Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel efficient bioflocculant QZ-7 for the removal of heavy metals from industrial wastewater
Zayed M. M. Abu Tawila ab,
Salmah Ismail*a,
Salem S. Abu Amrc and
Emad K. Abou Elkhairb
ab,
Salmah Ismail*a,
Salem S. Abu Amrc and
Emad K. Abou Elkhairb
aInstitute of Biological Science, Faculty of Science, University of Malaya, 50603, Kuala Lumpur, Malaysia. E-mail: salmah_r@um.edu.my; Tel: +60379672143
bBiology Department, Faculty of Science, Al-Azhar University, Gaza, Palestine
cMalaysian Institute of Chemical & Bioengineering Technology, Universiti Kuala Lumpur, (UniKL, MICET), 78000, Melaka, Malaysia
First published on 4th September 2019
Abstract
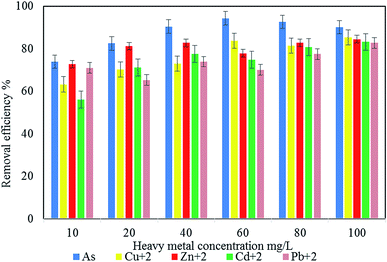
In this study, a novel bioflocculant QZ-7 was produced from Bacillus salmalaya 139SI for industrial wastewater treatment. Biochemical analysis, FTIR, scanning electron microscopy-energy dispersive X-ray spectroscopy, and thermogravimetric analysis were performed. A synthetic wastewater sample was used to validate the performance of the prepared OZ-7 for the adsorption efficiency of As, Zn2+ Pb2+, Cu2+, and Cd2+ under optimal experimental conditions such as initial metal concentrations, pH, contact time (h) and QZ-7 adsorbent dosage (mg mL−1). The maximum removal efficiency for Zn2+ (81.3%), As (78.6%), Pb2+ (77.9%), Cu2+ (76.1%), and Cd2+ (68.7%) was achieved using an optimal bioflocculant dosage of 60 mg L−1 at 2 h shaking time, 100 rpm and pH 7. Furthermore, the obtained optimum experimental conditions were validated using real industrial wastewater and the removal efficiencies of 89.8%, 77.4% and 58.4% were obtained for As, Zn2+ and Cu2+, respectively. The results revealed that the prepared bioflocculant QZ-7 has the capability to be used for the removal of heavy metals from industrial wastewater.
1. Introduction
Resources of fresh drinking water are globally considered as the most significant reservoirs. For survival, fresh drinking water must be accessible to all living organisms on the planet.1,2 Increasing world population, developing industries, and long droughts decrease the available water resources.3 Inefficient treatment of industrial wastewater results in the contamination of natural water resources by high level of refractory pollutants.4 Metals are the most abundant chemical elements found in the earth's crust; they are also frequently found in foods at low concentrations. Thus, their nutritional or toxicological hazard differs according to the types of metals and their concentrations.5 Anthropogenic industries, such as agriculture and mining, lack environment-friendly techniques and along with natural disasters, such as earthquakes, storms, and volcanoes, discharge toxic substances in water.6 Wastewater pollution containing heavy metal ions has raised a wide concern for environmental sustainability, calling for the development of high-efficiency and low-cost materials for wastewater treatment.7 These contaminants are characterized into three main groups: organic, inorganic, and biological elements.6 Waste production from mines results in vast environmental complications, in which draining from tailings and rock waste discharges can increase the concentration of acid and noxious heavy metals. Heavy metals persist in trace quantities in the environment and at small concentrations, they play significant roles in the living organisms; however, they can cause toxicity if their concentrations exceed the recommended level. Toxic metal contamination is considered as one of the pivotal environmental problems, which is generally prevalent in industrialized cities, where the increase in the toxic metal concentration beyond the recommended level can increase many health problems such as carcinogenic diseases and Alzheimer's or Huntington's disease.7 For example, heavy metals such as arsenic, lead, mercury, copper, cadmium, nickel, zinc, and chromium act as the primary noxious wastes in fresh water reservoirs because of their harmful, non-biodegradable, and persistent nature.3 Industrial development is the basic source of heavy metals that pass through various environment paths, including water, soil, air, and the biosphere. Heavy metals are easily absorbed by seafood, vegetables and eventually by the human body because of their high solubility in the aquatic environment.3 The combination of heavy metals with the sulfhydryl groups of proteins prevents enzyme activity, and such combination occurs in contaminated ecosystems, leading to consequences for the human health.8 Thus, in living organisms, the strong affinity of arsenite to methanethiol (mercaptan) groups, such as