Open Access Article
Open Access ArticleThe dynamic CO2 adsorption of polyethylene polyamine-loaded MCM-41 before and after methoxypolyethylene glycol codispersion†
Xia Wang *a,
Wulan Zenga,
Hongyan Zhanga,
Dan Lia,
Hongjing Tianb,
Xiude Huc,
Qian Wu
*a,
Wulan Zenga,
Hongyan Zhanga,
Dan Lia,
Hongjing Tianb,
Xiude Huc,
Qian Wu *a,
Chunling Xin*a,
Xiaoyu Caoa and
Wenjing Liua
*a,
Chunling Xin*a,
Xiaoyu Caoa and
Wenjing Liua
aDepartment of Chemistry and Chemical Engineering, Weifang University, Weifang 261061, Shandong, China. E-mail: xiawangwfu@163.com
bCollege of Chemical Engineering, Qingdao University of Science & Technology, Qingdao 266042, China
cState Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, China
First published on 28th August 2019
Abstract
To reduce the cost of CO2 capture, polyethylene polyamine (PEPA), with a high amino density and relatively low price, was loaded into MCM-41 to prepare solid sorbents for CO2 capture from flue gases. In addition, methoxypolyethylene glycol (MPEG) was codispersed and coimpregnated with PEPA to prepare composite sorbents. The pore structures, surface functional groups, adsorption and regeneration properties for the sorbents were measured and characterized. When CO2 concentration is 15%, for 30, 40 and 50 wt% PEPA-loaded MCM-41, the equilibrium adsorption capacities were respectively determined to be 1.15, 1.47 and 1.66 mmol g−1 at 60 °C; for 30 wt% PEPA and 20 wt% MPEG, 40 wt% PEPA and 10 wt% MPEG, and 50 wt% PEPA and 5 wt% MPEG codispersed MCM-41, the equilibrium adsorption capacities were respectively determined to be 1.97, 2.22 and 2.25 mmol g−1 at 60 °C; the breakthrough and equilibrium adsorption capacities for 50 wt% PEPA and 5 wt% MPEG codispersed MCM-41 respectively reached 2.01 and 2.39 mmol g−1 at 50 °C, all values showed a significant increase compared to PEPA-modified MCM-41. After 10 regenerations, the equilibrium adsorption capacity for codispersed MCM-41 was reduced by 5.0%, with the regeneration performance being better than that of PEPA-loaded MCM-41, which was reduced by 7.8%. The CO2-TPD results indicated that the mutual interactions between PEPA and MPEG might change basic sites in MCM-41, thereby facilitating active site exposure and CO2 adsorption.
1. Introduction
Coal-fired power plants are undoubtedly large CO2 emission points all over the world, in which the capture of CO2 has been extensively researched; Bui et al. has reviewed the progress for carbon capture and storage (CCS) and pointed out the way forward.1–3 Solid amine adsorption technology, with weak corrosivity, highly efficient adsorption performance and low regeneration energy consumption, is recognized as an effective method.4–7So far the study of amine-loaded solid sorbents mainly focuses on immobilizing amines onto the inner surface of porous supporting materials, such as metal–organic frameworks (MOFs),8–13 mesoporous molecular sieves,14–22 silicas23–31 and so on.32–40 Chen et al. incorporated polyethyleneimine (PEI) into mesoporous zeolite 13X prepared by pore-expanding original micropores; due to PEI-dispersion in mesopores, the CO2 adsorption capacity for PEI-modified 13X reached 80 mg g−1 at 100 °C under a pure CO2 atmosphere.16 Saha immobilized PEI in silicas and polymethyl methacrylate (PMMA) to prepare type I adsorbents, grafted different amounts of aminosilanes in mesoporous SBA-15 to prepare type II adsorbents and performed breakthrough adsorption experiments. Both sorbents showed significant adsorption capacities and consistent regenerability during 3–5 adsorption–desorption cycles.5 Cheng et al. incorporated amines into silicas with trimodal pore structures, and the nanocomposite sorbents suggested rapid adsorption kinetics and good adsorption performance, with an adsorption capacity of 172 mg g−1 being obtained for an amine loading amount of 70 wt%.25 To further improve the dispersion and stability of TEPA or PEI with high viscosity, auxiliary reagents with hydroxyl or ether groups were coloaded into supporting materials for preparing composite sorbents.41–44 Jung et al. coimpregnated PEI and 1,3-butadienediepoxide (BDDE) with hydroxyl groups in silicas to investigate the improved CO2 adsorption performance of PEI-loaded silicas, which was primarily attributed to following two aspects. On the one hand, synergistic combinations between hydroxyl groups and the PEI on the inner surface of the supporting materials occurred, which promoted the dispersion and thermostability of PEI in silicas. On the other hand, after coimpregnation, the chemisorption