Open Access Article
Open Access ArticleRetracted Article: Berberine alleviates amyloid beta-induced injury in Alzheimer's disease by miR-107/ZNF217
Jing Wang†
 *a and
Dong Jin†b
*a and
Dong Jin†b
aDepartment of Acupuncture, The Second Affiliated Hospital of Tianjin University of TCM, No. 69 Zengchan Road, Hebei District, 300211, Tianjin, China. E-mail: msxqmd@sina.com; Tel: +86-022-60637096
bDepartment of Traditional Chinese Medicine, PingJin Hospital, 300211, Tianjin, China
First published on 13th August 2019
Abstract
Berberine plays a neuroprotective role in neurodegenerative disorders, including Alzheimer's disease (AD). However, the underlying mechanism by which berberine inhibits AD progression remains largely unclear. The AD model was established using PC12 cells after treatment of amyloid beta (Aβ)25-35. Cells were transfected with microRNA (miRNA)-107 mimic, inhibitor, zinc finger protein 217 (ZNF217) overexpression or corresponding negative controls. Cell viability, apoptosis and inflammatory cytokine secretion were measured by MTT, flow cytometry or enzyme linked immunosorbent assay, respectively. The expressions of miR-107, ZNF217 and phosphorylated tau (p-Tau) were detected by quantitative real-time polymerase chain reaction or Western blot. The association between miR-107 and ZNF217 was explored by luciferase reporter assay and RNA immunoprecipitation. Berberine attenuated Aβ25-35-induced viability suppression in PC12 cells. Moreover, berberine inhibited the Aβ25-35-induced increase of inflammatory cytokine expression, apoptosis and p-Tau level in PC12 cells. miR-107 expression was reduced in Aβ25-35-treated PC12 cells and its overexpression alleviated Aβ25-35-induced injury, which was further weakened by combination with berberine. ZNF217 was a target of miR-107 and its addition reversed miR-107-mediated inhibition of inflammatory injury, apoptosis and phosphorylation of tau. Besides, ZNF217 protein level was decreased by berberine via regulating miR-107 in Aβ25-35-treated PC12 cells. Berberine protected against Aβ25-35-induced inflammatory injury, apoptosis and phosphorylation of tau by regulating miR-107 and ZNF217, indicating berberine as a promising neuroprotective agent for therapeutics of AD.
Introduction
Alzheimer's disease is one of the most common neurodegenerative diseases and lowers life quality by inducing dementia with the increasing aged population in China.1 The abnormal accumulation of amyloid beta (Aβ) peptides and hyperphosphorylation of tau protein (p-Tau) contribute to cognitive impairment leading to AD development.2 With the advance in AD pathogenesis, many therapeutic approaches are promising for AD treatment, including therapies targeted at Aβ, tau, apolipoprotein-E function, neuroinflammation and oxidative stress.3 However, their application in clinical practice needs to be further optimized.Berberine is a protoberberine alkaloid isolated from several medical plants, which exerts various pharmacological activities in therapeutics of cancers and multiple diseases.4 Moreover, berberine shows better therapeutic potential against neurodegenerative diseases, such as Parkinson's disease and AD.5 The emerging evidence suggests that berberine plays an important neuroprotective effect on mice with severe traumatic brain injury by reducing impairment of learning and memory skill.6 Additionally, berberine is found to ameliorate Aβ pathology and tau phosphorylation in vitro and in vivo and decrease cognitive impairment in AD mouse model.7 However, the underlying mechanism allows the neuroprotective role of berberine in AD remains largely unknown.
microRNAs (miRNAs) are a class of noncoding RNAs and plays important roles in cognitive impairment.8 Moreover, miRNAs have been reported as promising tools for prognosis and therapeutics of AD.9 A novel miRNA, miR-107, is found to inhibit cell apoptosis and improve spatial memory in mice with neurodegeneration.10 Wang et al. reported that miR-107 could attenuate traumatic brain injury and neurodegenerative disease progression by regulating granulin.11 More particularly, miR-107 is reported to be dysregulated in AD.12 An emerging evidence suggests that zinc finger protein 217 (ZNF217) might be implicated in Aβ-induced neurotoxicity in AD model of PC12 cells.13 We hypothesized that miR-107 and ZNF-217 might participate in berberine-mediated neuroprotective role in AD. In this study, we established cellular AD model using Aβ25-35-treated PC12 cells and investigated the potential role in inflammatory injury, apoptosis and accumulation of p-Tau. Furthermore, we explored the interaction between berberine and miR-107/ZNF217 in AD model.
