Open Access Article
Open Access ArticleOn the road to cost-effective fossil fuel desulfurization by Gordonia alkanivorans strain 1B †
Marta Pacheco ,
Susana M. Paixão
,
Susana M. Paixão *,
Tiago P. Silva and
Luís Alves
*,
Tiago P. Silva and
Luís Alves *
*
LNEG – Instituto Nacional de Energia e Geologia, IP, Unidade de Bioenergia, Estrada do Paço do Lumiar, 22, 1649-038, Portugal. E-mail: susana.alves@lneg.pt; luis.alves@lneg.pt
First published on 14th August 2019
Abstract
Biodesulfurization (BDS) is an ecofriendly process that uses microorganisms to efficiently remove sulfur from fossil fuels. To make the BDS process economically competitive with the deep hydrodesulfurization process, which is currently used in the oil industry, it is necessary to improve several factors. One crucial limitation to be overcome, common within many other biotechnological processes, is the cost of the culture medium. Therefore, an important line of work to make BDS scale-up less costly is the optimization of the culture medium composition aiming to reduce operating expenses and maximize biocatalyst production. In this context, the main goal of this study was on the minimization of inorganic key components of sulfur-free mineral (SFM) medium in order to get the maximal production of efficient desulfurizing biocatalysts. Hence, a set of assays was carried out to develop an optimal culture medium containing minimal amounts of nitrogen (N) and magnesium (Mg) sources and trace elements solution (TES). These assays allowed the design of a SFMM (SFM minimum) medium containing 85% N-source, 25% Mg-source and 25% TES. Further validation consisted of testing this minimized medium using two carbon sources: the commercial C-source (glucose + fructose) versus Jerusalem artichoke juice (JAJ) as a cheaper alternative. SFMM medium allowed microbial cells to almost duplicate their specific desulfurization rate (q2-HBP) for both tested C-sources, namely from 2.15 to 3.39 μmoL g−1 (DCW) h−1 for Fru + Glu and from 1.91 to 3.58 μmoL g−1 (DCW) h−1 for JAJ, achieving a similar net 2-hydroxybiphenyl produced per g of consumed sugar (∼17 μmoL g−1). These results point out the great advantage of using cheaper culture medium that in addition enhances the bioprocess effectiveness, paving the way to a sustainable scale-up for fossil fuel BDS.
I. Introduction
With the emergence of hybrid and non-petroleum-based engines, it was expected that oil consumption would decrease drastically. However, this effect was only observed in developed countries where alternative energy sources are well established and the population economic power allows access to these new technologies.1 In developing countries, the demand for cheaper, reliable and comfortable means of transportation is high; therefore, vehicles with internal combustion engines are the preferred choice. Moreover, the recent advances of the technology necessary to remove oil from difficult-to-drill sites, such as shale formations or oil sands, have revolutionized the energy market, bringing oil prices to a historical low in 2015,2 making oil an attractive and well-known energy source. However, the combustion of fossil fuels releases many hazardous components such as CO2, NOx, SOx and other sulfur compounds. As a response to the increasing concern with the environmental and health problems related with the sulfur levels in fossil fuels, countries have begun to implement strict sulfur concentration limits in on-road and off-road gasoline and diesel requirements. These will become stricter in the foreseeable future towards ultra-low sulfur fuels, approaching zero sulfur emissions from burned fuels. Therefore, the efficiency of the desulfurization technologies becomes a key point.3Sulfur (S) is a major component of crude oil, along with carbon and hydrogen, ranging from 0.03 to 7.89% (g g−1), and between 500 and 5000 mg L−1 in the distillate fraction used to make diesel oil.4,5 High viscosity and density crudes (such as shale oils) tend to have higher sulfur concentrations 3.09% (g g−1), especially in its organic forms (e.g. sulfides and thiophenic compounds) that can account for 75% of total sulfur. Nowadays, the most used sulfur removing process in the refineries is the hydrodesulfurization (HDS), which uses high temperatures; high pressures conjugated with elemental hydrogen and advanced metal catalysts to remove sulfur from crude oil. However, some types of organic sulfur molecules are very recalcitrant to this process, because the sulfur molecule is enclosed within an aromatic ring. Biodesulfurization (BDS) is a biotechnological process corresponding to HDS, since it removes sulfur from complex compounds without the need for high temperatures or pressures, elemental hydrogen or metal catalysts. In the last decades, BDS has drawn wide attention because of its green processing of fossil fuels. Since BDS is more efficient and less expensive than HDS in removing sulfur from refractory heterocyclic compounds present in crude oil, it could be used in oil refineries as a complement to achieve ultra-low sulfur diesel.3 Indeed, BDS can be used to desulfurize heavy oils, like shale oils, which have high thiophene concentration.
The preferred microorganisms for BDS are those that remove the sulfur from the hydrocarbons through 4S pathway metabolism, regulated by a dsz operon, in which the calorific value of the fuel is preserved. One of those microorganisms is the bacterium Gordonia alkanivorans strain 1B, isolated by Alves et al.,6 from oil contaminated soil samples from Parque das Nações (former Petrogal location, Lisbon, Portugal). In the last decade, several works have demonstrated the great potential of G. alkanivorans strain 1B towards fossil fuel BDS.3,7–12
However, this bioprocess still has a few limitations, such as the cost of the culture medium for the biocatalysts production.12–16 In order to reduce the production costs, it is important to search for cheaper carbon sources which can contribute to produce less expensive microbial biomass. Due to their low cost, the utilization of agroindustrial residues or other abundant materials as carbon sources is one of the most followed strategies in biotechnology processes.10–12,17 Silva et al.12 described an optimized dibenzothiophene desulfurization process by G. alkanivorans strain 1B using Jerusalem artichoke juice (JAJ) as carbon source (∼25 g L−1 total sugars), achieving high desulfurization results (2-hydroxybiphenyl (2-HBP) production rate = 28.2 μM h−1 and q2-HBP = 5.06 μmoL g−1 (DCW) h−1), and highlighted JAJ potential as a promising alternative carbon source towards further scale-up assays.