ratio for amine and CO2 molecules changed from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the CO2 adsorption efficiency improved.41 Mafra et al. performed two-dimensional solid-state nuclear magnetic resonance (NMR) experiments on amine-modified silicas after adsorbing CO2; the results showed that all chemisorbed CO2 molecules participated in hydrogen bonding interactions with either neighboring alkylamines or surface silanols, and the work helped one to understand the configuration of chemisorbed CO2.27 Afonso et al. prepared primary amine-modified mesoporous silicas and performed NMR experiments; different conformations of carbamic acid, alkylammonium carbamate as well as hydrogen bonds were confirmed using a density functional theory (DFT) model. The study also provided well understanding of the chemisorbed CO2 in amine-oxide hybrid surfaces at a molecular-level.28
1, and the CO2 adsorption efficiency improved.41 Mafra et al. performed two-dimensional solid-state nuclear magnetic resonance (NMR) experiments on amine-modified silicas after adsorbing CO2; the results showed that all chemisorbed CO2 molecules participated in hydrogen bonding interactions with either neighboring alkylamines or surface silanols, and the work helped one to understand the configuration of chemisorbed CO2.27 Afonso et al. prepared primary amine-modified mesoporous silicas and performed NMR experiments; different conformations of carbamic acid, alkylammonium carbamate as well as hydrogen bonds were confirmed using a density functional theory (DFT) model. The study also provided well understanding of the chemisorbed CO2 in amine-oxide hybrid surfaces at a molecular-level.28
In this work, PEPA, with similar amino density but a lower price than tetraethylenepentamine (TEPA) and PEI (the price for PEPA, TEPA and PEI were respectively ¥68 per 500 mL, ¥199 per 500 mL and ¥1199 per 500 mL), was impregnated into mesoporous MCM-41 to prepare solid amine sorbents, and MPEG was also codispersed with PEPA to prepare composite sorbents. The pore structures, surface functional groups and desorption properties for MCM-41 before and after loading were characterized; the adsorption and regeneration performances were also studied. In addition, the improvement in the adsorption performance of PEPA-loaded MCM-41 due to the MPEG was also discussed.
2. Experimental
2.1 Materials
MCM-41 was provided by the Tianjin Nanhua Catalyst Company in China, polyethylene polyamine (PEPA) and methoxypolyethylene glycol (MPEG) were bought from Shanghai Aladdin Bio-Chem Technology Co., Ltd in China, and anhydrous ethanol was supplied by Sinopharm Chemical Reagent Co., Ltd in China. N2 (99.999%) and the simulated flue gas, with a volume ratio of 85% N2 (99.999%) to 15% CO2 (99.999%), were distributed by the Anqiu Hengan Gas Factory.2.2 Preparation of PEPA-loaded MCM-41 before and after MPEG codispersion
The preparation of PEPA-loaded MCM-41 adopted a wet impregnation method under ultrasound-assistance.15 A calculated volume of PEPA was dissolved in anhydrous ethanol in a beaker, with the solution being sonicated for 30 min; then, MCM-41 with a mass of 1.0 g was quickly added and the beaker was continuously sonicated for another 120 min. The mixture was dried in an electric drying oven at 90 °C for 24 h and named as MCM-41-PEPAa, where a is the weight loading amount of PEPA in the sorbent.For PEPA and MPEG codispersed MCM-41, the preparation process was as above, and a calculated amount of PEPA and MPEG were first codispersed in anhydrous ethanol before impregnating into MCM-41. The corresponding composite sorbents were named as MCM-41-PEPAa-Mb, with M representing MPEG and a, b separately representing the weight loading amounts of PEPA, MPEG in the composite sorbents.
2.3 Characterization
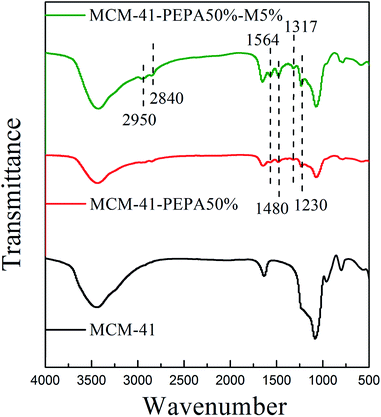
The N2 adsorption–desorption isotherms for PEPA-loaded MCM-41 before and after MPEG codispersion were performed using an ASAP 2460 (Micromeritics, America) at an analysis bath temperature of 77 K. The BET surface area was measured and calculated by the Brunauer–Emmett–Teller (BET) equation; the pore volume was estimated from the N2 adsorption amount at a relative pressure of P/P0 = 0.99; the pore size distribution curves were determined from the Barrett–Joyner–Halenda (BJH) desorption branch.The surface functional groups for PEPA-loaded MCM-41 before and after MPEG codispersion were measured using Fourier-transform infrared spectroscopy (FT-IR) with a TENSOR-27 (Bruker, Germany) over a frequency range of 4000 to 500 cm−1.