peptides, proteins, and amino acids, involves the types of enzymes that can cause severe noxiousness to humans.9 Environmental systems (abiotic methods) have been utilized for removing heavy metals from polluted locations. Abiotic methods include adsorption, the use of activated carbon, reverse osmosis, chemical precipitation, and ion exchange. Flocculating materials are commonly applied in numerous fields, including water treatment, fabric industry, cosmetics, drug treatment, and those related to fermentation development.10 In literature, several applications and techniques have been reported for industrial wastewater treatment. Several specialists have worked on advanced competent treatment skills. Treatment technologies are principally based on physicochemical, advanced oxidation, or electrochemical methods.4 Recently, a biological remediation technique using a permeable reactive barrier (PRB) has been identified as the most cost effective and safe technique; it involves organic, (date seed, Moringa oleifera and wood chips) and inorganic (limestone; CaCO3) carbonaceous materials in varying proportions for nitrate (NO3) remediation.11 Phytoremediation is a potential cost-effective technology for remediating heavy metal-contaminated soils. For example, Pennisetum sp. was found to be the best species for Cu and Cd removal.12 Also, the biomasses of Elsholtzia splendens and Sedum plumbizincicola were found to be good species for Cu and Cd removal.12 The obtained results showed that the functionalized halloysite nanotubes enhanced the adsorption capability of the clay mineral and it made nanoclay a good candidate for heavy metal removal from aqueous solutions.13 Physicochemical procedures comprise adsorption, ion exchange, membrane filtration, and chemical precipitation. Electrochemical methods are characterized by electro-coagulation, electro-deposition, and electro-flotation.3 Photo-catalysis is a unique feature of innovative oxidation techniques. Recently, nanotechnology has been applied in the treatment of wastewater. Among these potential approaches, those with cost-effectiveness, environment-friendliness, and no extra contaminant features are favored.3 Flocculant agents are categorized into three groups: organic polymer flocculants, such as polyacrylamide derivatives and polyacrylic acid; inorganic flocculants, such as polyaluminum chloride and aluminum sulfate; and microbial bioflocculants, which include glycoprotein, polysaccharides, and proteins.14,15 Wang et al. (2011)16 reported that the flocculants adsorbed onto the surface of suspended particles are essential for the application of vigorous attractive forces to control electrostatic repulsive force. Flocculating methods have an immediate effect on the suspended particle size, that is, a larger size results in more rapid settling down of particles.17Zhang et al. (2014)18 reported that the selection of the flocculant type presents a significant effect on the capacity of the flocculating techniques, potency of the accumulated particles, and the degree of bond stability as a consequence of the flocculation process. However, regardless of the significant effectiveness of the flocculating practices in water treatment, the main disadvantage of flocculation is the production of breakable flocs at low temperatures; these flocs can scatter the applied physical force.1
Therefore, “green technology” bioflocculants have attracted increasing methodical and technical consideration in environmental wastewater treatment because they are non-hazardous to humans, possess biodegradable constituents, and become free from pollutants by intermediate deprivation.19 In addition, bioflocculants have been generally involved in different applications, such as cleaning up of wastewater, decontamination of organic substance components from sugar, dewatering, dredging, pulp and paper industry, and condensation of mine wastes.14,20 A high cost prevents the advancement of bioflocculants for marketable use.18,21 Therefore, industries of large-scale production and utilization of bioflocculants as prospective alternatives of their artificial counterpart remain to be achieved. The present work aimed to evaluate the potential capability of a polymeric bioflocculant QZ-7 in removing heavy metals from industrial wastewater. In this research, the bioflocculant QZ-7 was produced from Bacillus salmalaya strain 139SI.22
Different bioflocculant dosages, initial heavy metal concentrations, pH values, and reaction times were examined and evaluated using synthetic wastewater samples. The performances obtained under the optimal operational conditions were compared with those of the actual wastewater samples.
