.3 to 7.0 and CECs of between 30 and 53 cmol kg−1 (Tables 1 and S4 of ESI†). The relatively young inceptisol at Farm B was also slightly acidic with pH ≈ 6.3 but the CEC was significantly lower at about 8 cmol kg−1. The base-rich mollisol of Farm C had the highest pH value of about 7, combined with the lowest CEC of between 1.7 and 2.9 cmol kg−1 (Tables 1 and S4 of ESI†).3.2. Cd concentrations and isotope compositions of soils
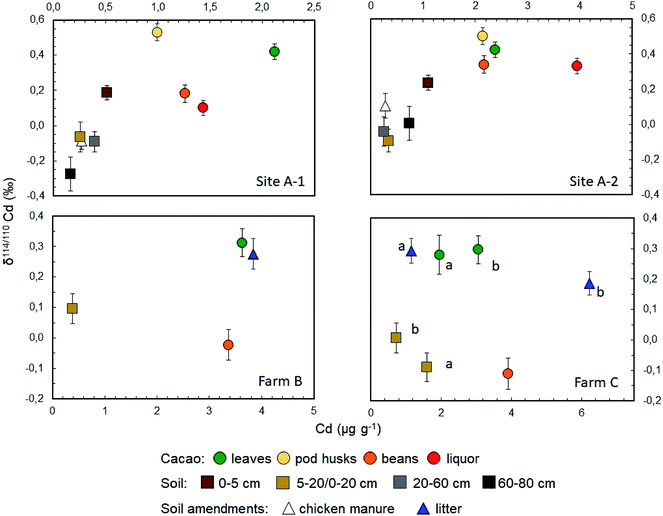
The Cd concentrations of the soils vary by roughly an order of magnitude, from 0.167 mg kg−1 at 60–80 cm depth of Site A-1 to 1.60 mg kg−1 at 0–20 cm of location C-a (Fig. 1, 2 and Table 2). A marked variability in concentrations is thereby seen both within the soil profiles of sites A-1 and A-2 (by about a factor of ×3 to ×4) as well as between the proximal sites A-1, A-2 and C-a, C-b. This variability may reflect relatively heterogeneous Cd concentrations even at small length scales in the field or the relatively small sample aliquots (of 100–200 mg) that were digested for analysis.In contrast to the concentrations, the Cd isotope compositions of most soil samples show only limited variability (Fig. 1, 2 and Table 2). In detail, 6 of the 10 soil samples have δ114/110Cd values of between 0.0‰ and −0.1‰ and are identical within analytical error. One of the remaining samples is isotopically light with δ114/110Cd = −0.27‰ whilst the other three are topsoils with δ114/110Cd values of between 0.10‰ and 0.24‰ (Fig. 1, 2 and Table 2).
3.3. Cd concentrations and isotope compositions of cacao plant samples
All cacao leaves are enriched in Cd relative to the soils, with concentrations between 1.95 to 3.63 mg kg−1. They are also characterized by nearly identical heavy Cd isotope compositions with δ114/110Cd values from about 0.3‰ to 0.4‰ (Fig. 1 and Table 2). The two cacao pod husks also have variable Cd concentrations akin to the leaves, with 0.99 to 2.14 mg kg−1 Cd, and similar or slightly higher δ114/110Cd of about 0.5‰ (Fig. 1 and Table 2).The Cd concentrations of the four cacao bean samples resemble the leaves, with concentrations of 1.26 to 3.92 mg kg−1 but the Cd isotope compositions are more variable (Fig. 1 and Table 2). In detail, the two bean samples from Farm A have positive δ114/110Cd values that are distinct, considering the analytical errors, at 0.18 ± 0.05‰ and 0.34 ± 0.07‰. In contrast, the bean samples from Farms B and C display lighter Cd isotope compositions, with δ114/110Cd values that are identical, within uncertainty, at −0.02 ± 0.05‰ and −0.11 ± 0.05‰.
3.4. Cd concentrations and isotope compositions of additional samples
Two samples of chicken manure from Farm A, which was used as organic fertilizer, have relatively low Cd concentrations of about 0.27 mg kg−1 and δ114/110Cd values similar to the soils, with results of −0.09‰ and 0.11‰ (Fig. 1 and Table 2). The tree litter from Farms B and C, displays Cd systematics that resemble but are slightly more variable than the fresh cacao leaves. In detail, the Cd concentrations vary between 1.15 to 6.23 mg kg−1 whilst the δ114/110Cd values range from 0.19‰ to 0.29‰. Finally, the cacao liquors from Farm A have higher Cd concentrations than the beans from the same sampling sites but essentially identical δ114/110Cd values (Fig. 1 and Table 2).4. Discussion
Compared to most other recent studies of Cd concentrations in cacao and soil – cacao systems, the current investigation features results for a relatively small number of samples but these data are coupled with novel Cd isotope results. As such, the discussion primarily focuses on the latter results.4.1. Cd distribution and isotope fractionation in the soils
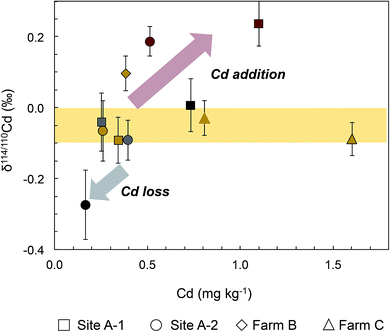
The variable Cd concentrations of the soils are in accord with previously reported total Cd concentrations for soils from different locations in Ecuador.5,8,15 Notably, the new soil Cd data show no systematic variation with soil type or soil properties and, for the soil profiles from Farm A, with depth.The eight soil samples from the two profiles of Farm A are, however, in accord with a rough positive correlation of δ114/110Cd with Cd concentration, and two of the three 0–20 cm soil samples from Farms B and C are also in accord with such a trend (Fig. 2). The small dataset obtained here does not allow a rigorous evaluation of the inferred correlation but some interpretations can be developed. Notably, the majority of soils cluster at Cd concentrations of 0.2 to 0.8 mg kg−1 and they have δ114/110Cd values of between −0.1‰ and 0.0‰, similar to the estimated composition of the upper continental crust (with δ114/110Cd ≈ 0).46,47 Compared to this, the 60–80 cm soil from site A-2 has a lower Cd concentration and lower δ114/110Cd of −0.27 ± 0.10‰ (Fig. 2 and Table 2). The latter observation is most readily explained by loss of Cd from the solid soils to pore waters by leaching during weathering, as soil solutions are expected to be enriched in heavy Cd isotopes relative to the residual solid.34,48,49 The dissolved Cd is subsequently removed from the soils by drainage of the pore waters and Cd uptake by plants.34
The scattered δ114/110Cd versus Cd trend of the soil samples is, furthermore, governed by the relatively high Cd concentrations and δ114/110Cd values (of 0.19‰ to 0.24‰) for the two 0–5 cm topsoils from Farm A. In addition, a relatively high δ114/110Cd of 0.10‰ is also observed for the 0–20 cm soil of Farm B (Fig. 2 and Table 2). As the two 0–5 cm soils from Sites A-1 and A-2 have Cd concentrations that clearly exceed the deeper soil layers, it is likely that they record Cd addition to the soil surface. The relatively high δ114/110Cd of the Cd-rich topsoils is, furthermore, in accord with the interpretation that the added Cd is derived from plant material that decomposed at the surface whilst other possible sources of added Cd are unlikely. The latter conclusion is discussed in the following for (i) chicken manure, (ii) aerosols, (iii) river sediments and (iv) mineral P fertilizers as plausible Cd sources.