Materials and methods
Cell culture and treatment
Rattus norvegicus pheochromocytoma PC12 cells (American Tissue Culture Collection, Manassas, VA, USA) were cultured in DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco), and 1% penicillin/streptomycin (Gibco) at 37 °C with 5% CO2. To construct cellular AD model in vitro, PC12 cells were treated with 10 μM of aggregated Aβ25-35 for 24 h. Aβ25-35 was purchased from Sigma (St. Louis, MO, USA), dissolved in distilled water and pre-aggregated through incubating at 37 °C for 7 days prior to use. To assay the role of berberine, PC12 cells were exposed to different concentrations of berberine (Sigma) for 24 h before the treatment of Aβ25-35.Cell viability
PC12 cells (1 × 104 per well) were seeded into 96-well plates, cultured at 37 °C overnight and then incubated with different concentrations of berberine for 24 h, followed by incubating with or without 10 μM Aβ25-35 for 24 h. At the ending point, cell medium was changed with fresh medium containing 0.5 mg ml−1 MTT solution (Sigma) and maintained at 37 °C for another 4 h. Subsequently, the medium was discarded and 100 μl DMSO (Sigma) was added to each well to solubilize the formazan. The cell viability was analyzed according to the absorbance at 490 nm using a microplate reader (Bio-Rad, Hercules, CA, USA) and normalized to non-treated group.Cell transfection
miR-107 mimic (miR-107), mimic negative control (miR-NC), miR-107 inhibitor (anti-miR-107), inhibitor negative control (anti-miR-NC), pcDNA-ZNF217 overexpression vector (ZNF217) and pcDNA empty vector were obtained from Genepharma (Shanghai, China). PC12 cells were maintained in 6-well plates to reach 70% confluence and then transfected with 40 nM oligonucleotides or vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After transfection of 24 h, transfection efficacy was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) or Western blot.Enzyme linked immunosorbent assay (ELISA)
Transfected or non-transfected PC12 cells (1 × 105 per well) were seeded into 12-well plates overnight and then treated with 1 μM berberine or 10 μM Aβ25-35 for 24 h. The cell culture medium was collected for analysis of IL-1β, IL-6 and TNF-α expressions using special human ELISA Kit (Invitrogen) referring to the manufacturer's instructions. The secretion levels of these inflammatory cytokines were measured by the representative standard curve according to the intensity of color at 450 nm with a microplate reader.Cell apoptosis
After treatment of 10 μM Aβ25-35 for 24 h, PC12 cells were digested and collected for apoptosis analysis by flow cytometry using Annexin V-FITC/PI apoptosis detection kit (Solarbio, Beijing, China) according to the manufacturer's instructions. The apoptotic cells (Annexin V-FITC+/PI+/−) were analyzed through a flow cytometer (BD Biosciences, San Jose, CA, USA).Western blot