Indeed, Jerusalem artichoke (Helianthus tuberosus) is an herbaceous perennial tuberous plant that accumulates high concentrations of inulin in its tubers. Inulin is a linear polymer in which a variable number of D-fructose units are linked by β(2 → 1) bonds, terminated by a D-glucose residue through a sucrose-type linkage at the reducing end α(2 → 1). So, this polysaccharide can be easily converted into a sugar-rich juice (JAJ ≥ 80% fructose) and further used as carbon source.
In this context, this work consisted on the minimization of inorganic key components (N-source; Mg-source and micronutrients) of G. alkanivorans strain 1B culture medium aiming to get a total sugar consumption (C-source: 10 g L−1 total reducing sugars) and by the further validation of Jerusalem artichoke juice as a cheaper alternative C-source in order to achieve a significant decrease on the overall production costs of BDS biocatalysts.
II. Materials and methods
A. Jerusalem artichoke juice
Jerusalem artichoke tubers cultivated in a forest soil at Oleiros, Portugal were harvested and further processed, as described in Silva et al.12 and in Paixão et al.,18 to produce Jerusalem artichoke juice (JAJ). The JAJ was hydrolyzed at pH 2 and 55 °C for 48 hours to convert inulin into fructose and glucose and then filtered (0.20 μm membrane filters). For total sulfate precipitation, sterile BaCl2 at 0.5% (w/v) was added to a filter sterilized juice adjusted to pH 8.73.12 This JAJ was shaken vigorously, incubated at 30 °C for 36 hours and then filtered to remove all the BaSO4 (0.45 μm membranes) and again filter sterilized (0.20 μm membrane filters). So, the precipitated JAJ contained ∼0 mg L−1 of sulfate, and a total sugar concentration of 160 g L−1, of which 80% was fructose and 20% glucose, indicating complete inulin conversion.B. Microorganism and culture media
The microorganism used in this study was the bacterium G. alkanivorans strain 1B, isolated in our laboratory.6 The basal salts medium used for cultivation of this microorganism was a sulfur-free mineral (SFM) culture medium containing 1.22 g L−1 NH4Cl, 2.55 g L−1 KH2PO4, 2.55 g L−1 Na2HPO4·2H2O and 0.17 g L−1 MgCl2·6H2O. This medium was supplemented with 0.50 mL L−1 of a sulfur-free trace elements solution (TES)9 and its final pH was adjusted to 7.5, before being autoclaved at 121 °C, 1 atm for 15 min. Pure carbon sources, namely glucose and fructose, were dissolved in Millipore water in 50% (w/v) concentrated solutions. Pure carbon sources and Jerusalem artichoke juice (JAJ) were filter sterilized before being added to the culture medium in aseptic conditions, to an initial concentration of about 10 g L−1 total reducing sugars.Prior the BDS assays, the bacterial inoculum was prepared by growing strain 1B with fructose (10 g L−1) as the only carbon source and 250 μM DBT as the only sulfur source, within 150 mL culture medium in 500 mL Erlenmeyer, at 30 °C and 150 rotations per minute (rpm) for 72 hours, in an orbital-shaker.
C. Biodesulfurization assays: culture medium minimization
Aiming to minimize the concentrations of key inorganic components for a full consumption of 10 g L−1 of C-source without limiting neither the growth nor the desulfurization ability of strain 1B, a set of BDS assays were performed testing different formulations of the SFM culture medium aforementioned. The key culture medium inorganic components minimized were: nitrogen-source (NH4Cl), magnesium-source (MgCl2·6H2O) and TES, ranging from 0% to 75% of the SFM original amounts. The details of the tested medium formulations, F#1 to F#13, are presented in Table 1, being F#1 the original SFM medium (control assay). In all tested formulations, the carbon and sulfur sources concentration were maintained constant [10 g L−1 of total reducing sugars in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 glucose/fructose (to mimic the sugar concentration of the Jerusalem artichoke juice); 250 μM of dibenzothiophene (DBT) as sulfur source]. Following, validation assays (F#14 and F#15) were performed for the designed minimized culture medium (SFMM medium) using mix glucose/fructose vs. JAJ as C-source.