(i) Chicken manure, which is added to the soils at Farm A for fertilization, has Cd concentrations and δ114/110Cd values that are lower compared to the topsoils (Fig. 2 and Table 2). As such, it likely has only a limited impact on topsoil Cd budgets.
(ii) A recent isotopic study investigated diverse marine aerosols with Cd budgets that varied in origin between primarily natural (most likely from mineral dust) to dominantly anthropogenic. Despite this, 11 of the 12 investigated aerosols had very similar δ114/110Cd values of between −0.1‰ and 0.1‰. One particularly Cd-rich sample, most likely from anthropogenic sources, featured δ114/110Cd ≈ −0.5‰.50 The latter result is in accord with data for fumes and ash from metal smelting operations, which are also enriched in light Cd isotopes, most likely due to preferential release of isotopically light Cd in high-temperature processes.51–54 Together, this suggests that atmospheric additions of Cd, for example from volcanic emanations in the Andes, forest fires or the slash and burn farming practices that are common in the Amazon region55 and industrial sources (e.g., oil refinery or shipping port in Esmeraldas), are probably not responsible for the enrichment of isotopically heavy Cd in the topsoils of Farms A and B.
(iii) It is also unlikely that the topsoil enrichment of Cd results from riverborne sediments, which might have been emplaced during flooding by the river at Farm A. This follows from the inference that such sediments will likely feature δ114/110Cd values similar to or slightly lower than average upper continental crust, for which data are available from a few studies.46,47 Support for this inference, is provided by the limited variability of Cd isotope compositions found so far in silicate rocks and clastic sediments,46,56 as well as the observation that silicate weathering appears to deplete residual rocks in isotopically heavy Cd (Fig. 2; ref. 31, 33, 34 and 48). Given an average upper continental crust with δ114/110Cd = −0.01‰ ± 0.04‰, river sediments with similar or lower δ114/110Cd values are unable to account for the heavy Cd isotope compositions of the two Farm A topsoils (Fig. 2 and Table 2).
(iv) Mineral P fertilizers can feature high Cd contents but are an improbable source for the observed topsoil Cd enrichment as such fertilizers were not used at any of the farms for the past years. Furthermore, Cd isotope data for numerous different mineral P fertilizers reveal that the great majority are characterized by δ114/110Cd values of less than 0.20‰.33–35 Use of such fertilizers in the more distant past is thus unlikely to have caused the observed high δ114/110Cd = 0.24‰ for the 0–5 cm topsoil at Site A-2 (Fig. 2 and Table 2).
Given these results, it is most likely that the enrichment of Cd characterized by positive δ114/110Cd values in the topsoils of Farms A and B are due to addition of the element from decomposing cacao tree litter. This conclusion is reasonable as tree litter and cacao leaves feature both high Cd concentrations and relatively heavy Cd isotope compositions, with δ114/110Cd values of about 0.2‰ to 0.3‰ (Fig. 1 and Table 2). Notably, it is common practice to leave pod husks and leaves in cacao fields after harvest for organic fertilization and it was previously suggested that the rapid decomposition of such material in tropical conditions may represent an important source of Cd to the uppermost soil layer.6,8 The interpretation is further supported by data for birch leaves from Sweden, which were also found to have ubiquitously heavy Cd isotope compositions with a mean δ114/110Cd of 0.70 ± 0.20‰ (1sd, n = 83).57
Of further interest in this context are recent detailed studies of soil-wheat agricultural systems in Switzerland, which also identified Cd enrichments characterized by heavy isotope compositions in topsoils.31,33 Based on a detailed soil mass balance, it was concluded that these Cd enrichments are unlikely to be of recent origin but rather reflect long-term ‘plant-pumping’, whereby uptake of Cd by plants and trees, subsequent decay of the organic matter with relatively high δ114/110Cd and release of this Cd into the topsoil over long timescales is responsible for the Cd enrichment. In the absence of a detailed soil mass balance for the cacao sites of this study, it is therefore uncertain whether the observed topsoil Cd enrichments have a long-term origin, which predates cacao cultivation or reflect recent agricultural practices, such as the use of leaf litter as a soil supplement. This question can be addressed but this would require a detailed investigation of the Cd budget and mass balance of the soils, including relevant input and output fluxes of the element.