Cell protein was extracted using RIPA lysis buffer and quantified using BCA protein assay kit (Beyotime, Shanghai, China). The equal amounts of denatured proteins were separated by SDS-PAGE gel electrophoresis, transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) and blocked with 5% BSA. The membranes were incubated with rabbit primary antibodies against p-Tau (ab109390, Abcam, Cambridge, MA, USA), ZNF217 (PA5-40738, Invitrogen) or β-actin (ab8227, Abcam) overnight at 4 °C and then interacted with appropriate second antibody (ab6721, Abcam) for 2 h at room temperature. The protein bands were visualized using enhanced chemiluminescence chromogenic substrate (Beyotime) and the relative expressions of protein were analyzed by Image Lab software (Bio-Rad) with β-actin as a loading control and normalized to corresponding control group.qRT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and reverse transcribed using TaqMan microRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The qRT-PCR was performed with SYBR master mix kit (Takara, Otsu, Japan) on Real-Time PCR Detection System (Bio-Rad) following the procedure: 95 °C for 5 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 1 min. The relative expressions of targeted RNAs were analyzed with U6 or GAPDH as internal control using 2−ΔΔCt method.14 The special primers were listed as follows: miR-107 forward primer: 5′-GTTAAGTCAGAGCGGGGCTT-3′, reverse primer: 5′-CACTCCGCTTTTTCAGTGCC-3′; U6 forward primer: 5′-CTCGCTTCGGCAGCACA-3′, reverse primer: 5′-AACGCTTCACGAATTTGCGT-3′; ZNF217 forward primer: 5′-GTTGTTCCATTCCGAGCTACA-3′, reverse primer: 5′-GGTAGGCCGGTGTTGCATTA-3′; GAPDH forward primer: 5′-AACGACCCCTTCATTGACCTC-3′, reverse primer: 5′-CCTTGACTGTGCCGTTGAACT-3′.Luciferase reporter assay
The fragment of 3′ UTR of ZNF217 containing putative binding sites of miR-107 predicted by starBase was amplified by PCR and cloned into the luciferase gene downstream of pmirGLO vectors (Promega, Madison, WI, USA) to generate wild type luciferase reporter vector, named as ZNF217-WT. The mutant luciferase reporter construct was generated by mutated the seed sites to GUUAAU using a site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA), named as ZNF217-MUT. For luciferase reporter assay, PC12 cells were seeded in 24-well plates and co-transfected with 40 nM miR-107 or miR-NC and 100 ng corresponding luciferase reporter constructs using Lipofectamine 2000. After the post-transfection for 48 h, dual-luciferase assay kit (Promega) was applied for the luciferase activity assay.RNA immunoprecipitation (RIP)