8 glucose/fructose (to mimic the sugar concentration of the Jerusalem artichoke juice); 250 μM of dibenzothiophene (DBT) as sulfur source]. Following, validation assays (F#14 and F#15) were performed for the designed minimized culture medium (SFMM medium) using mix glucose/fructose vs. JAJ as C-source.
| Set assays | Medium formulations (F#) | SFM culture medium composition | Carbon source (10 g L−1)c | Sulfur source (250 μM) | ||
|---|---|---|---|---|---|---|
| Components minimized | ||||||
| NH4Cl (g L−1) | MgCl2·6H2O (g L−1) | TES (mL L−1) | ||||
| a SFM medium – sulfur-free mineral medium (default medium for shake-flask assays).b SFMM medium – SFM minimum medium for shake-flask.c 10 g L−1 Total sugars; 8 Fru: fructose 8 g L−1; 2 Glu: glucose 2 g L−1; JAJ: sulfate precipitated Jerusalem artichoke juice. | ||||||
| Control (aSFM medium) | #1 | 1.22 | 0.17 | 0.50 | 8 Fru + 2 Glu | DBT |
| #1′ | JAJ | |||||
| N-source minimization | #2 | 0.92 (75%) | 0.17 | 0.50 | 8 Fru + 2 Glu | DBT |
| #3 | 0.61 (50%) | |||||
| #4 | 0.31 (25%) | |||||
| Mg-source minimization | #5 | 1.22 | 0.13 (75%) | 0.50 | ||
| #6 | 0.09 (50%) | |||||
| #7 | 0.04 (25%) | |||||
| TES minimization | #8 | 1.22 | 0.17 | 0.38 (75%) | ||
| #9 | 0.25 (50%) | |||||
| #10 | 0.13 (25%) | |||||
| #11 | 0.03 (5%) | |||||
| #12 | 0.01 (2.5%) | |||||
| #13 | 0 (0%) | |||||
| Minimized medium (bSFMM) | #14 | 1.04 (85%) | 0.04 (25%) | 0.13 (25%) | 8 Fru + 2 Glu | DBT |
| #15 | JAJ | |||||
BDS assays were performed in 500 mL Erlenmeyer-flasks with about 150 mL of culture medium formulations (F#1 to F#15), inoculated with 2% (v/v) of bacterial inoculum prepared as above described, and incubated at 30 °C and 150 rpm from about 96 to 124 h, in an orbital-shaker. All BDS assays were carried out in triplicates.
D. Analytical methods
The culture growth was monitored by determining the sugars consumption (glucose/fructose) using high-performance liquid chromatography (HPLC) instrumentation, the optical density at 600 nm (OD600nm) and the dry cell weight (DCW), as described by Fernandes et al.19DBT desulfurization was evaluated by measuring 2-HBP production, which is the final product of the DBT desulfurization through the 4S-pathway. The samples (0.750 mL) were acidified with 0.025 mL of HCl (4 M) and then a liquid–liquid extraction with ethyl acetate was performed on a vortex (5 minutes), in order to extract 2-HBP and DBT. After phase separation, the organic phase was analyzed by gas-chromatography (GC) in a gas-chromatograph (Chrompack, Model CP9001, Middelburg, The Netherlands) equipped with a flame ionization detector. A 10% CP-5 CB on 100/120 MESH Chromosorb W-HP column was used with nitrogen as the carrier gas. The chromatograph oven start temperature was 130 °C for 3 minutes and the end temperature 230 °C maintained for 3 minutes (heating rate of 6 °C min−1). The injector and detector temperatures were set for 280 and 290 °C, respectively. In all GC measurements 4-methyl-DBT was used as internal standard to minimize variations.
E. Statistical validation
Unless indicated otherwise, the results presented in graphs are mean values derived from three replicates (i.e. 3 independent BDS assays performed in the same conditions). The corresponding standard deviation (n = 3) is represented as error bars.To validate the statistical significance of the results observed with the cultures grown with the final minimized formulations (F#14 and F#15), and compare them with the initial formulations (F#1 and F#1′), an ANOVA (single factor) test was used, and a significant difference was considered at a level of p < 0.05.
III. Results and discussion
A. Biodesulfurization assays: culture medium minimization
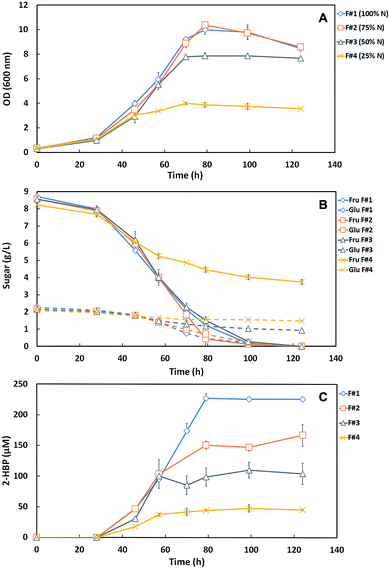
This study consisted on the optimization of the SFM culture medium for the maximum production of efficient desulfurizing biocatalysts (G. alkanivorans strain 1B active cells) taking into account the lowest operation costs towards a future BDS process scale-up. In this context, key components of the SFM medium used to grow strain 1B (NH4Cl, MgCl2 and TES) were reduced to a minimal amount while aiming to have no limitation both on the full consumption of the carbon source (10 g L−1) and on the biodesulfurization ability. The phosphate compounds of the culture medium were not altered since, in the shake-flask growth assays, their presence is crucial for pH maintenance (buffer effect). Indeed, the reduction of their concentration would imply a sharp pH decrease on culture medium and consequently a decrease of BDS effectiveness.Hence, in the first set of BDS assays, three formulations with different amounts of N-source (NH4Cl), namely 75% (F#2), 50% (F#3) and 25% (F#4) of the amount in the original medium (1.22 g L−1 = 100% N) were tested against the original SFM medium as a control (F#1, default SFM medium), for comparison. Fig. 1A and B shows the time course profiles of cellular growth (OD600nm) and sugar consumption for the four formulations (F#1 to F#4, Table 1) containing different N-source concentrations. The bacterial culture attained maximal OD600nm (9.98 – F#1; 10.39 – F#2) with complete sugar consumption (10 g L−1) for both F#1 and F#2 formulations. However, for the remaining formulations (F#3 and F#4) a lower growth was observed. For F#3, an OD600 of 7.87 was attained, with a complete fructose consumption but with a remaining of 0.9 g L−1 of glucose; and for F#4 was attained an OD600nm of only 4, remaining more than half of the total sugars (3.7 g L−1 Fru and 1.5 g L−1 Glu) in culture medium.