2. Material and methods
2.1 Materials
Bioflocculant QZ-7 was produced from B. salmalaya 139SI.22 Heavy metal compounds including arsenite, lead acetate, copper sulfate, cadmium chloride, and zinc sulfate were used to prepare heavy metal stock solutions, and they were of analytical grade. Solutions of 1 M of HCl and NaOH were used for pH adjustment. Nitric acid was used for digestion process. Industrial wastewater samples were obtained from the Perlis state of Malaysia. Inductively coupled plasma mass spectrometry (AAS; Model AA 6300, Shimadzu, Japan) was performed to determine heavy metal concentration before and after adsorption. pH of the prepared solution was measured through a digital pH meter (BP3001 Trans instrument) using a calibrated electrode with standard buffer solutions.2.2 Characterization of bioflocculant QZ-7
2.3 Factors affecting bioflocculant performance
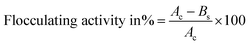 | (1) |
2.4 Factors affecting heavy metal adsorption
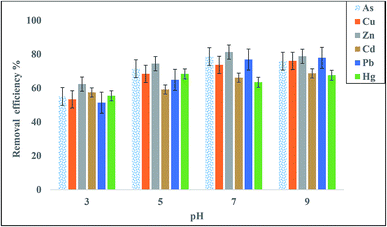
Removal of heavy metals using a bioflocculant was measured according to the work of Lin and Harichund.28 The metal salts used comprised copper sulfate, lead acetate, sodium arsenate, zinc sulfate, and cadmium chloride (Sigma Co). The effects of different initial concentrations of heavy metals (20, 40, 60, 80, and 100 mg L−1), bioflocculant concentrations (20, 40, 60, 80, and 100 mg L−1), and pH values (3, 5, 7, and 9) on metal adsorption were examined. QZ-7 solutions (5 mL) were added through a dialysis tube in flasks having 100 mL of each metal-salt solution and kept for 24 h at room temperature with shaking at 100 rpm. Next, 2 mL of each solution was filtered through an Amicon filter (Centrifree) and then acidified with 1% nitric acid solution for residual metal determination. The metal amounts removed from the samples tested, that is, the bound polymers were measured previously, whereas those that remained after 24 h were detected using inductively coupled plasma mass spectrometry (AAS; Model AA 6300, Shimadzu, Japan); the removal percentage rate for each element was calculated.2 Control (5 mL of deionized water) solutions were also prepared in the dialysis tube for different metal-salt solutions. Removal percentage (R) is expressed by eqn (2):| R = ((Ci − Ce)/Ci) × 100 | (2) |
2.5 Removal of heavy metals from industrial wastewater using bioflocculant QZ-7
Wastewater from the rubber industry was selected for the experiments. Removal efficiency analysis was performed as stated by the standard methods for examination of water and wastewater.29 Filtered wastewater samples (100 mL) were poured into a dialysis tube; different bioflocculant concentrations (20, 40, and 60 mg L−1) were added and stirred with a magnetic stirrer for 15 min at 100 rpm, slowly stirred for 5 min at 50 rpm, and allowed to settle for 15 min. The volume of the supernatant was filtered through a filter paper and used for heavy metal analysis before and after bioflocculant treatment through inductively coupled plasma mass spectrometry (AAS; Model AA 6300, Shimadzu, Japan); the metal removal percentage rate of each element was also calculated.30 Controls (5 mL of deionized water) were also prepared in the dialysis tube for different metal-salt solutions. Removal percentage (R) was expressed as eqn (2).2.6 Statistical analysis