The Magna RIP Kit (Millipore) was used for argonaute 2 (Ago2) RIP assay to probe the link between miR-107 and ZNF217 following the protocol. In brief, PC12 cells were transfected with miR-107 or miR-NC for 24 h and then interacted with magnetic beads (Thermo Fisher) bounded with anti Ago2 (CST) or IgG antibody. The ZNF217 mRNA in beads was detected by qRT-PCR and normalized to a positive control (input).Statistical analysis
The experiments were repeated 3 times. All results were presented as the means ± standard deviation (S.D.). The differences were analyzed by student's t test or one-way ANOVA followed by Dunnett's test using GraphPad Prism 7 (GraphPad Inc., San Diego, CA, USA). The difference was statistically significant when P < 0.05.Results
Berberine inhibits Aβ25-35-induced injury in PC12 cells
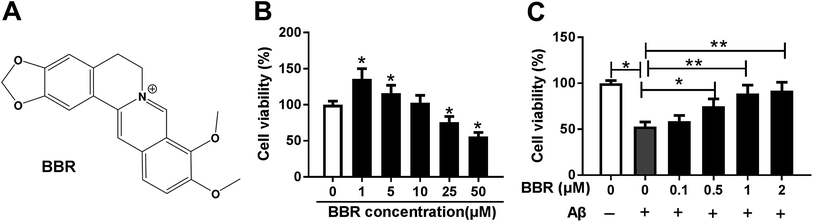
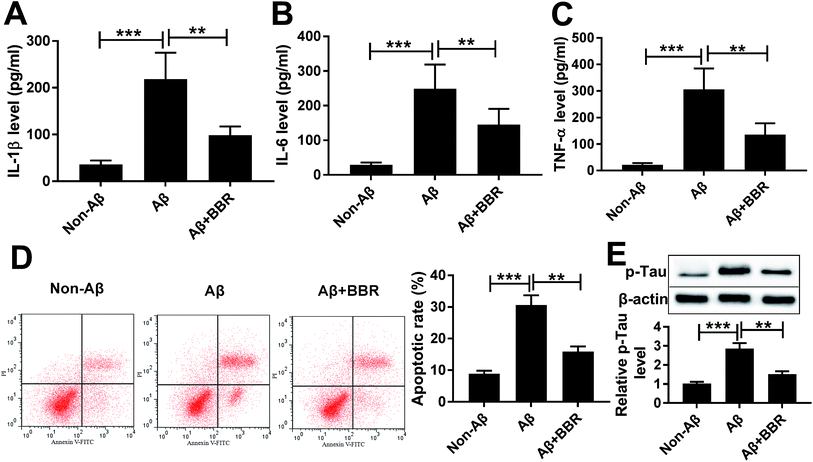
To explore the role of berberine in AD, PC12 cells were treated with different concentrations of berberine for 24 h. The chemical structure of berberine was shown in Fig. 1A. MTT assay showed that PC12 cell viability was obviously promoted by treatment of 1 and 5 μM berberine but inhibited by 25 and 50 μM berberine (Fig. 1B). To analyze the potential neuroprotective role of berberine, low concentrations of berberine was used for exposure of Aβ25-35-treated PC12 cells. Results exhibited that treatment of Aβ25-35 significantly inhibited cell viability, which was progressively restored by low doses of berberine treatment (Fig. 1C). To further explore the effect of berberine on AD processes, PC12 cells were treated with 1 μM berberine and 10 μM Aβ25-35 for 24 h. The data of ELISA assay revealed that treatment of Aβ25-35 led to obvious increase of IL-1β, IL-6 and TNF-α in serum of PC12 cells, which was markedly weakened by berberine (Fig. 2A–C). Moreover, treatment of berberine significantly mitigated Aβ25-35-induced apoptosis production in PC12 cells (Fig. 2D). Besides, Western blot analysis displayed exposure of berberine greatly decreased Aβ25-35-induced phosphorylation of tau (Fig. 2E).Berberine suppresses Aβ25-35-induced injury by up-regulating miR-107
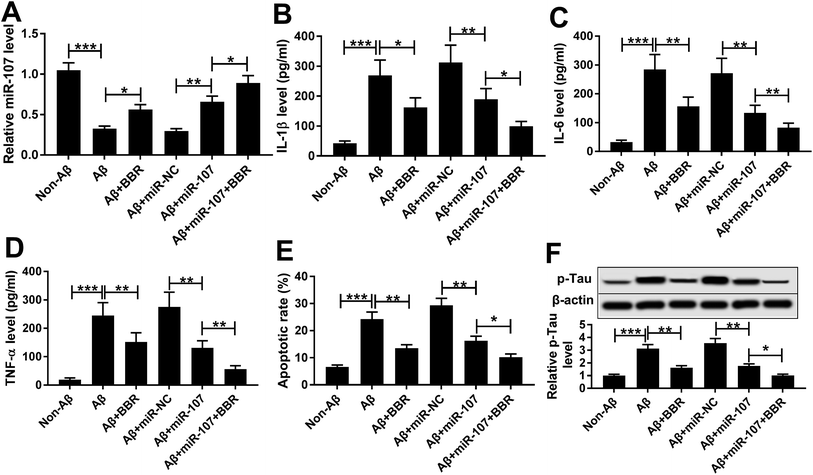
The expression of miR-107 was notably decreased in PC12 cells after treatment of Aβ25-35 compared with that in non-treated group (Fig. 3A). To explore the biological function of miR-107 in AD progression, PC12 cells were transfected with miR-107 mimic or miR-NC and then treated with Aβ25-35 for 24 h. Transfection efficacy was validated by qRT-PCR with the results of elevated miR-107 level in miR-107 transfection group compared with that in miR-NC group in Aβ25-35-treated PC12 cells (Fig. 3A). Moreover, overexpression of miR-107 specially ameliorated Aβ25-35-induced increase of IL-1β, IL-6 and TNF-α secretion, apoptosis and phosphorylation of tau (Fig. 3B–F). To explore whether berberine-induced inhibition of AD progression was modulated by miR-107, transfected cells were treated with berberine and Aβ25-35 for 24 h. Results showed that berberine treatment up-regulated miR-107 expression and further contributed to miR-107-mediated inhibitive role in Aβ25-35 injury in PC12 cells (Fig. 3).ZNF217 is a target of miR-107