These results indicate that the amount of the N-source greatly influenced the bacterial behavior. F#4 (25% N) was clearly insufficient to ensure normal cell growth, resulting in an abrupt growth stop when all other nutrients were still in excess. Comparatively to F#4, F#3 (50% N) allowed a growth enhancement, almost doubling the maximum OD600nm attained (from 4 to 7.87), but without the full consumption of the available sugars. In F#3, fructose was fully consumed but most of the glucose was left untouched. This preferential consumption of fructose was expected since strain 1B is a fructophilic microorganism.9 F#2 (75% N) was the only formulation that allowed complete growth and sugar consumption, achieving a similar growth curve to the one obtained with 100% N (F#1, original medium).
The desulfurization ability of the strain 1B depends mostly on cell physiology, which is influenced by the amount of nutrients and carbon in the culture medium. In Fig. 1C is presented the DBT desulfurization by strain 1B grown in the formulations F#1 to F#4, with decreasing amounts of N-source (100–25%). These results point out for the limitation of desulfurization ability by strain 1B due to the lack of N-source in the culture medium. In F#1, the bacterial culture presented a maximum 2-HBP production of 227.2 μM and a maximum specific 2-HBP production rate (q2-HBP) of 2.5 μmoL g−1 (DCW) h−1, achieving a total consumption of the 250 μM of DBT by the end of the growth. In F#2, the q2-HBP obtained was 2.4 μmoL g−1 (DCW) h−1 with a maximum 2-HBP of 167.1 μM and a remaining 27 μM of DBT. In F#3 and F#4, the q2-HBP obtained was 2.25 μmoL g−1 (DCW) h−1 and 1.41 μmoL g−1 (DCW) h−1, and the maximum 2-HBP produced was 110.1 μM and 47.8 μM, respectively. Relatively to DBT, 69 μM and 202 μM were not consumed in F#3 and F#4, respectively. The desulfurization results for both F#3 and F#4 formulations can be supported by their respective data for cellular growth and sugar consumption, showed in Fig. 1A and B. In fact, a NH4Cl concentration ≤75% of the original amount (F#2 to F#4) seems to not be sufficient for strain 1B to achieve maximum growth with total C-source consumption and full desulfurization of the 250 μM DBT. The overall growth, sugar consumption profiles and q2-HBP were very similar for F#1 and F#2; however a maximum 2-HBP production of 167.1 μM was observed in F#2. In contrast, in F#1 the maximum 2-HBP production was 227.2 μM (∼60 μM higher), despite of the consumption of almost all DBT (223 μM) in the F#2. Hence, these results point to the need of using a concentration of NH4Cl >75% of the original amount as the minimal N-source towards a minimized SFM medium.
Since nitrogen is a major component of enzymes and functions as a co-factor for innumerous essential proteins, it is expected that insufficient amounts will lead to deficiencies in cellular processes.20,21 This fact can support the sequential decrease in the desulfurization rate and, consequently, the maximum 2-HBP production observed in the tested formulations, namely from F#1 (100% N) to F#4 (25% N). Only in F#1 (default medium), the N amount was sufficient to support the complete cell growth, using the totality of C-source available, and the biodesulfurization of all the DBT. In the other formulations (F#2 to F#4), with decreasing NH4Cl concentrations, increasing limitations at different levels (e.g. growth rate, sugar consumption, 2-HBP production or q2-HBP) were observed.
Therefore, based on results from Fig. 1B and C, 85% N was extrapolated as an appropriate amount to be further tested as minimal N-source within a SFM minimized medium (F#14 and F#15 in Table 1).
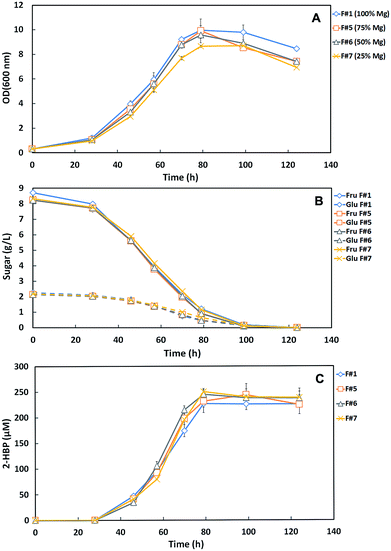
Fig. 2A and B shows the time course profiles of cellular growth (OD600nm) and sugar consumption for the tested medium formulations with the different Mg-source concentrations: F#1 (100% Mg = control with original medium), F#5 (75% Mg), F#6 (50% Mg) and F#7 (25% Mg). These results show that strain 1B growth and sugar consumption profiles for F#5 and F#6 were very similar to those observed for F#1. A total consumption of the 10 g L−1 total sugars, within 120 h, was observed for these three formulations achieving an OD600nm around 10. However, when F#7 (25% Mg) was used as culture medium, the strain 1B only attained a maximum OD600nm of 8.66 with an overall growth profile slightly below the others, despite having a similar maximal growth rate (μMax ∼ 0.06 h−1 to all). Moreover, strain 1B in F#7 medium consumed all the C-source (Fru + Glu) with the same sugar consumption profile as the other formulations. This fact contrasts with what was observed when N-source was limiting, where accumulation of sugars was detected.