Statistical analyses for the data collected were analysed using SPSS statistical version 25 software. Minimum three measurements were made to detect variability and to avoid biases. One-way ANOVA, a multiple comparison post-hoc test, was used for the determination of differences in each assay for cases of equality of variance assumed or not assumed. Significant differences were analysed through analysis of variance ANOVA for the basis of making conclusion and predication.31,323. Results and discussion
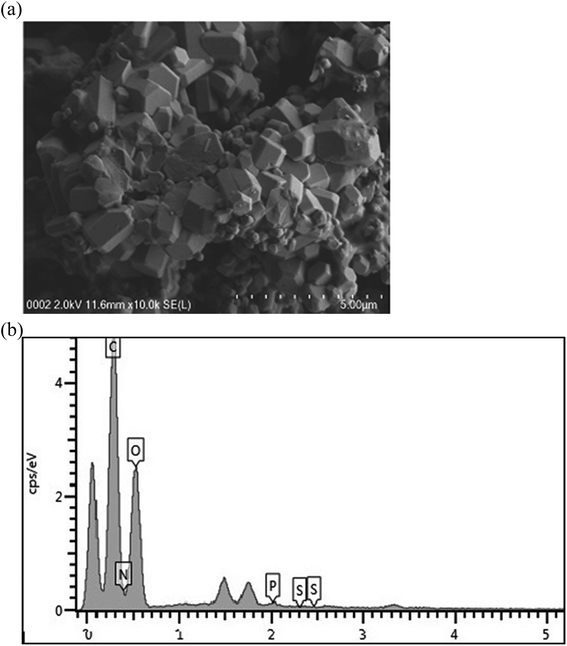
3.1 Characterization of bioflocculant QZ-7
| Component type | Analytical method | Occurrence |
|---|---|---|
| Polysaccharide | Anthrone test | + |
| Protein | Bradford test | + |
| Uronic acid | Carbazole-sulfuric acid test | + |
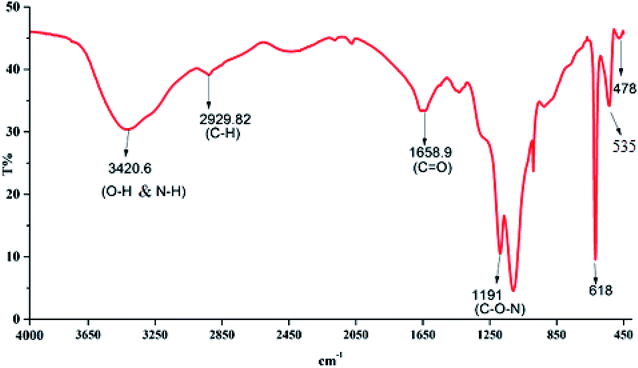
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the –CONH group in proteins and amino sugars.36 The band numbers between 1187 and 900 cm−1 indicated significantly different sugar derivatives.37 The band at 1109.66 cm−1 was ascribed to the asymmetrical stretching of the C–O–C ester linkage. The presence of a β-glycoside bond between the sugar monomers was indicated by small absorption bands at 618.50 cm−1, 535.79 cm−1 and 478.32 cm−1. The presence of –OH, NH–, COOH– and COO– groups in the bioflocculant molecules is important for determining the flocculating activity, while the H+ and OH− groups on the surface of the suspended particles may form hydrogen bonds when the bioflocculant chains approach the surface of the particles.38,39
O stretching in the –CONH group in proteins and amino sugars.36 The band numbers between 1187 and 900 cm−1 indicated significantly different sugar derivatives.37 The band at 1109.66 cm−1 was ascribed to the asymmetrical stretching of the C–O–C ester linkage. The presence of a β-glycoside bond between the sugar monomers was indicated by small absorption bands at 618.50 cm−1, 535.79 cm−1 and 478.32 cm−1. The presence of –OH, NH–, COOH– and COO– groups in the bioflocculant molecules is important for determining the flocculating activity, while the H+ and OH− groups on the surface of the suspended particles may form hydrogen bonds when the bioflocculant chains approach the surface of the particles.38,39
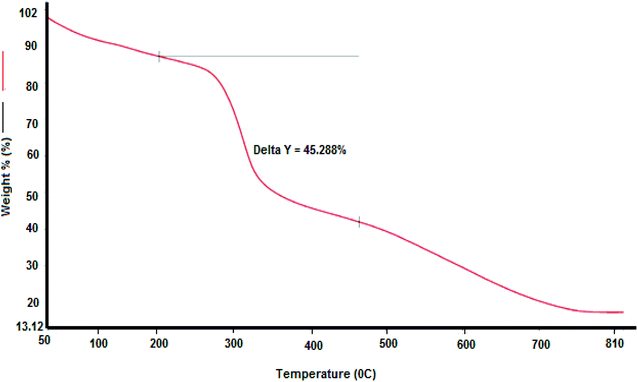
The first weight decrease may be due to the loss of available moisture or the presence of carboxyl and hydroxyl groups in the protein portions related to the glycoprotein-like molecules of QZ-7. Analogous observations were reported by Wang et al.16 with respect to the bioflocculant gained from the mixed consortium of bacteria.