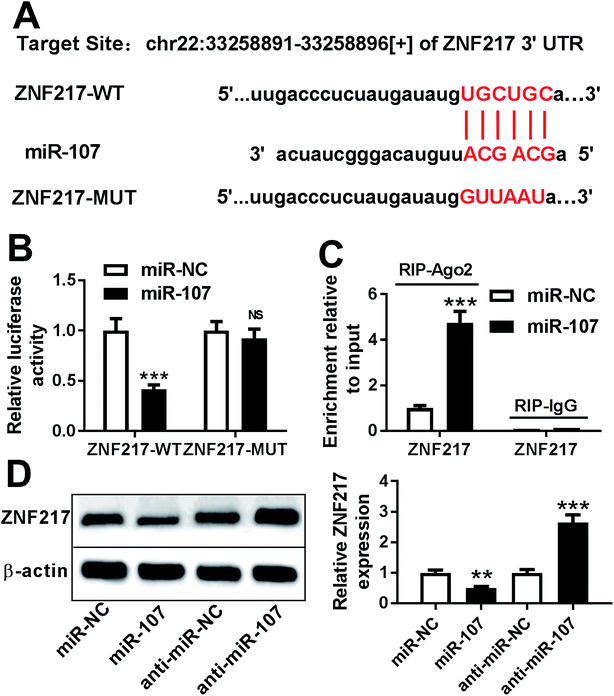
miRNA usually exerts its functions through regulating the related targets. Bioinformatics analysis provided the putative binding sites between miR-107 and 3′ UTR of ZNF217, suggesting that ZNF217 might be a target of miR-107 (Fig. 4A). To identify this prediction, we transfected WT or MUT luciferase reporter vector into PC12 cells and measured the luciferase activity. The results of luciferase reporter assay revealed that transfection of miR-107 mimic led to obvious loss of luciferase activity in PC12 cells transfected with ZNF217-WT compared with miR-NC, while it showed little effect on ZNF217-MUT group (Fig. 4B). Moreover, RIP assay presented more enrichment of ZNF217 in miR-107-transfected PC12 cells than that in miR-NC group after Ago2 RIP, while IgG failed to display the efficacy of enrichment (Fig. 4C). Besides, the effect of miR-107 on ZNF217 protein level was analyzed in PC12 cells and the results showed that the abundance of ZNF217 protein was significantly reduced by transfection of miR-107 mimic but enhanced by inhibition of miR-107 (Fig. 4D).miR-107 suppresses Aβ25-35-induced injury by regulating ZNF217
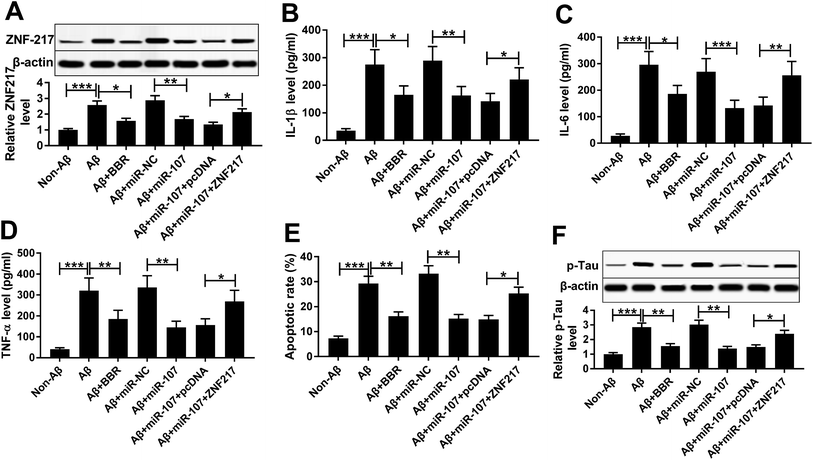
Western blot assay demonstrated that ZNF217 protein level was abnormally enhanced in PC12 cells after treatment of Aβ25-35, but inhibited by introduction of miR-107 or berberine (Fig. 5A). To explore whether ZNF217 was required for miR-107-mediated AD progression, PC12 cells were co-transfected with miR-107 mimic and ZNF217 overexpression vector or empty vector and then treated with Aβ25-35 for 24 h. Western blot experiment also confirmed the transfection efficacy with the result which ZNF217 protein abundance was notably rescued by introduction of ZNF217 (Fig. 5A). Moreover, ZNF217 overexpression reversed miR-107-mediated suppressive effect on secretion of IL-1β, IL-6 and TNF-α in Aβ25-35-treated PC12 cells (Fig. 5B–D). In addition, restoration of ZNF217 weakened the effect of miR-107 on cell apoptosis under Aβ25-35 (Fig. 5E). Besides, the phosphorylation of tau inhibited by miR-107 was remarkably triggered by addition of ZNF217 in Aβ25-35-treated PC12 cells (Fig. 5F).Berberine decreases ZNF217 protein expression by regulating miR-107 in Aβ25-35-treated PC12 cells
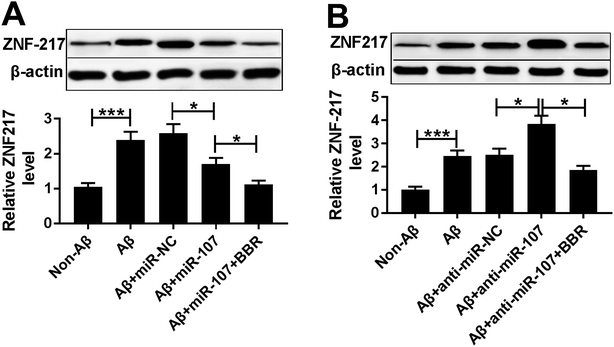