Fig. 2C presents the desulfurization curves (2-HBP production) by the strain 1B grown in the different Mg-source medium formulations. As already described in the N-source minimization assays, for the control formulation (F#1) a q2-HBP of 2.45 μmoL g−1 (DCW) h−1 and a maximum 2-HBP of 227.2 μM were achieved. For the other test formulations, the respective q2-HBP and maximum 2-HBP obtained were: 2.67 μmoL g−1 (DCW) h−1 and 244.0 μM (F#5), 3.01 μmoL g−1 (DCW) h−1 and 244.8 μM (F#6), and 3.37 μmoL g−1 (DCW) h−1 and 250.0 μM (F#7). In all these formulations, strain 1B was capable of fully desulfurize the amount of DBT provided (250 μM).
These results show an evident enhancement of the maximum specific desulfurization rate (q2-HBP) with the decrease of the Mg-source/C-source ratio, from 2.45 μmoL g−1 (DCW) h−1 (ratio 100% Mg per 10 g L−1 C in F#1) to 3.37 μmoL g−1 (DCW) h−1 (ratio 25% Mg per 10 g L−1 C in F#7). This fact is in agreement with results previously reported in studies of DBT desulfurization by strain 1B. Alves and Paixão9 reported a q2-HBP of 2.12 μmoL g−1 (DCW) h−1 by strain 1B when SFM medium with a ratio 100% Mg per 10 g L−1 Fru was used and Silva (23) reported a q2-HBP of 6.57 μmoL g−1 (DCW) h−1 by strain 1B when SFM medium with a ratio 100% Mg per 25 g L−1 Fru was used. These results also pointed out for the stimulatory effect on desulfurization by the decrease of Mg-source/C-source ratio.
Hence, F#7 (25% Mg) allowed a similar maximum growth rate, despite the lower value for maximum OD600nm, and the enhancement of desulfurization. Therefore, it was stipulated as an appropriate amount to be further tested as minimal Mg-source within a SFM minimized medium.
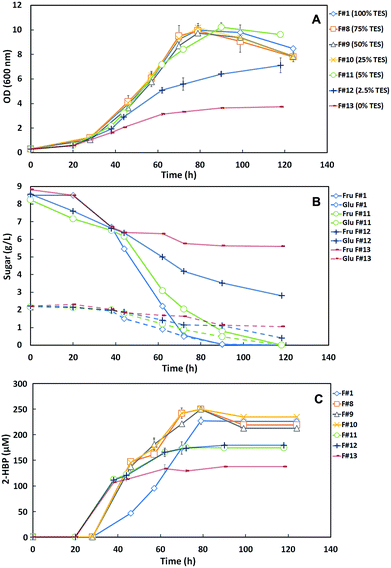
These results show that the media formulations F#1 (100% TES), F#8 (75% TES), F#9 (50% TES) and F#10 (25% TES) induced a similar behavior on strain 1B and consequently the growth curves and the sugar consumption profiles were identical. Indeed, for the control (F#1) and for F#8 to F#10 formulations a maximum OD600nm ∼ 10 was attained, with total sugar consumption (10 g L−1) within 124 h. In the absence of the TES (F#13) the maximum OD600nm achieved by strain 1B was only 3.73 with an accumulation of 6.6 g L−1 of sugars. In F#12 (2.5% TES), the bacterium was able to attain an OD600nm of 7.11 but still accumulated 3.2 g L−1 of total sugars. However, in F#11 (5% TES), a maximum OD600nm of 10.22 with a total sugar consumption was achieved, similarly to that observed for medium formulations with ≥25% TES (F#1, F#8 to F#10). These results indicate that a TES concentration ≥5% is required for maximum growth of strain 1B and full C-source consumption. However, when 5% TES was used the sugar consumption rate was somewhat slower than for ≥25% TES, prolonging the fermentation from 90–100 h to a total of 118 h.
Fig. 3C presents the desulfurization results as the time course profiles of 2-HBP production by strain 1B cultivated in the different formulations with decreasing TES concentrations. These results show a desulfurization enhancement with the decrease of TES concentration up to 25% in the culture medium. However, for concentrations <25% TES, namely in F#11 (5%), F#12 (2.5%) and F#13 (0%), the reduction of trace elements was limiting and the desulfurization stopped within about 60 h, attaining lower levels of 2-HBP production. At 0% TES the strain 1B desulfurization was probably mediated by trace elements available from culture medium used to grow the bacterium. Table 2 summarizes the main metabolic parameters (maximum q2-HBP and maximum 2-HBP produced) for the DBT desulfurization by G. alkanivorans strain 1B grown in the formulations with decreasing TES concentrations. In agreement with the results presented in Fig. 3C, the q2-HBP values for F#8 (2.80 μmoL g−1 (DCW) h−1), F#9 (3.19 μmoL g−1 (DCW) h−1) and F#10 (3.02 μmoL g−1 (DCW) h−1) were higher than the value of the control (F#1, 2.45 μmoL g−1 (DCW) h−1). In overall, these results seem to indicate that there is some inhibitory effect caused by the TES, since a sequential decrease of TES concentration up to 25% (F#10) produces cells with higher desulfurization ability than the control. In fact, F#10 with 25% TES induces an enhancement of about 23% of the specific desulfurization rate in comparison with the control (100% TES). Nevertheless, most of the trace elements are essential for strain 1B growth. Therefore, an excessive reduction of these micronutrients may result in a poor microbial growth and consequently cause the incomplete desulfurization of the DBT present in culture medium. This can be due to an alteration on essential enzymes structure, which need the nutrients supplied in the TES to function properly.24,25
| Medium formulations | F#1 (100% TES) | F#8 (75% TES) | F#9 (50% TES) | F#10 (25% TES) | F#11 (5% TES) | F#12 (2.5% TES) | F#13 (0% TES) |
|---|---|---|---|---|---|---|---|
| q2-HBP (μmol g−1 (DCW) h−1) | 2.45 | 2.80 | 3.19 | 3.02 | 1.32 | 1.26 | 0.68 |
| 2-HBP produced (μM) | 227.2 | 250.0 | 250.0 | 250.0 | 179.5 | 173.7 | 133.5 |
Since 25% TES (F#10) was the lowest concentration tested that allowed maximum cell growth and in addition it has stimulated desulfurization, this was the chosen amount as the most appropriated to be further tested as minimal TES within a SFM minimized medium.