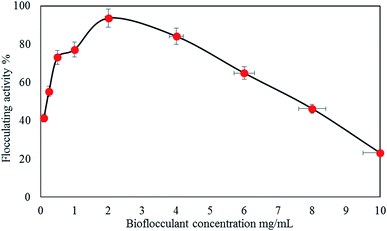
3.2 Factors affecting flocculating performance of bioflocculant
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 405.203 405.203 |
7 | 2343.600 | 2014.895 | 0.000 |
| Within groups | 18.610 | 16 | 1.163 | ||
| Total | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423.813 423.813 |
23 |
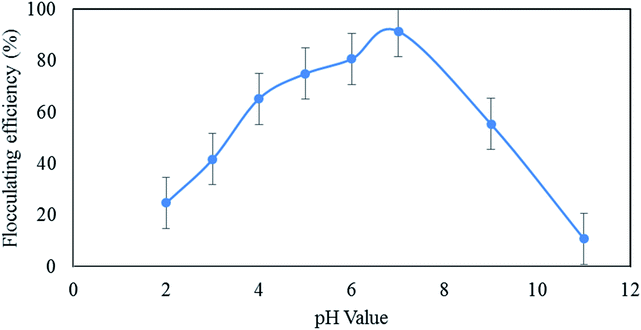
Table 3 shows that the result obtained is in accordance with the reported findings for the flocculation performance of different concentrations of bioflocculants with significant differences (p < 0.00).
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.856 241.856 |
8 | 1530.232 | 834.503 | 0.000 |
| Within groups | 33.007 | 18 | 1.834 | ||
| Total | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274.863 274.863 |
26 |
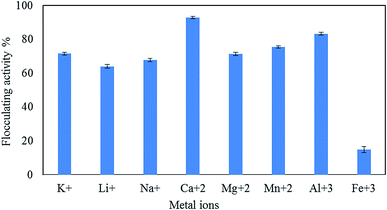
The statistical analysis presented in Table 4 indicates that there is a significant difference (p < 0.000) between different cations.
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327.947 327.947 |
7 | 1618.278 | 1016.387 | 0.000 |
| Within groups | 25.475 | 16 | 1.592 | ||
| Total | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353.422 353.422 |
23 |
3.3 Application of bioflocculant QZ-7
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 2555.898 | 6 | 425.983 | 274.912 | 0.000 |
| Within groups | 21.693 | 14 | 1.550 | ||
| Total | 2577.591 | 20 |
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 740.568 | 5 | 148.114 | 35.041 | 0.000 |
| Within groups | 50.723 | 12 | 4.227 | ||
| Total | 791.291 | 17 |
Based on the multiple analyses of the effect of heavy metal concentration on the bioflocculant adsorption efficiency shown in Table 7, there is a significant difference (p < 0.05) for the different concentrations of heavy metals.