4.2. Cd distribution and isotope fractionation in the cacao tissues
For all investigated cacao samples, the Cd concentrations significantly exceed those observed for the soils from the same location. Using the measured or calculated Cd soil concentrations for 0–20 cm as a reference (Table 2), Cd shows enrichment factors of between about ×2 to ×10 in both leaves and beans, whereby the leaf and bean enrichment factors are roughly similar for all 4 sites (Table 3). The observed Cd concentrations and enrichment factors are in accord with data from previous investigations of cacao plantations in Ecuador, which considered a larger number of samples and locations.8,15| Sampling site | A-1 | A-2 | B | Cb |
|---|---|---|---|---|
| a The quoted errors for Δ114/110Cd are derived by propagating the uncertainties of the individual δ114/110Cd results (Table 2; see ESI for details).b The Cd enrichment and apparent isotope fractionations for Farm C samples were calculated using the weighted mean Cd concentrations and isotope compositions from Sites C-a and C-b. Only a single mixed bean sample (encompassing beans from C-a and C-b) was analyzed. | ||||
| Cd enrichment factors [Cd] in A/[Cd] in B | ||||
| Leaves/soil 0–20 cm | 6.5 | 4.5 | 9.5 | 2.1 |
| Beans/soil 0–20 cm | 3.9 | 4.1 | 8.8 | 3.2 |
| Beans/leaves | 0.6 | 0.9 | 0.9 | 1.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Apparent Cd isotope fractionation Δ114/110CdA–B | ||||
| Leaves–soil 0–20 cm | 0.41 ± 0.09 | 0.38 ± 0.13 | 0.22 ± 0.07 | 0.35 ± 0.07 |
| Beans–soil 0–20 cm | 0.17 ± 0.08 | 0.30 ± 0.10 | −0.12 ± 0.07 | −0.05 ± 0.07 |
| Beans–leaves | −0.24 ± 0.07 | −0.08 ± 0.13 | −0.34 ± 0.07 | −0.40 ± 0.08 |
For the samples analysed here, the observed Cd enrichment factors show no systematic variation with cultivar, as the CCN-51 hybrids of Site A-2 reveal Cd enrichments that are intermediate to those observed for the Nacional hybrids from Site A-1 and the Nacional cacao of Farms B and C (Table 3). Instead, it appears that the enrichment of Cd in the cacao plants is, at least in part, governed by soil pH, which is known to have an impact on the extent to which soil-bound Cd is available to plants.5,8,15 In detail, the cacao leaves and beans of Farm C, with the highest observed soil pH of about 7.1, show the lowest Cd enrichment factors of about ×2 to ×3 (Table 3). In contrast, the highest enrichment factors of about ×9 to ×10 were found at Farm B, where the 0–20 cm soil has the lowest pH value of 6.3. Finally, intermediate Cd enrichments of about ×2 to ×6 were observed for the cacao plants from Farm A, where the soils have intermediate pH values of about 6.5 to 6.8. This conclusion, however, should be viewed with caution, due to the small number of samples investigated here and the limited variation in soil pH.
The Cd isotope compositions of the cacao tissues also reveal systematic distributions. In particular, the cacao leaves and pod husks consistently display Cd isotope compositions, which are heavier compared to the soil from the same location (Fig. 1). This observation is particularly clear if data for the 0–5 cm soils from Sites A-1 and A-2 are disregarded, as these samples most likely have higher δ114/110Cd than deeper soils due to admixture of leaf-derived Cd. In the following, measured or calculated 0–20 cm soil samples are therefore used for reference (Table 2), in accord with the observation that most cacao roots are situated at this depth.41,42 Using these data, the isotope fractionation between the cacao leaves and soils from Sites A1, A2 and farm C is identical with Δ114/110Cdleaf–soil of between 0.35 ± 0.07‰ to 0.41 ± 0.09‰, and a similar value of 0.22 ± 0.07 is observed for Farm B.
An enrichment of isotopically heavy Cd relative to soils is also seen for the cacao pod husks from Sites A-1 and A-2, which have δ114/110Cd values that agree with the leaves to within less than 0.1‰ (Fig. 1 and Table 2). As such, the transfer of Cd from the soils to leaves and pods is associated with essentially the same isotope fractionation. Similarly, tree litter from sites B and C-b displays Cd isotope compositions that are identical to mature leaves picked from trees of the same site (Fig. 1 and Table 2). This suggests that the initial decomposition of the leaves is not associated with any substantial Cd isotope fractionation, in accord with the previous conclusion that Cd derived from decomposing tree litter delivers isotopically heavy Cd to topsoils. Notably, these results contrast with Zn isotope data acquired for Typha latifolia, as heavy Zn isotopes were found to be enriched in mature and decaying relative to fresh leaves.58 These contrasting observations may reflect that Zn, as an essential micronutrient, is intensively translocated within plants via the phloem whilst such translocation is expected to be less important for non-essential Cd.59
The cacao beans feature more complex Cd isotope systematics. At Farms B and C, the Nacional beans display the lowest δ114/110Cd coupled with negative Δ114/110Cdbean–leaf values of −0.34‰ and −0.40 (Tables 2 and 3). In contrast, the CCN-51 hybrid beans from Site A-2 harbor the highest δ114/110Cd and no resolvable Cd isotope fractionation between beans and leaves with Δ114/110Cdbean–leaf ≈ −0.08 ± 0.13. Finally, the Nacional hybrid beans of Site A-1 have δ114/110Cd, Δ114/110Cdbean–leaf and Δ114/110Cdbean–soil values that are intermediate to those recorded at Sites A1 and Farms B, C (Tables 2 and 3).
Notably, the cacao leaves and beans from Farms B and C display identical δ114/110Cd values, within error, and identical leaf–soil and bean–leaf Cd isotope fractionation (Fig. 1, Tables 2 and 3). As the trees of these farms are all from the Nacional cultivar but vary in age between 6 years and 10 to 20 years, the results suggest that tree age may not have a substantial impact on the uptake and translocation of Cd by cacao plants.