B. Minimized medium: commercial versus alternative C-source
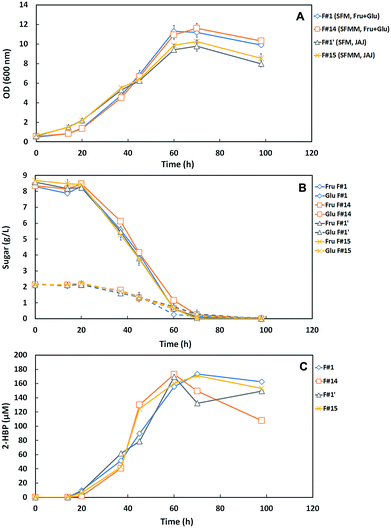
Using the N-source, Mg-source and TES minimal concentrations, determined in the prior assays, a SFM minimum (SFMM) medium containing 85% N, 25% Mg and 25% TES was designed (Fig. S1†). Further, two formulations (F#14 vs. F#15, Table 1) were tested in comparison with the correspondent original medium (F#1 vs. F#1′) to evaluate the whole effect of the collective nutrients reduction and to validate the minimized culture medium towards a cost-effective DBT desulfurization process by G. alkanivorans strain 1B.Indeed, as already referred, it is also crucial the replacement of the expensive commercial C-sources (glucose/fructose) by alternative and more sustainable C-sources when the goal is to reduce the overall production costs. In this context, based on the information previously reported by Silva et al.12 highlighting the great potential of JAJ as a sustainable alternative C-source that enhances the DBT desulfurization ability of strain 1B, the validation assays were performed by testing the SFMM medium into two formulations using a different C-source: F#14 with Fru + Glu versus F#15 with JAJ, simultaneously with their respective control (F#1 vs. F#1′, Table 1). In these assays, 10 g L−1 of total sugars (Fru + Glu or JAJ) were used as C-source and 250 μM DBT as S-source.
Fig. 4A–C presents the cellular growth (OD600nm), the sugar consumption and biodesulfurization profiles for strain 1B grown in both minimized medium formulations (F#14 and F#15) and respective controls (F#1 and F#1′). Comparing the growth profiles (Fig. 4A), it can be observed that cells grown using the commercial C-source (Fru + Glu) attained higher OD600nm values and higher growth rates than those obtained with JAJ. Using the commercial sugars as C-source, strain 1B attained a maximum OD600nm of ∼11 with both formulations, while cells grown with JAJ only attained a value of ∼10. The highest OD600nm value was observed on F#14 (11.61) after 70 hours. Growth rates were higher for both media with Fru + Glu as C-source (F#1 and F#14), however in all formulations the sugar consumption presented similar profiles, with total consumption within 98 h (Fig. 4B). These results were further validated by a single factor ANOVA test, which confirmed that the differences in maximum OD600nm obtained between the two carbon sources were statistically significant, with a level of p < 0.005.
Based on the results from Fig. 4, the main metabolic parameters associated to the growth and desulfurization by strain 1B in these four tested formulations (F#1 vs. F#14; F#1′ vs. F#15) are summarized in Table 3. These results demonstrate that independently of the C-source used, the minimized medium for N-source, Mg-source and TES (SFMM medium) enhanced the desulfurization by strain 1B in comparison with the original culture medium (SFM medium), namely from 2.15 μmoL g−1 (DCW) h−1 (F#1) to 4.39 μmoL g−1 (DCW) h−1 (F#14) for Fru + Glu and from 1.91 μmoL g−1 (DCW) h−1 (F#1′) to 3.58 μmoL g−1 (DCW) h−1 (F#15) for JAJ. So, a biodesulfurization increase of about 2-fold was attained when the minimized medium was used instead the more expensive SFM medium. The difference observed in desulfurization rates between the SFM medium (F#1, F#1′) and the SFMM medium (F#14, F#15) was statistically validated by a single factor ANOVA test, with a level of p < 0.05.