| Sum of squares | df | Mean square | F | Sig. | |
|---|---|---|---|---|---|
| Between groups | 563.465 | 5 | 112.693 | 26.520 | 0.000 |
| Within groups | 50.991 | 12 | 4.249 | ||
| Total | 614.457 | 17 |
3.4 Removal of heavy metals from industrial wastewater using bioflocculant QZ-7
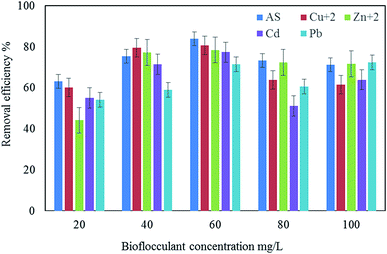
Rapid initial adsorption of the heavy metals can be achieved by the affinity action of amino, carboxyl and hydroxyl groups present in a bioflocculant.30 The treatment of industrial effluents with the bioflocculant QZ-7 at different concentrations, i.e., 20, 40, and 60 mg L−1 showed effective flocculation with concomitant reduction in heavy metals. Similarly, Fig. 10 depicts the effect of adsorbent dosage on the adsorption of heavy metals by bioflocculant QZ-7. For example, As removal increased from 71.9% to 89.85% with the amount of adsorbent concentration, whereas Zn2+ removal also increased from 63.85% to 77.4%. On the other hand, Cu2+ removal slightly increased from 53.2% to 58.4%.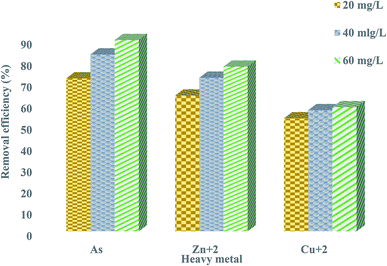 | ||
| Fig. 10 Removal efficiency of heavy metals using 20 mg L−1, 40 mg L−1 and 60 mg L−1 of bioflocculant QZ-7. | ||
4. Conclusion
The present study investigated the performance of a new polymeric bioflocculant QZ-7 for the removal of heavy metals from industrial wastewater. The removal efficiency for As3+, Cu2+, Zn2+, Cd2+, and Pb2+ was evaluated under varying experimental conditions, such as pH, incubation time, initial concentration of metal ions, and adsorbent dosage. Maximum flocculating efficiency (92.9%) was achieved for Ca2+ at pH 7.0 ± 0.2 followed by Al3+ (83.3%); Mn2+, K+, and Mg2+ (over 70%); Na+ and Li+ (above 64%). The maximum adsorptions of Zn2+ (81.2%) and As (78.6%) were detected at pH 7, whereas the maximum removal of Pb2+ (77.9%), Cu2+ (76.1%), and Cd2+ (68.7%) was obtained at pH 9. The results showed that the bioflocculant QZ-7 can be applied for the removal of heavy metals from industrial wastewater within the concentration range from 20 mg L−1 to 60 mg L−1. The bioflocculant was effective in the removal of As, Zn2+, and Cu2+. The findings established that the efficiency of heavy metal removal depends on the dose of low-cost adsorbent and bioflocculant concentrations. The optimum pH range for heavy metal adsorption ranged from 7 to 9.Funding
This study was supported by the University of Malaya, UMRG Programme under Grant No. RP023A-14 AFR.Conflicts of interest
There are no conflicts to declare.References
- K. Okaiyeto, U. U. Nwodo, S. A. Okoli, L. V. Mabinya and A. I. Okoh, MicrobiologyOpen, 2016, 5, 177–211 CrossRef PubMed.
- R. Gourdon, S. Bhende, E. Rus and S. S. Sofer, Biotechnol. Lett., 1990, 12, 839–842 CrossRef CAS.
- A. Azimi, A. Azari, M. Rezakazemi and M. Ansarpour, ChemBioEng Rev., 2017, 4, 37–59 CrossRef CAS.
- A. Mukherjee, T. Mandal, A. Ganguly and P. K. Chatterjee, ChemBioEng Rev., 2016, 3, 86–96 CrossRef CAS.
- M. Anastácio, A. M. dos Santos, M. Aschner and L. Mateus, Toxicol. Rep., 2018, 5, 434–439 CrossRef PubMed.
- V. K. Gupta and I. Ali, Environmental water: advances in treatment, remediation and recycling, Newnes, 2012 Search PubMed.
- F. Wang and Z. Guo, Appl. Clay Sci., 2019, 175, 115–123 CrossRef CAS.
- K. Nogawa and T. Kido, Toxicology of metals, 1996, pp. 353–369 Search PubMed.
- M. Altun, E. Sahinkaya, I. Durukan, S. Bektas and K. Komnitsas, J. Hazard. Mater., 2014, 269, 31–37 CrossRef CAS PubMed.