4.3. Cd isotope fractionation in other plants
It is of interest to compare the Cd isotope data obtained for cacao with results acquired for other plants in recent studies. These comparative data are, however, limited to the cereals wheat and barley as well as perennial Cd-tolerant and accumulator plants. Notably, the more complex architecture and physiology of cacao trees60,61 is expected to be associated with distinct internal Cd fluxes between the plant organs, whilst different molecular mechanisms of Cd transport and sequestration might also be relevant.The enrichment of isotopically heavy Cd in the leaves, pods and beans of cacao is in accord with observations made for wheat, whereby the straw and grains of the latter were found to have higher δ114/110Cd values than the soils.31 In part, this isotopic difference reflects that the plant-available (PA) Cd pool of soils typically appears to be characterized by higher δ114/110Cd than bulk soils, with a fractionation factor of up to Δ114/110CdPA–soil ≈ 0.5‰.31,33 As such, bulk wheat plants have Cd isotope compositions that closely resemble the plant-available Cd of soils but with significant internal Cd isotope fractionation, whereby successively higher δ114/110Cd values are observed for the roots, the straw and the grains. Amongst these compartments, only the grains are consistently enriched in isotopically heavy Cd relative the plant-available soil Cd, with an apparent fractionation of Δ114/110CdPA–grain ≈ 0.1–0.5‰.31,33 In contrast, the roots and straw of wheat have Cd isotope compositions that are lighter than or roughly similar to the plant-available Cd of soils, respectively.
Different systematics were identified for the Cd-tolerant plant Ricinus communis L. and the Cd accumulator Solanum nigrum L. In both cases, the plants were enriched in light Cd isotopes compared to the hydroponic nutrient solution, and the largest fractionations were observed between the roots and the hydroponic solution, with Δ114/110Cdroot–solution ≈ −0.4‰.36 However, clear differences in isotope fractionation patterns were observed within the plants. In detail, the Cd accumulator displayed only little or no isotope fractionation between the roots, stems and leaves. In contrast, the Cd-tolerant R. communis revealed variable isotope fractionation between stems and roots but a consistent enrichment of isotopically light Cd in the leaves relative to stems, with Δ114/110Cdleaf–stem of between −0.04‰ and −0.33‰.
4.4. Cd isotope fractionation between cacao leaves and soil
Considering the literature results, it is likely that the relatively consistent isotope fractionation about 0.2‰ to 0.4‰ observed between cacao leaves and the bulk soil of all four sites (Table 3) reflects a combination of (i) enrichment of isotopically heavy Cd in the plant-available Cd relative to the total Cd budget of the soils as well as (ii) sequestration of isotopically light Cd in the cacao roots and thus selective translocation of heavy Cd isotopes to the shoots. This conclusion is reasonable even though no data were acquired here for roots or soil solutions, as enrichments of (i) isotopically heavy Cd in soil solutions or leachates relative to bulk soils and (ii) isotopically light Cd in roots relative to the plant-available Cd seem to be consistent characteristics of plant-soil systems investigated to date.31,33Given these constraints, the fairly consistent Δ114/110Cdleaf–soil values observed here most likely reflect isotopically similar pools of plant-available Cd in soils, which are a consequence of the relatively similar mean δ114/110Cd and pH values observed for the soil profiles of all four sites (Tables 1 and 2). In addition, they also require that the sequestration of isotopically light Cd in the roots produces a residual, isotopically heavier pool of Cd in the plants which, after translocation, is incorporated in leaves that have consistent δ114/110Cd values of between 0.28‰ and 0.42‰ (Fig. 1 and Table 2). The observation of similar leaf Cd isotope compositions and Δ114/110Cdleaf–soil values may thus indicate that the three studied cacao cultivars employ similar molecular strategies of Cd sequestration in the roots. Notably, this mechanism might resemble the Cd sequestration processes that are employed by wheat plants. This conclusion follows from the finding that wheat cultivated on two soils with Cd concentrations and pH values similar to those seen here (at 0–20 cm) displayed Cd isotope fractionations between soils and stems (Δ114/110Cdstem–soil) of 0.27‰ and 0.45‰.33 For wheat, this isotope fractionation was inferred to reflect mechanisms to avoid Cd accumulation in grains, which encompass sequestration of isotopically light Cd in roots.
4.5. Cd isotope fractionation between cacao beans and leaves
Whilst the Cd isotope fractionation between leaves and soil is similar for the cacao of all four sites there are clear differences in the bean–leaf fractionation Δ114/110Cdbean–leaf observed for the plants (Tables 2 and 3). Even though the sample numbers are limited, it is conceivable that these differences in Cd isotope compositions and fractionations may be linked to distinct cacao cultivars, as different cultivars may feature distinct molecular pathways by which Cd is partitioned and sequestered within the plants. This tentative inference is in accord with previous elemental studies, which revealed significant differences in the partitioning of Cd between plant organs for different cultivars of cacao,7,30 and other crops.26–29 Also notable is that the enrichment of light Cd isotopes in beans of the relatively pure Nacional cultivar (Tables 2 and 3) is in accord with observations made for Cd-tolerant and Cd accumulator plants, as these also revealed an enrichment of light Cd isotopes in stems and leaves relative to the hydroponic solution in which they were cultured.62 The latter observation was thereby linked to the particular molecular mechanisms employed by these plants to cope with high levels of toxic Cd. As such, it would be of interest to interrogate the tentative inference of the current study in a more thorough follow-up investigation.5. Conclusions
Results are presented from a pilot investigation that employed coupled Cd isotope and concentration measurements to study the distribution and cycling of Cd in soil – cacao systems of Ecuador. The results provide two particularly interesting observations.First, most of the soil samples from 0 to 80 cm depth have relatively uniform δ114/110Cd of about −0.1‰ to 0‰. However, one sample from 60–80 cm is depleted in Cd and isotopically lighter, in accord with a weathering signature. Furthermore, two 0–5 cm topsoils have high Cd concentrations coupled with heavy Cd isotope compositions of δ114/110Cd ≈ 0.2%. This distinct isotopic signature suggests that the enrichment is unlikely to be derived from organic or mineral P fertilizers, natural or anthropogenic atmospheric contributions or sediments from a nearby stream. Rather, the Cd additions are probably sourced from decomposing tree litter. Further study is required, however, to ascertain whether the Cd enrichments reflects recent use of tree litter as fertilizer in the cacao plantations or natural accumulation of Cd from decomposing tree material over a much longer timescale.