X-ray diffraction patterns were collected using the ASAP 2020 V4.01 X-ray diffractometer with Cu Ka radiation (λ = 0.154 nm) in the range of 2θ = 10 − 80°.
Temperature-programmed desorption (TPD) experiments were conducted using a fully automatic multipurpose instrument: a TP-5080 (Xianquan, China). CO2 adsorption was performed at 60 °C, and then the temperature was elevated at a rate of 10 K min−1 in N2.
The thermostability for TEPA and PEPA was determined using a NETZSCH, STA 449F3 (Netzsch, Germany). 10 milligrams of the sample was placed in an alumina pan and N2 was passed at a flow rate of 40 mL min−1 at room temperature; then, the temperature was heated to 700 °C at a rate of 10 K min−1.
2.4 CO2 adsorption and regeneration experiments
CO2 adsorption and regeneration experiments were performed on our self-assembled fixed-bed reactor, in which the inner diameter and length of the quartz tube were 0.8 and 40 cm, respectively.18 The sorbent with a weight of 1.0 g was filled into the reactor, and N2 was passed in at a flow rate of 30 mL min−1; then, the temperature was increased to 100 °C and held constant for 60 min; the temperature was then decreased to a certain adsorption temperature, and the inlet gas was switched to the simulated flue gas with a flow rate of 30 mL min−1, where in the CO2 adsorption process began. Meanwhile, the CO2 concentration at the reactor outlet (C) was detected by on-line gas chromatography. When C doesn't change and equals the CO2 concentration at the inlet (C0), an adsorption process finishes, which is called equilibrium adsorption stage, with the adsorption amount at this stage being the equilibrium adsorption capacity; when C is equal to 5% of C0, a breakthrough adsorption stage is reached, in which the adsorption time and amount are called the breakthrough time and breakthrough adsorption capacity. The adsorption capacity was integrally calculated from the breakthrough curve as discussed previously.15After completing the equilibrium adsorption, the inlet gas was switched to N2 and the temperature was increased to 100 °C to perform the desorption process. When CO2 was not checked at the outlet, the desorption process was completed and the sorbent was regenerated. Ten adsorption–desorption regenerations were performed in this study.