| Medium formulations | Fru + Glu | JAJ | ||
|---|---|---|---|---|
| F#1b – SFM | F#14 – SFMM | F#1′ – SFM | F#15 – SFMM | |
| a Max – maximum.b This was a new F#1 control that was repeated in simultaneously with the assays for F#14; F#1′ and F#15. | ||||
| Maxa OD600nm | 11.32 | 11.61 | 9.8 | 10.27 |
| Max biomass (g L−1) | 4.17 | 4.18 | 4.68 | 4.94 |
| μMax (h−1) | 0.072 | 0.071 | 0.054 | 0.056 |
| Max q2-HBP (μmol g−1 (DCW) h−1) | 2.15 | 4.39 | 1.91 | 3.58 |
| Max 2-HBP produced (μM) | 173.42 | 173.21 | 169.49 | 171.17 |
| 2-HBP produced/C-source consumed (μmol g−1 sugar) | 17.34 | 17.32 | 16.95 | 17.12 |
In addition, also based on Table 3 results, comparing the results by C-source (i.e. F#1 vs. F#1′; F#14 vs. F#15), it can be observed that for both culture media (SFM and SFMM) the growth rates (μMax) were lower when JAJ was used as C-source. This behavior can be due to the presence of an inhibitory compound, which can be formed during the JAJ acidic hydrolysis with hydrochloric acid, since the same behavior is not observed if enzymatic hydrolysis is used instead;23,26 and/or due to the use of the nutrients available on JAJ (a complex C-source) not only to cell growth, but also towards secondary metabolism, such as pigment production.19,27 But, despite of the slower growth rates in JAJ, only slight differences were observed in the maximum OD values attained when compared with those for Fru + Glu, and moreover the net biomass produced by G. alkanivorans strain 1B was even higher in JAJ, and this fact influences the respective specific 2-HBP production rate (q2-HBP) values, which are lower than for Fru + Glu (Table 3: F#1 vs. F#1′; F#14 vs. F#15). Indeed, the maximum value of 2-HBP produced by strain 1B in the four formulations (F#1, F#1′, F#14 and F#15) was quite similar (169.49–173.42 μM), as well as the overall 2-HBP produced per g of consumed sugar (16.95–17.34 μmoL g−1), but the 2-HBP was produced faster in SFMM with Fru + Glu (60 h) due to the higher net production rate. In SFMM with JAJ, the maximum 2-HBP only was attained after 70 h, ∼16.7% more time (Fig. 4C).
In overall, these results point out for the benefit of using a cheaper culture medium that enhances the bioprocess effectiveness. In fact, per each liter of the miniaturized BDS culture medium (SFMM medium), there is a net saving of about 15% N-source; 75% Mg-source and 75% TES in comparison with these nutrients in SFM medium. Based on data from Alves et al.3 and considering a process scale-up scenario for the production of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L of biocatalysts/bioreactor, a saving in expenses of about 700€ in N-source, 200€ in Mg-source and 75€ TES (metals solution) is attained if SFMM medium is used instead SFM medium.
000 L of biocatalysts/bioreactor, a saving in expenses of about 700€ in N-source, 200€ in Mg-source and 75€ TES (metals solution) is attained if SFMM medium is used instead SFM medium.
When working on a larger scale, besides the costs associated strictly with the production of microbial biocatalysts, it is also necessary to account for possible left overs, which can represent a burden to environment (e.g. nutrient-rich wastewaters; biomass wastes). Using this SFMM medium and foreseeing a full C-source (sugars) consumption, most of the salts present in the water after recovering the biocatalyst should be at residual concentrations, except for the phosphate buffer, which is not consumed by the bacteria, and would thus require further removal. So, the production of less contaminated wastewaters can translate into a reduction of treatment costs, increasing the overall efficiency of the industrial process.
Moreover, if a suitable cheaper alternative C-source is also addressed this may turn the overall bioprocess more sustainable and cost-effective, since the C-source is usually one of the most important and expensive nutrients into the culture medium.16 Several alternative C-sources were already tested for BDS studies with strain 1B, but JAJ was the one with the most promising results,9–12,17 and for that reason JAJ (>80% fructose) should be better exploited as the optimal alternative C-source for the fructophilic bacterium, specially using an SSF approach.26
In addition to the cost reduction within the biocatalysts production step, high added-value byproducts, such as the carotenoids from strain 1B,19,27 may be also exploited for their potential benefits to turn BDS process economically viable.16,27–29 Indeed, if successfully implemented, the ecofriendly BDS technology could result in less environmental issues than the current industry's solution (HDS) to accomplish the stringent European sulfur limits for transportation fuels (<10 ppm, ultra-low sulfur program). BDS option involves a CO2 emission reduction of 70 to 80%, smaller residue production and a lower net energy consumption, which in turn is translated in lower operational cost a in reduced capital cost, about two thirds of that for HDS.3,13,30
IV. Conclusions
A microbial approach for petroleum desulfurization could be beneficial for a deep desulfurization in which the classical hydroprocessing methods are costly and non-selective. In this context, the utilization of desulfurizing microorganisms that can grow in low nutrient culture media without vitamins and other growth promoters (e.g. yeast extract, peptone, etc) is an advantage for BDS upgrade since it may reduce the biocatalyst production costs significantly. Herein, a minimized culture medium (SFMM medium), accounting a C-source of 10 g L−1 (total sugars), was proposed for a cost-effective BDS by G. alkanivorans strain 1B. This SFMM medium, which allowed an improved desulfurization (2-fold higher q2-HBP), contains less 25% of N-source; 75% of Mg-source and 75% of TES than the original default medium (SFM medium), which implies net savings of about 975€ if a process scale-up scenario for the production of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L of biocatalysts/bioreactor is considered. Significant additional savings may be still achieved within biocatalysts production process if cheaper alternative C-sources (e.g. JAJ) are further exploited.