Second, the beans of the three investigated cacao cultivars appear to show differences in Cd isotope signatures. In detail, beans from CCN-51 hybrid plants show no resolvable Cd isotope fractionation relative to the leaves whilst the relatively pure Nacional cultivar has beans that display lower δ114/110Cd than the leaves, with Δ114/110Cdbean–leaf values of −0.34‰ and −0.40‰. As such, the data may suggest that the Nacional and CCN-51 cultivars differ in the molecular mechanisms that are employed for the translocation and sequestration of Cd between cacao leaves, pods and beans.
In summary, these results suggest that further, more comprehensive, coupled Cd isotope and concentration studies should be carried out to confirm whether such analyses indeed provide useful constraints on the origin and cycling of Cd in soil – cacao systems and the partitioning of Cd within cacao plants. In particular, the isotopic data may be of utility for molecular and genetic studies,63 which intend to identify the transporter proteins that govern Cd accumulation and partitioning in cacao plants, as well as for breeding efforts and the development of farming practices that aim to reduce the relatively high Cd concentrations of cacao beans grown on Cd-rich soils.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the Olga Triballat Institute, the French Cooperative Ethiquable and by the Isotopes Axe of the Observatoire Midi-Pyrénées (OMP-Toulouse). The analytical work in the UK was supported by STFC-GCRF funding to MR. Rémi Freydier from the Platform AETE-ISO, OSU OREME at the Montpellier University is thanked for the Q-ICP-MS measurements. We are grateful to Nicolas Eberhart from Ethiquable for his help with sample collection in Esmeraldas and the cacao farmers for their support in the field, especially to Belia Vaca and Galo Rodriguez. The critical but constructive comments by four reviewers of an earlier manuscript version and two referees of the current text were immensely helpful in crafting a significantly improved article.References
- Agency for Toxic Substances Disease Registry (ATSDR), Toxicological profile for cadmium, US. Department of Health and Human Services, September 2012, https://www.atsdr.cdc.gov/toxprofiles/tp5-c6.pdf Search PubMed.
- S. He, X. Yang, Z. He and V. C. Baligar, Morphological and physiological responses of plants to cadmium toxicity: a review, Pedosphere, 2017, 27(3), 421–438, DOI:10.1016/S1002-0160(17)60339-4.
- The European Comission, Comission Regulation (EU) No 488/2014 of 12 May 2014 Amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs, Off. J. Eur. Union, 2014 Search PubMed.
- D. Bertoldi, A. Barbero, F. Camin, A. Caligiani and R. Larcher, Multielemental fingerprinting and geographic traceability of Theobroma cacao beans and cocoa products, Food Control, 2016, 65, 46–53, DOI:10.1016/j.foodcont.2016.01.013.
- E. Chavez, Z. L. He, P. J. Stoffella, R. S. Mylavarapu, Y. C. Li, B. Moyano and V. C. Baligar, Concentration of cadmium in cacao beans and its relationship with soil cadmium in Southern Ecuador, Sci. Total Environ., 2015, 533, 205–214, DOI:10.1016/j.scitotenv.2015.06.106.
- A. Gramlich, S. Tandy, C. Gauggel, M. López, D. Perla, V. Gonzalez and R. Schulin, Soil cadmium uptake by cocoa in Honduras, Sci. Total Environ., 2018, 612, 370–378, DOI:10.1016/j.scitotenv.2017.08.145.
- E. Arévalo-Gardini, C. O. Arévalo-Hernández, V. C. Baligar and Z. L. He, Heavy metal accumulation in leaves and beans of cacao (Theobroma cacao L.) in major cacao growing regions in Peru, Sci. Total Environ., 2017, 605–606, 792–800, DOI:10.1016/j.scitotenv.2017.06.122.
- F. Barraza, E. Schreck, T. Lévêque, G. Uzu, F. López, J. Ruales, J. Prunier, A. Marquet and L. Maurice, Cadmium bioaccumulation and gastric bioaccessibility in cacao: a field study in areas impacted by oil activities in Ecuador, Environ. Pollut., 2017, 229, 950–963, DOI:10.1016/j.envpol.2017.07.080.
- G. Ramtahal, I. C. Yen, I. Bekele, F. Bekele, L. Wilson, K. Maharaj and L. Harrynanan, Relationships between cadmium in tissues of cacao trees and soils in plantations of Trinidad and Tobago, Food Nutr. Sci., 2016, 07(1), 37–43, DOI:10.4236/fns.2016.71005.
- B. Kruszewski, M. W. Obiedziński and J. Kowalska, Nickel, cadmium and lead levels in raw cocoa and processed chocolate mass materials from three different manufacturers, J. Food Compos. Anal., 2018, 66, 127–135, DOI:10.1016/j.jfca.2017.12.012.
- B. A. Zarcinas, C. F. Ishak, M. J. McLaughlin and G. Cozens, Heavy metals in soils and crops in Southeast Asia: 1. Peninsular Malaysia, Environ. Geochem. Health, 2004, 26(3–4), 343–357, DOI:10.1007/s10653-005-4669-0.
- A. Gramlich, S. Tandy, C. Andres, J. Chincheros Paniagua, L. Armengot, M. Schneider and R. Schulin, Cadmium uptake by cocoa trees in agroforestry and monoculture systems under conventional and organic management, Sci. Total Environ., 2017, 580, 677–686, DOI:10.1016/j.scitotenv.2016.12.014.