3. Results and discussions
3.1 Characterization
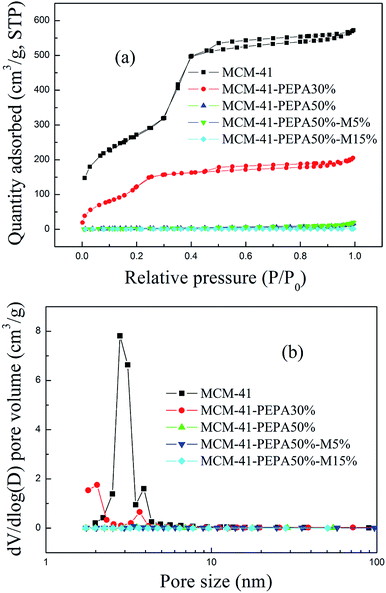 | ||
| Fig. 1 (a) N2 adsorption–desorption isotherms and (b) pore size distribution curves for MCM-41 before and after loading. | ||
| Sorbent | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| MCM-41 | 956.15 | 0.98 | 3.10 |
| MCM-41-PEPA30% | 545.21 | 0.31 | 2.30 |
| MCM-41-PEPA50% | 10.23 | 0.023 | 8.91 |
| MCM-41-PEPA50%-M5% | 11.34 | 0.027 | 9.68 |
| MCM-41-PEPA50%-M15% | 2.33 | 0.0026 | 4.39 |
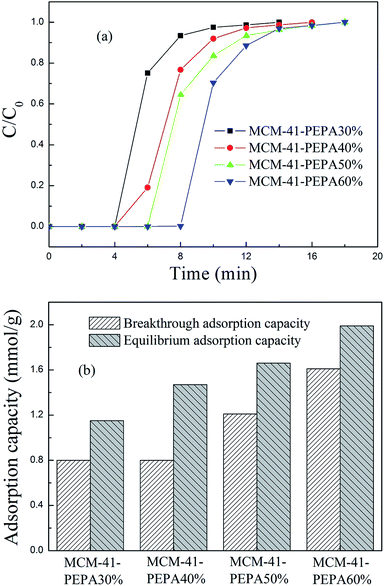
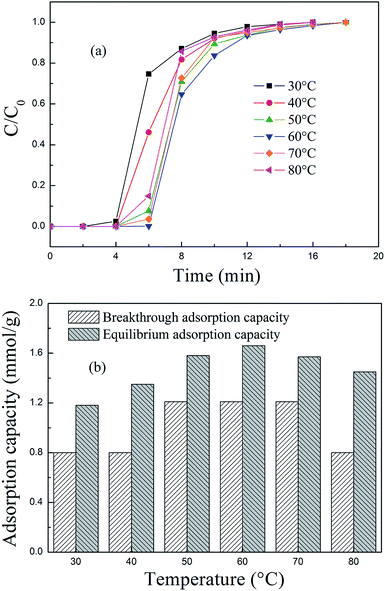
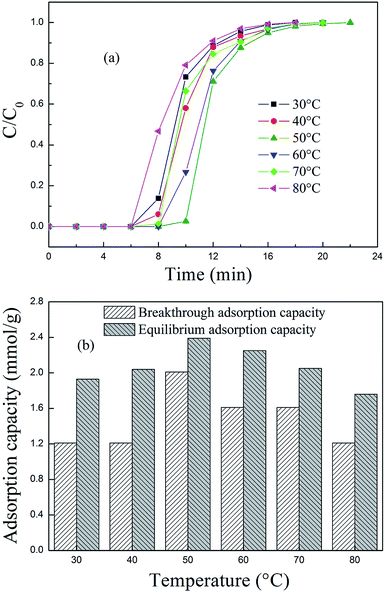
3.2 PEPA-loaded MCM-41 for CO2 adsorption
3.3 PEPA and MPEG codispersed MCM-41 for CO2 adsorption
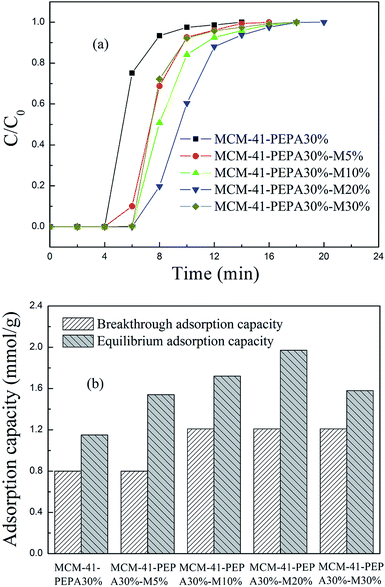 | ||
| Fig. 5 The (a) breakthrough adsorption curves and (b) adsorption capacities for 30 wt% PEPA and different ratios of MPEG codispersed MCM-41 at 60 °C. | ||
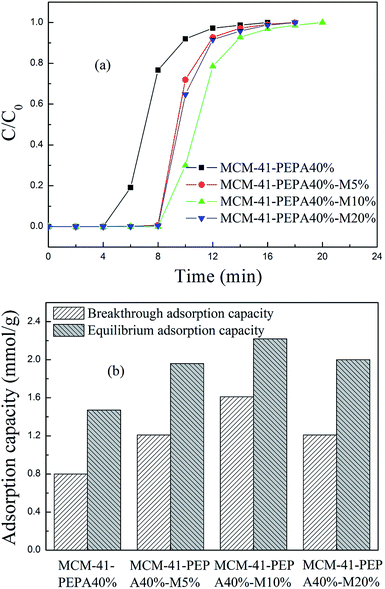 | ||
| Fig. 6 The (a) breakthrough adsorption curves and (b) adsorption capacities for 40 wt% PEPA and different ratios of MPEG codispersed MCM-41 at 60 °C. | ||
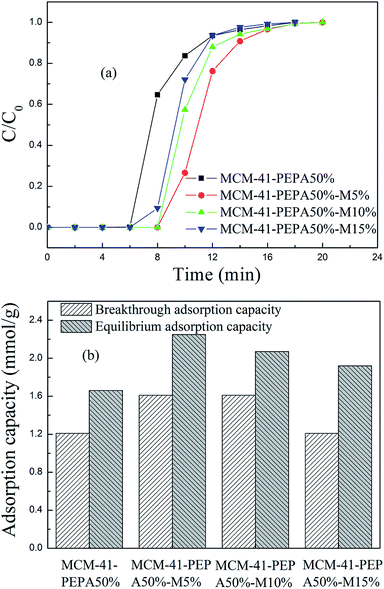 | ||
| Fig. 7 The (a) breakthrough adsorption curves and (b) adsorption capacities for 50 wt% PEPA and different ratios of MPEG codispersed MCM-41 at 60 °C. | ||
However, the increasing of MPEG didn't cause the adsorption performance for PEPA-loaded MCM-41 to improve continuously, but showed a gradual reduction. MPEG improved the dispersion of easily agglomerated PEPA into the pore channels of MCM-41, but too much MPEG addition could also lead to channel blockage and a decrease in adsorption performance. Among the studied sorbents, 50 wt% PEPA and 5 wt% MPEG codispersed MCM-41 showed good adsorption performance at 60 °C.