To explore the association between berberine and ZNF217, the effect of berberine on ZNF217 protein level was evaluated in Aβ25-35-treated PC12 cells. PC12 cells were transfected with miR-107 mimic or anti-miR-107 and then treated with 1 μM berberine and 10 μM Aβ25-35 for 24 h. Western blots analysis showed that treatment of berberine significantly exacerbated miR-107-mediated inhibition of ZNF217 expression at protein level in Aβ25-35-treated PC12 cells (Fig. 6A). Moreover, knockdown of miR-107 evidently elevated ZNF217 protein level in Aβ25-35-treated PC12 cells, which was reversed by exposure of berberine (Fig. 6B).Discussion
The accumulation of Aβ contributes to AD pathogenesis, which wreaks havoc on health of elderly people worldwide. The existence of Aβ in brain is a key neuropathological feature in AD, and Aβ25-35 oligomers are a fragment of full length Aβ1-42, which are easier to spread in brain to induce neurotoxicity. A number of investigators have used Aβ25-35-treated PC12 cells as important cellular model of AD.15–18 In this study, PC12 cells were treated with Aβ25-35 and results showed that Aβ25-35 treatment led to viability inhibition and increase of inflammatory response, apoptosis and p-Tau level, predicting the successful establishment of AD model in vitro. This study investigated the neuroprotective effect of berberine and first validated the interaction between berberine and miR-107/ZNF217 in AD.Berberine is found in such plants as Hydrastis canadensis (goldenseal), Coptis chinensis (coptis or golden thread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), and Berberis aristata (tree turmeric).19 Moreover, it could be easily obtained from these plants such as Coptis chinensis (5.2–7.7%)20 or by total synthesis.21 Moreover, berberine exhibits antibacterial, antiasthma, anticancer, anti-inflammatory, and antidiabetic activities.22 Previous study showed that berberine at low dose range (1.25–5 μM) promoted cell proliferation but at high dose range (10–80 μM) inhibited cell proliferation.23 Similarly, in our study, we also found that low concentrations (1 and 5 μM) of berberine increased cell viability of PC12 cells and high concentrations (25 and 50 μM) of berberine suppressed the viability. Furthermore, berberine, a promising Chinese herbal medicine, exhibits better neuroprotective effect in neurodegenerative diseases. For example, Liang et al. reported that berberine protected against Aβ25-35-induced apoptosis by mitochondria-related caspase pathway in primary neuronal cells.24 He et al. suggested that berberine could attenuate cognitive impairment and hyperphosphorylation of tau protein by regulating nuclear factor kappa beta signaling.25 Furthermore, Xu et al. demonstrated that berberine could inhibit neuro-inflammatory response in Aβ25-35-induced AD model.26 In the present study, we also found that berberine alleviated Aβ25-35-induced neuronal injury, reflecting the neuroprotective function of berberine in AD progression. However, its mechanism remains poorly understood.