- K. L. M. Zug, H. A. Huamaní Yupanqui, F. Meyberg, J. S. Cierjacks and A. Cierjacks, Cadmium accumulation in Peruvian cacao (Theobroma cacao L.) and opportunities for mitigation, Water, Air, Soil Pollut., 2019, 230, 72, DOI:10.1007/s11270-019-4109-x.
- E. Anyimah-Ackah, I. W. Ofosu, H. E. Lutterodt and G. Darko, Exposures and risks of arsenic, cadmium, lead and mercury in cocoa beans and cocoa-based foods: a systematic review, Food Qual. Saf., 2019, 3(1), 1–8, DOI:10.1093/fqsafe/fyy025.
- D. Argüello, E. Chávez, F. Lauryssen, R. Vanderschueren, E. Smolders and D. Montalvo, Soil properties and agronomic factors affecting cadmium concentrations in cacao beans: A nationwide survey in Ecuador, Sci. Total Environ., 2018, 649, 120–127, DOI:10.1016/j.scitotenv.2018.08.292.
- M. Shahid, C. Dumat, S. Khalid, N. K. Niazi and P. M. C. Antunes, Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant System, in Reviews of Environmental Contamination and Toxicology, ed. P. de Voogt, Springer International Publishing, Cham, 2016, vol. 241, pp. 73–137. DOI:10.1007/398_2016_8.
- S. He, Z. He, X. Yang, P. J. Stoffella and V. C. Baligar, Soil biogeochemistry, plant physiology and phytoremediation of cadmium-contaminated coils, in Advances in Agronomy, Elsevier, 2015, vol. 134, pp. 135–225 Search PubMed.
- E. Chavez, Z. L. He, P. J. Stoffella, R. S. Mylavarapu, Y. C. Li and V. C. Baligar, Chemical speciation of cadmium: an approach to evaluate plant-available cadmium in Ecuadorian soils under cacao production, Chemosphere, 2016, 150, 57–62, DOI:10.1016/j.chemosphere.2016.02.013.
- S. Clemens, M. G. M. Aarts, S. Thomine and N. Verbruggen, Plant science: the key to preventing slow cadmium poisoning, Trends Plant Sci., 2013, 18(2), 92–99, DOI:10.1016/j.tplants.2012.08.003.
- Y. Song, L. Jin and X. Wang, Cadmium absorption and transportation pathways in plants, Int. J. Phytorem., 2017, 19(2), 133–141, DOI:10.1080/15226514.2016.1207598.
- T. Yoneyama, T. Gosho, M. Kato, S. Goto and H. Hayashi, Xylem and phloem transport of Cd, Zn and Fe into the grains of rice plants (Oryza sativa L.) grown in continuously flooded Cd-contaminated soil, Soil Sci. Plant Nutr., 2010, 56(3), 445–453, DOI:10.1111/j.1747-0765.2010.00481.x.
- L.-Z. Li, C. Tu, W. J. G. M. Peijnenburg and Y.-M. Luo, Characteristics of cadmium uptake and membrane transport in roots of intact wheat (Triticum aestivum L) seedlings, Environ. Pollut., 2017, 221, 351–358, DOI:10.1016/j.envpol.2016.11.085.
- L. Fontanili, C. Lancilli, N. Suzui, B. Dendena, Y.-G. Yin, A. Ferri, S. Ishii, N. Kawachi, G. Lucchini and S. Fujimaki, et al., Kinetic analysis of zinc/cadmium reciprocal competitions suggests a possible Zn-insensitive pathway for root-to-shoot cadmium translocation in rice, Rice, 2016, 9(1) DOI:10.1186/s12284-016-0088-3.
- B.-F. Yan, C. Nguyen, O. S. Pokrovsky, F. Candaudap, C. Coriou, S. Bussière, T. Robert and J. Y. Cornu, Contribution of remobilization to the loading of cadmium in durum wheat grains: impact of post-anthesis nitrogen supply, Plant Soil, 2018, 424(1–2), 591–606, DOI:10.1007/s11104-018-3560-6.
- N. Engbersen, A. Gramlich, M. Lopez, G. Schwarz, B. Hattendorf, O. Gutierrez and R. Schulin, Cadmium accumulation and allocation in different cacao cultivars, Sci. Total Environ., 2019, 678, 660–670, DOI:10.1016/j.scitotenv.2019.05.001.
- S. Sghayar, A. Ferri, C. Lancilli, G. Lucchini, A. Abruzzese, M. Porrini, T. Ghnaya, F. F. Nocito, C. Abdelly and G. A. Sacchi, Analysis of cadmium translocation, partitioning and tolerance in six barley (Hordeum vulgare L.) cultivars as a function of thiol metabolism, Biol. Fertil. Soils, 2015, 51(3), 311–320, DOI:10.1007/s00374-014-0977-9.
- M. F. Mengist, D. Milbourne, D. Griffin, M. J. McLaughlin, J. Creedon, P. W. Jones and S. Alves, Cadmium uptake and partitioning in potato (Solanum tuberosum L.) cultivars with different tuber-Cd concentration, Environ. Sci. Pollut. Res., 2017, 24(35), 27384–27391, DOI:10.1007/s11356-017-0325-3.
- L. Meng, T. Huang, J. Shi, J. Chen, F. Zhong, L. Wu and J. Xu, Decreasing cadmium uptake of rice (Oryza sativa L.) in the cadmium-contaminated paddy field through different cultivars coupling with appropriate soil amendments, J. Soils Sediments, 2019, 19(4), 1788–1798, DOI:10.1007/s11368-018-2186-x.
- Q. Xu, C. Wang, S. Li, B. Li, Q. Li, G. Chen, W. Chen and F. Wang, Cadmium adsorption, chelation and compartmentalization limit root-to-shoot translocation of cadmium in rice (Oryza sativa L.), Environ. Sci. Pollut. Res., 2017, 24(12), 11319–11330, DOI:10.1007/s11356-017-8775-1.