3.4 Regeneration
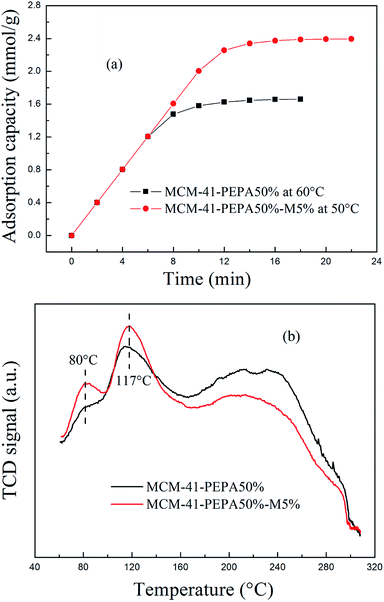
MCM-41-PEPA50% and MCM-41-PEPA50%-M5% were selected to further investigate the regenerability during 10 adsorption–desorption cycles. The adsorption temperatures for MCM-41-PEPA50% and MCM-41-PEPA50%-M5% were respectively 60 and 50 °C, and the desorption temperatures were all 100 °C, which were set according to previous studies.40 The equilibrium adsorption capacity data versus cycle number figures are depicted in Fig. 9. For both sorbents, the adsorption capacity remained stable during the first three cycles, and then showed a slight decrease. After 10 regenerations, the equilibrium adsorption capacity for MCM-41-PEPA50%-M5% was reduced by 5.0% compared to the fresh sorbent, being better than the corresponding value of 7.8% for MCM-41-PEPA50%. Thus, the introduction of MPEG improved the regenerability for PEPA-loaded MCM-41; the reason for this was attributed to the synergistic interactions between functional groups. The synergistic interactions enhanced the stability of PEPA in MCM-41, which reduced its loss during regenerations.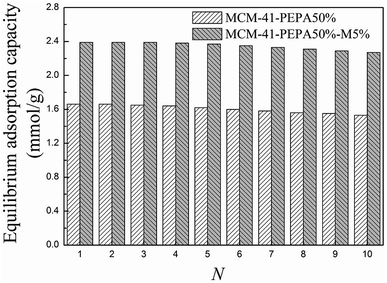 | ||
| Fig. 9 The equilibrium adsorption capacities for MCM-41-PEPA50% and MCM-41-PEPA50%-M5% during 10 adsorption–desorption cycles. | ||
The FT-IR and XRD spectra for MCM-41-PEPA50%-M5% before and after 10 cycles are shown in Fig. 3S and 4S.† Comparing with fresh MCM-41-PEPA50%-M5%, the characteristic peaks for the regenerated sample still retained, suggesting a good regenerability of MCM-41-PEPA50%-M5%; but the peak intensity showed slight decrease, which was due to the evaporation or degradation of the active composites during regenerations. The phenomenon was in accordance with the results from Fig. 9.