000 L of biocatalysts/bioreactor is considered. Significant additional savings may be still achieved within biocatalysts production process if cheaper alternative C-sources (e.g. JAJ) are further exploited.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by FEDER funds through POFC-COMPETE and by National funds through FCT (Fundação para a Ciência e a Tecnologia) in the scope of the projects Carbon4Desulf (FCOMP-01-0124-FEDER-013932) and GreenFuel (PTDC/EAM-AMB/30975/2017). Tiago P. Silva also acknowledges to FCT for his Ph.D. financial support (SFRH/BD/104977/2014).References
- OPEC, World Oil Outlook 2014, OPEC Secretariat, Vienna, Austria, 2014 Search PubMed.
- OPEC, World Oil Outlook 2017, OPEC Secretariat, Vienna, Austria, 2017 Search PubMed.
- L. Alves, S. M. Paixão, R. Pacheco, A. F. Ferreira and C. M. Silva, RSC Adv., 2015, 5, 34047–34057 RSC.
- M. Soleimani, A. Bassi and A. Margaritis, Biotechnol. Adv., 2007, 25, 570–596 CrossRef CAS PubMed.
- G. Mohebali and A. S. Ball, Microbiology, 2008, 154, 2169–2183 CrossRef CAS PubMed.
- L. Alves, R. Salgueiro, C. Rodrigues, E. Mesquita, J. Matos and F. M. Gírio, Appl. Biochem. Biotechnol., 2005, 120, 199–208 CrossRef CAS PubMed.
- L. Alves, M. Melo, D. Mendonça, F. Simões, J. Matos, R. Tenreiro and F. M. Gírio, Enzyme Microb. Technol., 2007, 40, 1598–1603 CrossRef CAS.
- L. Alves and S. M. Paixão, Bioresour. Technol., 2011, 102, 9162–9166 CrossRef CAS PubMed.
- L. Alves and S. M. Paixão, New Biotechnol., 2014, 31, 73–79 CrossRef CAS PubMed.
- L. Alves and S. M. Paixão, Appl. Biochem. Biotechnol., 2014, 172, 3297–3305 CrossRef CAS PubMed.
- T. P. Silva, S. M. Paixão, A. V. Teixeira, J. C. Roseiro and L. Alves, J. Chem. Technol. Biotechnol., 2013, 88, 919–923 CrossRef CAS.
- T. P. Silva, S. M. Paixão, J. C. Roseiro and L. Alves, J. Appl. Microbiol., 2015, 118, 609–618 CrossRef CAS.
- R. Vazquez-Duhalt, E. Torres, B. Valderrama and S. Le Borgne, Energy Fuels, 2002, 16, 1239–1250 CrossRef CAS.
- J. J. Kilbane, Curr. Opin. Biotechnol., 2006, 17, 305–314 CrossRef CAS PubMed.
- M. A. Dinamarca, A. Rojas, P. Baeza, G. Espinoza, C. Ibacache-Quiroga and J. Ojeda, Fuel, 2014, 116, 237–241 CrossRef CAS.
- S. M. Paixão, T. P. Silva, B. F. Arez and L. Alves, in Applying Nanotechnology to the Desulfurization Process in Petroleum Engineering, ed. T. Saleh, IGI Global, Hershey PA, 2016, vol. 13, pp. 390–425 Search PubMed.
- L. Alves, S. Marques, J. Matos, R. Tenreiro and F. M. Gírio, Chemosphere, 2008, 70, 967–973 CrossRef CAS PubMed.
- S. M. Paixão, L. Alves, R. Pacheco and C. M. Silva, Energy, 2018, 150, 468–481 CrossRef.
- A. S. Fernandes, S. M. Paixão, T. P. Silva, J. C. Roseiro and L. Alves, Bioprocess Biosyst. Eng., 2018, 41, 143–155 CrossRef CAS PubMed.
- R. M. Singh, Appl. Microbiol., 1971, 22, 131–132 CAS.
- J. P. Schimel and M. N. Weintraub, Soil Biol. Biochem., 2003, 35, 549–563 CrossRef CAS.
- M. Webb, J. Gen. Microbiol., 1949, 5, 485–495 CrossRef PubMed.
- T. P. Silva, MSc thesis, Faculty of Sciences of the Unversity of Lisbon, 2012.
- T. Ohshiro, T. Kojima, K. Torii, H. Kawasoe and Y. Izumi, J. Biosci. Bioeng., 1999, 88, 610–616 CrossRef CAS PubMed.
- L. Alves, J. Matos, R. Tenreiro and F. M. Gírio, J. Ind. Microbiol. Biotechnol., 2008, 35, 69–73 CrossRef CAS PubMed.
- S. M. Paixão, B. F. Arez, J. C. Roseiro and L. Alves, J. Environ. Manage., 2016, 182, 397–405 CrossRef PubMed.
- T. P. Silva, S. M. Paixão and L. Alves, RSC Adv., 2016, 6, 58055–58063 RSC.
- S. Bandyopadhyay, R. Chowdhury, C. Bhattacharjee and S. Pan, Fuel, 2013, 113, 107–112 CrossRef CAS.
- S. Bandyopadhyay, R. Chowdhury and C. Bhattacharjee, Green Chem. Lett. Rev., 2014, 7, 288–295 CrossRef CAS.
- J. M. Campos-Martin, M. C. Capel-Sanchez, P. Perez-Presas and J. L. G. Fierro, J. Chem. Technol. Biotechnol., 2010, 85, 879–890 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra03601f |
| This journal is © The Royal Society of Chemistry 2019 |