- C. Lewis, A. M. Lennon, G. Eudoxie and P. Umaharan, Genetic variation in bioaccumulation and partitioning of cadmium in Theobroma cacao L, Sci. Total Environ., 2018, 640–641, 696–703, DOI:10.1016/j.scitotenv.2018.05.365.
- M. Wiggenhauser, M. Bigalke, M. Imseng, M. Müller, A. Keller, K. Murphy, K. Kreissig, M. Rehkämper, W. Wilcke and E. Frossard, Cadmium isotope fractionation in soil–wheat systems, Environ. Sci. Technol., 2016, 50(17), 9223–9231, DOI:10.1021/acs.est.6b01568.
- M. Wiggenhauser, M. Bigalke, M. Imseng, A. Keller, M. Rehkämper, W. Wilcke and E. Frossard, Using isotopes to trace freshly applied cadmium through mineral phosphorus fertilization in soil-fertilizer-plant systems, Sci. Total Environ., 2019, 648, 779–786, DOI:10.1016/j.scitotenv.2018.08.127.
- M. Imseng, M. Wiggenhauser, A. Keller, M. Müller, M. Rehkämper, K. Murphy, K. Kreissig, E. Frossard, W. Wilcke and M. Bigalke, Towards an understanding of the Cd isotope fractionation during transfer from the soil to the cereal grain, Environ. Pollut., 2019, 244, 834–844, DOI:10.1016/j.envpol.2018.09.149.
- M. Imseng, M. Wiggenhauser, A. Keller, M. Müller, M. Rehkämper, K. Murphy, K. Kreissig, E. Frossard, W. Wilcke and M. Bigalke, Fate of Cd in agricultural soils: a stable isotope approach to anthropogenic impact, soil formation, and soil-plant cycling, Environ. Sci. Technol., 2018, 52(4), 1919–1928, DOI:10.1021/acs.est.7b05439.
- M. Salmanzadeh, A. Hartland, C. H. Stirling, M. R. Balks, L. A. Schipper, C. Joshi and E. George, Isotope tracing of long-term cadmium fluxes in an agricultural soil, Environ. Sci. Technol., 2017, 51(13), 7369–7377, DOI:10.1021/acs.est.7b00858.
- R. Wei, Q. Guo, G. Yu, J. Kong, S. Li, Z. Song, J. Hu, L. Tian, X. Han and C. P. Okoli, Stable isotope fractionation during uptake and translocation of cadmium by tolerant Ricinus communis and hyperaccumulator Solanum nigrum as influenced by EDTA, Environ. Pollut., 2018, 236, 634–644, DOI:10.1016/j.envpol.2018.01.103.
- Instituto Nacional de Meteorología e Hidrología (INAMHI), Anuario Meteorológico del Ecuador, no. 52-2012, 2012, http://www.serviciometeorologico.gob.ec/wp-content/uploads/anuarios/meteorologicos/Am/202012.pdf Search PubMed.
- Instituto Nacional de Meteorología e Hidrología (INAMHI), Climas del Ecuador, 2006, http://www.serviciometeorologico.gob.ec/gisweb/TIPO_DE_CLIMAS/PDF/CLIMAS/DEL/ECUADOR/2016.pdf Search PubMed.
- M. S. Beg, S. Ahmad, K. Jan and K. Bashir, Status, supply chain and processing of cocoa – a review, Trends Food Sci. Technol., 2017, 66, 108–116, DOI:10.1016/j.tifs.2017.06.007.
- P. Vargas Jentzsch, V. Ciobotă, W. Salinas, B. Kampe, P. M. Aponte, P. Rösch, J. Popp and L. A. Ramos, Distinction of Ecuadorian varieties of fermented cocoa beans using Raman spectroscopy, Food Chem., 2016, 211, 274–280, DOI:10.1016/j.foodchem.2016.05.017.
- J. Kummerow, M. Kummerow and W. Souza da Silva, Fine-root growth dynamics in cacao (Theobroma cacao), Plant Soil, 1982, 65(2), 193–201, DOI:10.1007/bf02374650.
- W. Niether, U. Schneidewind, M. Fuchs, M. Schneider and L. Armengot, Below- and aboveground production in cocoa monocultures and agroforestry systems, Sci. Total Environ., 2019, 657, 558–567, DOI:10.1016/j.scitotenv.2018.12.050.
- P. Fallavier, D. Babre and M. Breysse, Détermination de la capacité d’échange cationique des sols tropicaux acides, Agron. Trop., 1985, 40–4, 298–308 Search PubMed.
- K. Murphy, M. Rehkämper, K. Kreissig, B. Coles and T. van de Flierdt, Improvements in Cd stable isotope analysis achieved through use of liquid–liquid extraction to remove organic residues from Cd separates obtained by extraction chromatography, J. Anal. At. Spectrom., 2016, 31(1), 319–327, 10.1039/c5ja00115c.
- W. Abouchami, S. J. G. Galer, T. J. Horner, M. Rehkämper, F. Wombacher, Z. Xue, M. Lambelet, M. Gault-Ringold, C. H. Stirling and M. Schönbächler, et al., A Common reference material for cadmium isotope studies – NIST SRM 3108, Geostand. Geoanal. Res., 2013, 37(1), 5–17, DOI:10.1111/j.1751-908X.2012.00175.x.
- A.-D. Schmitt, S. J. G. Galer and W. Abouchami, Mass-dependent cadmium isotopic variations in nature with emphasis on the marine environment, Earth Planet. Sci. Lett., 2009, 277(1–2), 262–272, DOI:10.1016/j.epsl.2008.10.025.
- M. Rehkämper, F. Wombacher, T. J. Horner and Z. Xue, Natural and anthropogenic Cd isotope variations, in Handbook of Environmental Isotope Geochemistry, ed. M. Baskaran, Springer, Berlin Heidelberg, 2012, pp. 125–154, DOI:10.1007/978-3-642-10637-8_8.