The available evidence indicated the importance of miRNA-mRNA regulatory networks in berberine-addressed progression.27 Previous studies have revealed that berberine could regulate development of hepatosteatosis or breast cancer by modifying miRNA expression and its function.28 This study showed that berberine might improve miR-107 expression in Aβ25-35-treated PC12 cells, suggesting that miR-107 might be an important regulator in AD. qRT-PCR assay showed that the expression of miR-107 was reduced in PC12 cells after treatment of Aβ25-35, which is also in agreement with former work.29 In addition, previous effort elucidated that miR-107 could accelerate AD progression by varying pathways.30 The gain-of-function experiments disclosed that up-regulation of miR-107 attenuated Aβ25-35-induced inflammatory injury, apoptosis and hyperphosphorylation of tau protein, indicating the neuroprotective role of miR-107 in AD progression. What's more, introduction of berberine contributed to the effect of miR-107 on Aβ25-35-induced injury, revealing that berberine played the neuroprotective role by increasing miR-107 expression.
The function of miRNA is realized by regulating related targets. This study is the first time we identified ZNF217 as a functional target of miR-107 in PC12 cells by luciferase reporter assay and RIP. ZNF217, one member of the Krüppel-like family, has been reported as an oncogene in many cancers.31 Nevertheless, the effect of ZNF217 on AD progression is unclear but for the former work which implicated that ZNF217 might aggravate Aβ25-35-induced injury.13 In the present work, we found that restoration of ZNF217 reversed miR-107-mediated inhibition of Aβ25-35-induced injury, uncovering that miR-107 impeded AD progression by targeting ZNF217. Besides, treatment of berberine decreased ZNF217 protein expression in Aβ25-35-treated PC12 cells. These findings manifested that berberine might modulate miR-107 and ZNF217 to block inflammatory response, apoptosis and hyperphosphorylation of tau, leading to delay of AD progression. However, the data of in vivo experiments were absence in the present study. Hence, to better understanding the role of berberine and its mechanism, an animal model of AD or patients needed to be recruited in further study. Furthermore, the most of treatments in this study just lasted for 24 h, so that it was unclear whether it was irreversible or reversible. Hence, a longer period of treatment is needed to be performed in future work.
Conclusion
Our results indicated that miR-107 expression was decreased and ZNF217 was a target of miR-107 in AD. Moreover, berberine attenuated Aβ25-35-induced injury in PC12 cells, which might be associated with miR-107/ZNF217 axis. This study indicates berberine as a promising neuroprotective medicine for therapeutics of AD.Conflicts of interest
All authors declare that they have no conflicts of interest.Funding
None.Acknowledgements
Not applicable.References
- K. Li, S. Wei, Z. Liu, L. Hu, J. Lin, S. Tan, Y. Mai, W. Peng, H. Mai, Q. Hou and G. Tu, Iran. J. Public Health, 2018, 47, 1615–1626 Search PubMed.
- E. D. Roberson, K. Scearce-Levie, J. J. Palop, F. Yan, I. H. Cheng, T. Wu, H. Gerstein, G. Q. Yu and L. Mucke, Science, 2007, 316, 750–754 CrossRef CAS PubMed.
- J. Cao, J. Hou, J. Ping and D. Cai, Mol. Neurodegener., 2018, 13, 64 CrossRef CAS PubMed.
- Y. Jin, D. B. Khadka and W. J. Cho, Expert Opin. Ther. Pat., 2016, 26, 229–243 CrossRef CAS PubMed.
- W. Jiang, S. Li and X. Li, Sci. China: Life Sci., 2015, 58, 564–569 CrossRef CAS PubMed.
- J. Wang and Y. Zhang, Mol. Med. Rep., 2018, 17, 6881–6886 CAS.
- S. S. Durairajan, L. F. Liu, J. H. Lu, L. L. Chen, Q. Yuan, S. K. Chung, L. Huang, X. S. Li, J. D. Huang and M. Li, Neurobiol. Aging, 2012, 33, 2903–2919 CrossRef CAS PubMed.
- P. Piscopo, E. Lacorte, M. Feligioni, F. Mayer, A. Crestini, L. Piccolo, I. Bacigalupo, M. Filareti, E. Ficulle, A. Confaloni, N. Vanacore and M. Corbo, Ageing Res. Rev., 2019, 50, 131–141 CrossRef CAS PubMed.