3.5 Adsorption and desorption kinetics
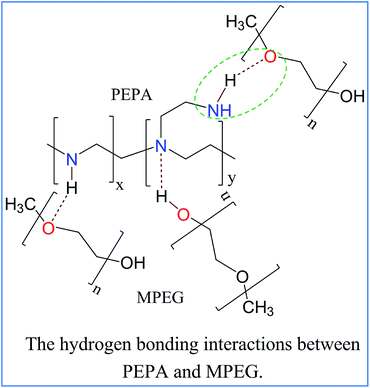
In MCM-41-PEPA50%-M5%, two strong desorption peaks appeared at both 80 and 117 °C, suggesting that the addition of MPEG changed the basic sites in the sorbents. In addition to the original amino sites from PEPA, other basic sites appeared at a much lower temperature of 80 °C, which were the amino groups participating in the hydrogen bonding interactions with the ether and hydroxyl groups from MPEG. The hydrogen bonding interactions helped with the dispersion and exposure of the amino groups, and the optimum adsorption temperature for codispersed MCM-41 was reduced to 50 °C. The CO2-TPD results also confirmed the mutual interactions between PEPA and MPEG, with similar phenomenon previously reported for NaNO3-promoted MgO for CO2 capture.47
4. Conclusions
PEPA, with a similar amino density but lower price than TEPA, was found to be more suitable for preparing amine-loaded MCM-41 sorbents, in which the breakthrough and equilibrium adsorption performance as well as regenerability were found to be comparable with that for TEPA-loaded MCM-41. To improve the exposure and dispersion of viscous PEPA in MCM-41, different mass ratios of MPEG were codispersed and coimpregnated into MCM-41 to prepare composite sorbents for CO2 capture. For 50 wt% PEPA and 5 wt% MPEG codispersed MCM-41, the breakthrough and equilibrium adsorption capacities were respectively 2.01 and 2.39 mmol g−1 at 50 °C, which were improved by 66% and 44% compared to the corresponding values for 50 wt% PEPA-loaded MCM-41 under the same operating conditions; after 10 regenerations, the equilibrium adsorption capacity was reduced by 5.0%, which is lower than the corresponding value of 7.8% for 60 wt% PEPA-loaded MCM-41.The codispersed sorbent of MCM-41-PEPA50%-M5% showed a rapid breakthrough adsorption stage, with a rate of 0.20 mmol g−1 min−1, and showed an adsorption capacity that was 84% of the equilibrium adsorption capacity, indicating a high adsorption efficiency. The CO2-TPD results indicated that the codispersed MCM-41 contained two kinds of basic sites, with one appearing at a lower temperature, which was attributed to the synergistic hydrogen bonding interactions between PEPA and MPEG.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The kind financial support from the Natural Science Foundation of Shandong Province (grant number ZR2017BEE038), the Foundation of State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (grant number 2017-K29), the Doctoral Research Program of Weifang University (grant number 2017BS07) and the Natural Science Foundation Joint Project of Shandong Province (grant number ZR2017LB025) is gratefully appreciated.References
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony and A. Boston, Carbon capture and storage (CCS): the way forward, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- S. M. Rafigh and A. Heydarinasab, Mesoporous chitosan-SiO2 nanoparticles: synthesis, characterization, and CO2 adsorption capacity, ACS Sustainable Chem. Eng., 2017, 5, 10379–10386 CrossRef CAS.
- L. Nie, Y. Mu, J. Jin, J. Chen and J. Mi, Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas, Chin. J. Chem. Eng., 2018, 26, 2303–2317 CrossRef.
- J. Wang, L. Huang, R. Yang, Z. Zhang, J. Wu, Y. Gao, Q. Wang, D. O'Hareb and Z. Zhong, Recent advances in solid sorbents for CO2 capture and new development trends, Energy Environ. Sci., 2014, 7, 3478–3518 RSC.
- A. Saha, Structure-function, recyclability and calorimetry studies of CO2 adsorption on some amine modified Type I & Type II sorbents, Int. J. Greenhouse Gas Control, 2018, 78, 198–209 CrossRef CAS.
- K. Sim, N. Lee, J. Kim, E.-B. Cho, C. Gunathilake and M. Jaroniec, CO2 adsorption on amine-functionalized periodic mesoporous benzenesilicas, ACS Appl. Mater. Interfaces, 2015, 7, 6792–6802 CrossRef CAS PubMed.
- R. Veneman, N. Frigka, W. Zhao, Z. Li, S. Kersten and W. Brilman, Adsorption of H2O and CO2 on supported amine sorbents, Int. J. Greenhouse Gas Control, 2015, 41, 268–275 CrossRef CAS.
- F. Martínez, R. Sanz, G. Orcajo, D. Briones and V. Yángüez, Amino-impregnated MOF materials for CO2 capture at post-combustion conditions, Chem. Eng. Sci., 2016, 142, 55–61 CrossRef.
- W. Li and S. Li, CO2 adsorption performance of functionalized metal-organic frameworks of varying topologies by molecular simulations, Chem. Eng. Sci., 2018, 189, 65–74 CrossRef CAS.
- S. Gaikwad, S.-J. Kim and S. Han, CO2 capture using amine-functionalized bimetallic MIL-101 MOFs and their stability on exposure to humid air and acid gases, Microporous Mesoporous Mater., 2019, 277, 253–260 CrossRef CAS.
- C. Chen, N. Feng, Q. Guo, Z. Xue, L. Ding, L. Wang, H. Wan and G. Guan, Surface engineering of a chromium metal-organic framework with bifunctional ionic liquids for selective CO2 adsorption: synergistic effect between multiple active sites, J. Colloid Interface Sci., 2018, 521, 91–101 CrossRef CAS PubMed.