- Y. Zhang, H. Wen, C. Zhu, H. Fan, C. Luo, J. Liu and C. Cloquet, Cd isotope fractionation during simulated and natural weathering, Environ. Pollut., 2016, 216, 9–17, DOI:10.1016/j.envpol.2016.04.060.
- L. E. Wasylenki, J. W. Swihart and S. J. Romaniello, Cadmium isotope fractionation during adsorption to Mn oxyhydroxide at low and high ionic strength, Geochim. Cosmochim. Acta, 2014, 140, 212–226, DOI:10.1016/j.gca.2014.05.007.
- L. Bridgestock, M. Rehkämper, T. van de Flierdt, K. Murphy, R. Khondoker, A. R. Baker, R. Chance, S. Strekopytov, E. Humphreys-Williams and E. P. Achterberg, The Cd isotope composition of atmospheric aerosols from the tropical Atlantic Ocean, Geophys. Res. Lett., 2017, 44(6), 2932–2940, DOI:10.1002/2017gl072748.
- A. E. Shiel, D. Weis and K. J. Orians, Evaluation of zinc, cadmium and lead isotope fractionation during smelting and refining, Sci. Total Environ., 2010, 408(11), 2357–2368, DOI:10.1016/j.scitotenv.2010.02.016.
- C. Cloquet, J. Carignan, G. Libourel, T. Sterckeman and E. Perdrix, Tracing source pollution in soils using cadmium and lead isotopes, Environ. Sci. Technol., 2006, 40(8), 2525–2530, DOI:10.1021/es052232+.
- E. Martinková, V. Chrastný, M. Francová, A. Šípková, J. Čuřík, O. Myška and L. Mižič, Cadmium isotope fractionation of materials derived from various industrial processes, J. Hazard. Mater., 2016, 302, 114–119, DOI:10.1016/j.jhazmat.2015.09.039.
- F. Fouskas, L. Ma, M. A. Engle, L. Ruppert, N. J. Geboy and M. A. Costa, Cadmium isotope fractionation during coal combustion: insights from two U.S. coal-fired power plants, Appl. Geochem., 2018, 96, 100–112, DOI:10.1016/j.apgeochem.2018.06.007.
- P. Artaxo, L. V. Rizzo, J. F. Brito, H. M. J. Barbosa, A. Arana, E. T. Sena, G. G. Cirino, W. Bastos, S. T. Martin and M. O. Andreae, Atmospheric aerosols in Amazonia and land use change: from natural biogenic to biomass burning conditions, Faraday Discuss., 2013, 165, 203, 10.1039/c3fd00052d.
- B. Gao, Y. Liu, K. Sun, X. Liang, P. Peng, G. Sheng and J. Fu, Precise determination of cadmium and lead isotopic compositions in river sediments, Anal. Chim. Acta, 2008, 612(1), 114–120, DOI:10.1016/j.aca.2008.02.020.
- N. Pallavicini, E. Engström, D. C. Baxter, B. Öhlander, J. Ingri and I. Rodushkin, Cadmium isotope ratio measurements in environmental matrices by MC-ICP-MS, J. Anal. At. Spectrom., 2014, 29(9), 1570–1584, 10.1039/c4ja00125g.
- A.-M. Aucour, J.-P. Bedell, M. Queyron, R. Tholé, A. Lamboux and G. Sarret, Zn speciation and stable isotope fractionation in a contaminated urban wetland soil – Typha latifolia system, Environ. Sci. Technol., 2017, 51(15), 8350–8358, DOI:10.1021/acs.est.6b02734.
- M. Wiggenhauser, M. Bigalke, M. Imseng, A. Keller, C. Archer, W. Wilcke and E. Frossard, Zinc isotope fractionation during grain filling of wheat and a comparison of zinc and cadmium isotope ratios in identical soil-plant systems, New Phytol., 2018, 219(1), 195–205, DOI:10.1111/nph.15146.
- F. Lahive, P. Hadley and A. J. Daymond, The physiological responses of cacao to the environment and the implications for climate change resilience. A review, Agron. Sustainable Dev., 2019, 230, 72, DOI:10.1007/s13593-018-0552-0.
- A.-A. F. De Almeida and R. R. Valle, Ecophysiology of the Cacao Tree, Braz. J. Plant Physiol., 2007, 19(4), 425–448, DOI:10.1590/S1677-04202007000400011.
- R. Wei, Q. Guo, H. Wen, C. Liu, J. Yang, M. Peters, J. Hu, G. Zhu, H. Zhang and L. Tian, et al., Fractionation of stable cadmium isotopes in the cadmium tolerant Ricinus communis and hyperaccumulator Solanum nigrum, Sci. Rep., 2016, 6, 24309, DOI:10.1038/srep24309.
- I. Ullah, Y. Wang, D. J. Eide and J. M. Dunwell, Evolution and functional analysis of natural resistance-associated macrophage proteins (NRAMPs) from Theobroma cacao and their role in cadmium accumulation, Sci. Rep., 2018, 8, 14412, DOI:10.1038/s41598-018-32819-y.
Footnotes |
| † Electronic supplementary information (ESI) available: Section 1: detailed information on Methods and materials, including study site locations, collection and initial treatment of cacao and soil samples, sample digestion, the Cd isotope measurements and error propagation. Fig. S1: locations of cacao Farms A, B and C in Ecuador. Table S1: microwave program used for digestion of cacao samples. Table S2: comparison of Cd concentrations measured by Q-ICP-MS for certified reference materials with reference values. Table S3: cadmium concentrations and isotope compositions obtained by MC-ICP-MS for reference and quality control materials and comparison with published results. Section 2 with Table S4: detailed physicochemical data for the soil samples. See DOI: 10.1039/c9ra05516a |
| ‡ Current affiliation: Instituto de Cultivos Tropicales (ICT), Tarapoto, Peru. |
| § Joint first authors. |
| This journal is © The Royal Society of Chemistry 2019 |