- B. Martinez and P. V. Peplow, Neural Regener. Res., 2019, 14, 242–255 CrossRef PubMed.
- W. Hu, L. Wen, F. Cao and Y. Wang, Curr. Alzheimer Res., 2019, 16, 135–145 CrossRef CAS PubMed.
- W. X. Wang, B. R. Wilfred, S. K. Madathil, G. Tang, Y. Hu, J. Dimayuga, A. J. Stromberg, Q. Huang, K. E. Saatman and P. T. Nelson, Am. J. Pathol., 2010, 177, 334–345 CrossRef CAS PubMed.
- P. Gupta, S. Bhattacharjee, A. R. Sharma, G. Sharma, S. S. Lee and C. Chakraborty, Curr. Alzheimer Res., 2017, 14, 1198–1206 CrossRef CAS PubMed.
- J. Wang, T. Zhou, T. Wang and B. Wang, Biomed. Pharmacother., 2018, 108, 707–715 CrossRef CAS PubMed.
- K. J. Livak and T. D. Schmittgen, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- L. Zhao, L. Zhu and X. Guo, Biomed. Pharmacother., 2018, 106, 77–82 CrossRef CAS PubMed.
- S. Luo, T. Lan, W. Liao, M. Zhao and H. Yang, Neurochem. Res., 2012, 37, 2787–2794 CrossRef CAS PubMed.
- Y. Zheng, F. You, Q. Li, J. Chen and H. Yang, Food Funct., 2016, 7, 4702–4708 RSC.
- Q. Zhang, W. Liu and G. Lu, J. Biosci., 2017, 42, 397–404 CrossRef CAS PubMed.
- T. Birdsall, Altern. Med. Rev., 1997, 2, 94–103 Search PubMed.
- J. Yin, J. Ye and W. Jia, Acta Pharm. Sin. B, 2012, 2, 327–334 CrossRef CAS.
- X. Feng, A. Sureda, S. Jafari, Z. Memarian, D. Tewari, G. Annunziata, L. Barrea, S. Hassan, K. Smeikal, M. Malanik, A. Sychrova, D. Barreca, L. Ziberna, M. Mahomoodally, G. Zengin, S. Xu, S. Nabavi and A. Shen, Theranostics, 2019, 9, 1923–1951 CrossRef CAS PubMed.
- N. Singh and B. Sharma, Front. Mol. Biosci., 2018, 5, 21 CrossRef PubMed.
- J. Bao, B. Huang, L. Zou, S. Chen, C. Zhang, Y. Zhang, M. Chen, J. Wan, H. Su, Y. Wang and C. He, PLoS One, 2015, 10, e0139298 CrossRef PubMed.
- Y. Liang, M. Huang, X. Jiang, Q. Liu, X. Chang and Y. Guo, Neurosci. Lett., 2017, 655, 46–53 CrossRef CAS PubMed.
- W. He, C. Wang, Y. Chen, Y. He and Z. Cai, Pharmacol. Rep., 2017, 69, 1341–1348 CrossRef CAS.
- J. Xu, W. Wu, H. Zhang and L. Yang, Exp. Ther. Med., 2018, 16, 4865–4872 CAS.
- Y. Yang, N. Zhang, K. Li, J. Chen, L. Qiu and J. Zhang, Drug Des., Dev. Ther., 2018, 12, 393–408 CrossRef CAS PubMed.
- C. H. Li, S. C. Tang, C. H. Wong, Y. Wang, J. D. Jiang and Y. Chen, Eur. J. Pharmacol., 2018, 825, 107–118 CrossRef CAS PubMed.
- P. T. Nelson and W. X. Wang, J. Alzheimer's Dis., 2010, 21, 75–79 CAS.
- W. X. Wang, B. W. Rajeev, A. J. Stromberg, N. Ren, G. Tang, Q. Huang, I. Rigoutsos and P. T. Nelson, J. Neurosci., 2008, 28, 1213–1223 CrossRef CAS PubMed.
- J. Li, L. Song, Y. Qiu, A. Yin and M. Zhong, Int. J. Clin. Exp. Pathol., 2014, 7, 3038–3047 CAS.
Footnote |
| † Equal contributors. |
| This journal is © The Royal Society of Chemistry 2019 |