- R. Huang, M. R. Hill, R. Babarao and N. V. Medhekar, CO2 adsorption in azobenzene functionalized stimuli responsive metal-organic frameworks, J. Phys. Chem. C, 2016, 120, 16658–16667 CrossRef CAS.
- M. Jia, Y. Feng, J. Qiu, X. Zhang and J. Yao, Amine-functionalized MOFs@GO as filler in mixed matrix membrane for selective CO2 separation, Sep. Purif. Technol., 2019, 213, 63–69 CrossRef CAS.
- G. Zhang, P. Zhao, L. Hao and Y. Xu, Amine-modified SBA-15(P): a promising adsorbent for CO2 capture, J. CO2 Util., 2018, 24, 22–33 CrossRef CAS.
- X. Wang, L. Chen and Q. Guo, Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture, Chem. Eng. J., 2015, 260, 573–581 CrossRef CAS.
- C. Chen, S.-S. Kim, W.-S. Cho and W.-S. Ahn, Polyethylenimine-incorporated zeolite 13X with mesoporosity for post-combustion CO2 capture, Appl. Surf. Sci., 2015, 332, 167–171 CrossRef CAS.
- G. Zhang, P. Zhao, L. Hao, Y. Xu and H. Cheng, A novel amine double functionalized adsorbent for carbon dioxide capture using original mesoporous silica molecular sieves as support, Sep. Purif. Technol., 2019, 209, 516–527 CrossRef CAS.
- X. Wang, Q. Guo, J. Zhao and L. Chen, Mixed amine-modified MCM-41 sorbents for CO2 capture, Int. J. Greenhouse Gas Control, 2015, 37, 90–98 CrossRef.
- J. Cheng, Y. Li, L. Hu, J. Liu, J. Zhou and K. Cen, CO2 absorption and diffusion in ionic liquid [P66614][Triz] modified molecular sieves SBA-15 with various pore lengths, Fuel Process. Technol., 2018, 172, 216–224 CrossRef CAS.
- C. Zhou, K. He, W. Lv, Y. Chen, S. Tang, C. Liu, H. Yue and B. Liang, Energy and economic analysis for post-combustion CO2 capture using amine-functionalized adsorbents in a temperature vacuum swing process, Energy Fuels, 2019, 33, 1774–1784 CrossRef CAS.
- M. G. Yıldız, T. Davran-Candan, M. E. Günay and R. Yıldırım, CO2 capture over amine-functionalized MCM-41 and SBA-15: exploratory analysis and decision tree classification of past data, J. CO2 Util., 2019, 31, 27–42 CrossRef.
- S. Loganathan and A. K. Ghoshal, Amine tethered pore-expanded MCM-41: a promising adsorbent for CO2 capture, Chem. Eng. J., 2017, 308, 827–839 CrossRef CAS.
- H. Zhang, A. Goeppert, S. Kar and G. K. S. Prakash, Structural parameters to consider in selecting silica supports for polyethylenimine based CO2 solid adsorbents. Importance of pore size, J. CO2 Util., 2018, 26, 246–253 CrossRef CAS.
- Q. Lai, Z. Diao, L. Kong, H. Adidharma and M. Fan, Amine-impregnated silicic acid composite as an efficient adsorbent for CO2 capture, Appl. Energy, 2018, 223, 293–301 CrossRef CAS.
- P. Zhao, G. Zhang, Y. Xu, Y.-K. Lv, Z. Yang and H. Cheng, Development of amine-functionalized silica foams with hierarchical pore structure for CO2 capture, Energy Fuels, 2019, 33, 3357–3369 CrossRef CAS.
- X. Li, Y. Ding, L. Guo, Q. Liao, X. Zhu and H. Wang, Non-aqueous energy-efficient absorbents for CO2 capture based on porous silica nanospheres impregnated with amine, Energy, 2019, 171, 109–119 CrossRef CAS.
- L. Mafra, T. Čendak, S. Schneider, P. V. Wiper, J. Pires, J. R. B. Gomes and M. L. Pinto, Structure of chemisorbed CO2 species in amine-functionalized mesoporous silicas studied by solid-state NMR and computer modeling, J. Am. Chem. Soc., 2017, 139, 389–408 CrossRef CAS PubMed.
- R. Afonso, M. Sardo, L. Mafra and J. R. B. Gomes, Unravelling the structure of chemisorbed CO2 species in mesoporous aminosilicas: a critical survey, Environ. Sci. Technol., 2019, 53, 2758–2767 CrossRef CAS PubMed.
- L. Mafra, T. Čendak, S. Schneider, P. V. Wiper, J. Pires, J. R. B. Gomes and M. L. Pinto, Amine functionalized porous silica for CO2/CH4 separation by adsorption: which amine and why, Chem. Eng. J., 2018, 336, 612–621 CrossRef CAS.