- H. Salehizadeh and N. Yan, Biotechnol. Adv., 2014, 32, 1506–1522 CrossRef CAS PubMed.
- M. Dahiru, A. B. N. Kartini and I. Yusoff, RSC Adv., 2019, 9, 15437–15447 RSC.
- L. Xu, X. Xing, J. Liang, J. Peng and J. Zhou, RSC Adv., 2019, 9, 993–1003 RSC.
- S. Cataldo, G. Lazzara, M. Massaro, N. Muratore, A. Pettignano and S. Riela, Appl. Clay Sci., 2018, 156, 87–95 CrossRef CAS.
- A. H. R. Aljuboori, A. Idris, N. Abdullah and R. Mohamad, Bioresour. Technol., 2013, 127, 489–493 CrossRef CAS PubMed.
- Z. Li, S. Zhong, H.-y. Lei, R.-w. Chen, Q. Yu and H.-L. Li, Bioresour. Technol., 2009, 100, 3650–3656 CrossRef CAS PubMed.
- L. Wang, F. Ma, Y. Qu, D. Sun, A. Li, J. Guo and B. Yu, World J. Microbiol. Biotechnol., 2011, 27, 2559–2565 CrossRef CAS.
- C. S. Lee, M. F. Chong, J. Robinson and E. Binner, Ind. Eng. Chem. Res., 2014, 53, 18357–18369 CrossRef CAS.
- D. Zhang, Z. Hou, Z. Liu and T. Wang, Int. J. Min. Sci. Technol., 2013, 23, 521–524 CrossRef CAS.
- C. Siah Lee, M. Fong Chong, J. P. Robinson and E. Binner, A Review on Development and Application of Plant-Based Bioflocculants and Grafted Bioflocculants, 2014 Search PubMed.
- H. Zhao, H. Liu and J. Zhou, Bioresour. Technol., 2013, 137, 226–232 CrossRef CAS PubMed.
- M. Fujita, M. Ike, S. Tachibana, G. Kitada, S. M. Kim and Z. Inoue, J. Biosci. Bioeng., 2000, 89, 40–46 CrossRef CAS.
- Z. Abu Tawila, S. Ismail, A. Dadrasnia and M. Usman, Molecules, 2018, 23, 2689 CrossRef PubMed.
- M. F. Chaplin and J. F. Kennedy, Carbohydrate analysis: a practical approach, IRL Press Ltd, 2nd edn, 1994 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- A. Ugbenyen and A. Okoh, Chem. Biochem. Eng. Q., 2013, 27, 511–518 CAS.
- J. Gao, H.-y. Bao, M.-x. Xin, Y.-x. Liu, Q. Li and Y.-f. Zhang, J. Zhejiang Univ., Sci., B, 2006, 7, 186–192 CrossRef CAS PubMed.
- W. Li, W. Zhou, Y. Zhang, J. Wang and X. Zhu, Bioresour. Technol., 2008, 99, 6893–6899 CrossRef CAS PubMed.
- J. Lin and C. Harichund, Afr. J. Microbiol. Res., 2011, 5, 599–607 CAS.
- A. P. H. Association and A. W. W. Association, Standard methods for the examination of water and wastewater, American Public Health Association, 1989 Search PubMed.
- B. Liu, X. Chen, H. Zheng, Y. Wang, Y. Sun, C. Zhao and S. Zhang, Carbohydr. Polym., 2018, 181, 327–336 CrossRef CAS PubMed.
- C. Zhu, C. Chen, L. Zhao, Y. Zhang, J. Yang, L. Song and S. Yang, J. Appl. Phycol., 2012, 24, 1245–1251 CrossRef CAS.
- X. Yong, W. Raza, G. Yu, W. Ran, Q. Shen and X. Yang, Bioresour. Technol., 2011, 102, 7548–7554 CrossRef CAS PubMed.
- A. Chen, C. Shang, J. Shao, Y. Lin, S. Luo, J. Zhang, H. Huang, M. Lei and Q. Zeng, Carbohydr. Polym., 2017, 155, 19–27 CrossRef CAS PubMed.