- X. Hou, L. Zhuang, B. Ma, S. Chen, H. He and F. Yin, Silanol-rich platelet silica modified with branched amine for efficient CO2 capture, Chem. Eng. Sci., 2018, 181, 315–325 CrossRef CAS.
- E. S. Sanz-Pérez, T. C. M. Dantas, A. Arencibia, G. Calleja, A. P. M. A. Guedes, A. S. Araujo and R. Sanz, Reuse and recycling of amine-functionalized silica materials for CO2 adsorption, Chem. Eng. J., 2017, 308, 1021–1033 CrossRef.
- X. Wang, W. Zeng, Q. Guo, Q. Geng, Y. Yan and X. Hu, The further activation and functionalization of semicoke for CO2 capture from flue gases, RSC Adv., 2018, 8, 35521–35527 RSC.
- E. Vilarrasa-García, J. A. Cecilia, D. C. S. Azevedo, C. L. Cavalcante and E. Rodríguez-Castellón, Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture, Microporous Mesoporous Mater., 2017, 249, 25–33 CrossRef.
- M. Parvazinia, S. Garcia and M. Maroto-Valer, CO2 capture by ion exchange resins as amine functionalised adsorbents, Chem. Eng. J., 2018, 331, 335–342 CrossRef CAS.
- F. Liu, S. Chen and Y. Gao, Synthesis of porous polymer based solid amine adsorbent: effect of pore size and amine loading on CO2 adsorption, J. Colloid Interface Sci., 2017, 506, 236–244 CrossRef CAS PubMed.
- Y. Kong, G. Jiang, Y. Wu, S. Cui and X. Shen, Amine hybrid aerogel for high-efficiency CO2 capture: effect of amine loading and CO2 concentration, Chem. Eng. J., 2016, 306, 362–368 CrossRef CAS.
- Y. Liu, B. Sajjadi, W.-Y. Chen and R. Chatterjee, Ultrasound-assisted amine functionalized graphene oxide for enhanced CO2 adsorption, Fuel, 2019, 247, 10–18 CrossRef CAS.
- S. Lawson, C. Griffin, K. Rapp, A. A. Rownaghi and F. Rezaei, Amine-functionalized MIL-101 monoliths for CO2 removal from enclosed environments, Energy Fuels, 2019, 33, 2399–2407 CrossRef CAS.
- W. Jung, J. Park and K. S. Lee, Kinetic modeling of CO2 adsorption on an amine-functionalized solid sorbent, Chem. Eng. Sci., 2018, 177, 122–131 CrossRef CAS.
- X. Wang, D. Wang, M. Song, C. Xin and W. Zeng, Tetraethylenepentamine-modified activated semicoke for CO2 capture from flue gas, Energy Fuels, 2017, 31, 3055–3061 CrossRef CAS.
- H. Jung, S. Jeon, D. H. Jo, J. Huh and S. H. Kim, Effect of crosslinking on the CO2 adsorption of polyethyleneimine-impregnated sorbents, Chem. Eng. J., 2017, 307, 836–844 CrossRef CAS.
- X. Wang, W. Zeng, M. Song, F. Wang, X. Hu, Q. Guo and Y. Liu, Polyetheramine improves the CO2 adsorption behavior of tetraethylenepentamine-functionalized sorbents, Chem. Eng. J., 2019, 364, 475–484 CrossRef CAS.
- M. B. Yue, L. B. Sun, Y. Cao, Z. J. Wang, Y. Wang, Q. Yu and J. H. Zhu, Promoting the CO2 adsorption in the amine-containing SBA-15 by hydroxyl group, Microporous Mesoporous Mater., 2008, 114, 74–81 CrossRef CAS.
- W. Yan, J. Tang, Z. Bian, J. Hu and H. Liu, Carbon dioxide capture by amine-impregnated mesocellular foam-containing template, Ind. Eng. Chem. Res., 2012, 51, 3653–3662 CrossRef CAS.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies, John Wiley & Sons, Chichester, UK, 2rd edn, 2001 Search PubMed.
- X. Wang, Q. Guo and T. Kong, Tetraethylenepentamine-modified MCM-41/silica gel with hierarchical mesoporous structure for CO2 capture, Chem. Eng. J., 2015, 273, 472–480 CrossRef CAS.
- M. L. T. Triviño, V. Hiremath and J. G. Seo, Stabilization of NaNO3-promoted magnesium oxide for high-temperature CO2 capture, Environ. Sci. Technol., 2018, 52, 11952–11959 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra05404a |
| This journal is © The Royal Society of Chemistry 2019 |