- H. Zhu, Y. Zhang, X. Yang, H. Liu, L. Shao, X. Zhang and J. Yao, J. Hazard. Mater., 2015, 296, 1–8 CrossRef CAS PubMed.
- R. Dobrowolski, A. Szcześ, M. Czemierska and A. Jarosz-Wikołazka, Bioresour. Technol., 2017, 225, 113–120 CrossRef CAS PubMed.
- M. Pathak, H. K. Sarma, K. G. Bhattacharyya, S. Subudhi, V. Bisht, B. Lal and A. Devi, Front. Microbiol., 2017, 8, 170 Search PubMed.
- J. Čopíková, A. S. Barros, I. Šmídová, M. Černá, D. H. Teixeira, I. Delgadillo, A. Synytsya and M. A. Coimbra, Carbohydr. Polym., 2006, 63, 355–359 CrossRef.
- U. Habiba, T. A. Siddique, T. C. Joo, A. Salleh, B. C. Ang and A. M. Afifi, Carbohydr. Polym., 2017, 157, 1568–1576 CrossRef CAS PubMed.
- D. Soto, J. Urdaneta, K. Pernia, O. León, A. Muñoz-Bonilla and M. Fernández-García, J. Polym. Environ., 2016, 24, 343–355 CrossRef CAS.
- H. Salehizadeh and S. Shojaosadati, Biotechnol. Adv., 2001, 19, 371–385 CrossRef CAS PubMed.
- G. M. Gadd, Curr. Opin. Biotechnol., 2000, 11, 271–279 CrossRef CAS PubMed.
- J. H. Yim, S. J. Kim, S. H. Ahn and H. K. Lee, Bioresour. Technol., 2007, 98, 361–367 CrossRef CAS PubMed.
- M. A. Hassan, T. P. Li and Z. Z. Noor, Journal of Chemical and Natural Resources Engineering, 2009, 4, 43–53 Search PubMed.
- Z. Liang, H. Baoping and L. Hong, Min. Sci. Technol., 2010, 20, 478–484 CAS.
- W.-X. Gong, S.-G. Wang, X.-F. Sun, X.-W. Liu, Q.-Y. Yue and B.-Y. Gao, Bioresour. Technol., 2008, 99, 4668–4674 CrossRef CAS PubMed.
- Y. Zheng, Z.-L. Ye, X.-L. Fang, Y.-H. Li and W.-M. Cai, Bioresour. Technol., 2008, 99, 7686–7691 CrossRef CAS PubMed.
- K. Okaiyeto, U. Nwodo, L. Mabinya and A. Okoh, Int. J. Environ. Res. Public Health, 2013, 10, 5097–5110 CrossRef CAS PubMed.
- C. Sekelwa, U. M. Anthony, M. L. Vuyani and O. I. Anthony, Afr. J. Microbiol. Res., 2013, 7, 2925–2938 CrossRef CAS.
- U. Nwodo, M. Agunbiade, E. Green, M. Nwamadi, K. Rumbold and A. Okoh, Materials, 2013, 6, 1237–1254 CrossRef CAS PubMed.
- P. Lodeiro, J. Barriada, R. Herrero and M. S. De Vicente, Environ. Pollut., 2006, 142, 264–273 CrossRef CAS.
- D. Sahoo, R. Kar and R. Das, Bioresour. Technol., 1992, 41, 177–179 CrossRef CAS.
- G. Bayramoğlu, S. Bektaş and M. Y. Arıca, J. Hazard. Mater., 2003, 101, 285–300 CrossRef.
- Y. Kacar, Ç. Arpa, S. Tan, A. Denizli, Ö. Genç and M. Y. Arıca, Process Biochem., 2002, 37, 601–610 CrossRef CAS.
- S. Das and S. Santra, New Frontiers of Environ. Biotechnol. Appl, 2007, vol. 3, pp. 1–10 Search PubMed.
- P. Puranik and K. Paknikar, Biotechnol. Prog., 1999, 15, 228–237 CrossRef CAS.
- K. Kavita, A. Mishra and B. Jha, Carbohydr. Polym., 2013, 94, 882–888 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |