Open Access Article
Open Access ArticleTwo step, one-pot sequential synthesis of functionalized hybrid polyheterocyclic scaffolds via a solid state melt reaction (SSMR)†
Manickam Bakthadoss *a,
Jayakumar Srinivasana,
Mir Ashiq Hussaina and
Duddu S. Sharada
*a,
Jayakumar Srinivasana,
Mir Ashiq Hussaina and
Duddu S. Sharada *b
*b
aDepartment of Chemistry, Pondicherry University, Pondicherry-605 014, India. E-mail: bhakthadoss@yahoo.com
bDepartment of Chemistry, Indian Institute of Technology Hyderabad, Kandi, 502285, Sangareddy, Telangana, India
First published on 6th August 2019
Abstract
A new one pot assembly of highly functionalized benzo[a]phenazinone fused chromene/bicyclic scaffolds via a domino Knoevenagel intramolecular hetero-Diels–Alder (IMHDA) strategy using a solid state melt reaction (SSMR) of 2-hydroxynaphthalene 1,4-dione, o-phenylenediamine, O-allyl salicylaldehyde/O-vinyl salicylaldehyde derivatives is reported. The formation of five new bonds (two C–C bonds and three C–O bonds), three six-membered rings, and three stereogenic centers in a one-pot manner is very attractive. Ease of reaction with short time, good yields with water as the only byproduct and work up free procedure are some of the excellent features of the present protocol.
Multicomponent reactions (MCRs)1 are known to construct complex structures from simple starting materials in a rapid and highly efficient manner where the production of wastes is minimized. Those multicomponent reactions which are carried out in a ‘one-pot’ MC sequential manner provide a high degree of reaction mass efficiency, which is crucial in the development of modern synthetic methodology for drug discovery and pharmaceutical programs.2 Enormous work has been carried out in the field of multicomponent reactions in the past decade where several MCRs have been developed and extensively used in natural product synthesis and drug discovery. The Ugi reaction is a prime example of a four-component reaction that has been found to be a powerful and efficient method for the preparation of α-amino amides both in academia and industry.3 Therefore, the development of new MCRs for the synthesis of biologically active molecules continues to attract considerable attention for their atom- and step economy features.
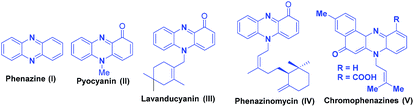
Heterocycles having nitrogen atom are widely available in nature and possess diverse and important biological activities.4 Phenazines are a group of organic compounds known to the mankind since past 150 years and are known to exhibit significant biological activities such as antimalarial, fungicidal, trypanocidal, antiplatelet, etc.5 A large number of drug molecules bearing the phenazine scaffold have been designed and evaluated in the recent years. The phenazines motif possess two dentate N atoms with three fused aromatic rings and an electron deficient conjugated system. The presence of the above features in phenazines give them an ability to form hydrogen bonds, ionic bonds, and π–π interactions with relative ease, and their use has also been demonstrated in supramolecular chemistry for molecular recognition (MR), supramolecular self-assembly (MS-A), and organic optic electronics materials.6 Few representative examples of phenazine and its derivatives are shown in the Fig. 1.
Chromenes and their derivatives are privileged scaffolds due to their ubiquitous presence in many natural products and synthetic molecules.7 Chromenes are also an important class of compounds displaying interesting biological activities against prostate cancer (DU-145) and breast cancer (MCF-7).8 Additionally, they have medicinal qualities such as antiviral and antimicrobial activity.9 Chromenes have also found their use in medicine, health-promoting agents, and photochromic materials.10
In recent years, the domino-Knoevenagel-hetero-Diels–Alder reaction (DKHDA) which was extensively studied by Tietze's group constitutes an important process for the preparation of complex compounds having interesting biological properties.11 The Diels–Alder reaction is important since it allows the formation of functionalized rings where there is complete control on regio-, diastereo-, and enantioselectivity. Moreover, the concerted nature of the Diels–Alder reactions allow the selective formation of up to three stereogenic centers in a single reaction step. Majority of the reactions reported on DKHDA have utilized 1,3-dicarbonyl compounds such as dimethyl barbituric acid, Meldrum's acid and 1,3-indanedione.12 Additionally, other active methylene compounds like 1-phenyl-3-methyl pyrazolone, 4-hydroxy-coumarin, dihydroindole-2-thione, benzoylacetonitrile, 4-hydroxydithiocoumarin have been also used;13 but phenazinones have never been employed in DKHDA reactions. To the best of our knowledge, there are no reports on intramolecular DKHDA reactions using phenazinone as the coupling partner. Due to our interest in the construction of bioactive fused chromenophenazinone derivatives and since this molecule contains a 1,3-dicarbonyl as well as a 1,3-imine moiety, it would be very challenging to control the regioselectivity of the molecule; therefore phenazinone scaffold can act as a model substrate for an intramolecular DKHDA. We envisaged that a series of angular polycyclic chromeno fused phenazinone derivatives could be obtained and it is possible that the final hybrid compounds will result in being more selective and efficient than chromene and phenazinone compounds in biological assays.
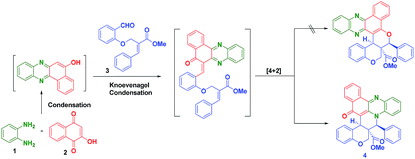
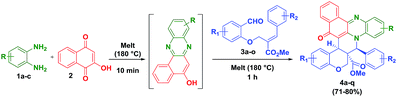
In this direction, we have decided to develop a new method for the preparation of novel benzo[a]phenazinone fused chromene/bicyclic scaffolds via a domino Knoevenagel intramolecular hetero-Diels–Alder (IMHDA) strategy using a solid state melt reaction (SSMR). As part of our research program for the development of new synthetic methods in heterocyclic chemistry14 and solid state melt reactions,15,16 we would like to report a simple and a general domino three-component reaction of 2-hydroxynaphthalene-1,4-dione, o-phenylenediamine and O-allylated/vinylated salicylaldehyde for the synthesis of pentacyclic benzo[a]phenazinone fused chromene/bicyclic scaffolds under environmentally benign reaction conditions (Scheme 1).
We have started this study by melting 2-hydroxynaphthalene-1,4-dione (2), o-phenylenediamine (1), and (E)-methyl 2-((2-formylphenoxy)methyl)-3-phenylacrylate (3) at 180 °C for 1 h in solvent free condition. Unfortunately, we could not obtain any desired product and the formation of a complex mixture was observed. To minimize the formation of the side products, the 2-hydroxynaphthalene-1,4-dione (2) and o-phenylenediamine (1) were first melted at 180 °C for 10 minutes to form the intermediate benzo[a]phenazin-5-ol. Subsequently, (E)-methyl 2-((2-formylphenoxy)methyl)-3-phenylacrylate 3a was added and the mixture was melted under the same temperature for 1 h which afforded the desired hybrid product chromene fused benzo[a]phenazinone 4a (ester moiety in ring junction) in 78% yield as shown in Table 1.
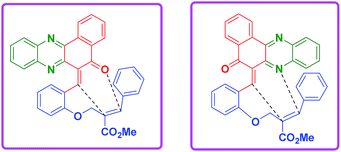
This two-step procedure allows the one-pot three-component reaction to be controlled, avoiding the separation of intermediates, as well as time-consuming and costly purification processes. It is also important to mention here that the reaction is not only diastereoselective, but also chemoselective. Among the two possible hetero diene such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N from phenazine ring, only the C
N from phenazine ring, only the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N diene is involved in the reaction which clearly shows the chemo-selective nature of the reaction (Fig. 2).
N diene is involved in the reaction which clearly shows the chemo-selective nature of the reaction (Fig. 2).
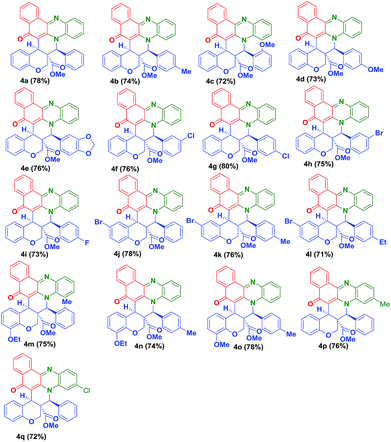
To expand the scope of this one pot reaction, by following the aforementioned procedure, we treated a variety of O-allylated salicylaldehyde derivatives (3b–o) and melted with o-phenylenediamine (1a–c), 2-hydroxynaphthalene-1,4-dione (2), which successfully yielded the desired fused novel benzo[a]phenazinone fused chromene derivatives 4b–q in 71–80% yields. The isolated yields of the pure products (4b–q) are summarized in Table 1. The reaction is highly diastereoselective and chemoselective in nature, which has been, clearly evidenced in the 1H NMR spectroscopy.
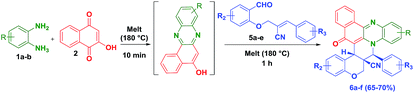
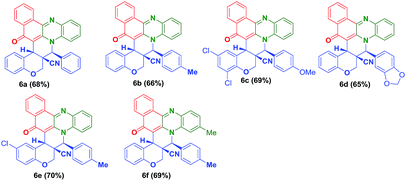
To check the generality of the reaction, we melted the O-allylated salicyldehyde derivatives (5a–e) bearing nitrile functionality, with o-phenylenediamine (1a–b) and 2-hydroxynaphthalene-1,4-dione (2) for 1 h at 180 °C which successfully provided the anticipated hybrid benzo[a]phenazinone fused chromene derivatives (6a–f) (angularly substituted nitrile moiety) in 65–70% yield (Table 2).
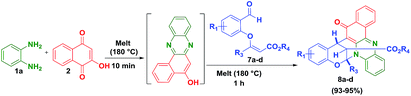
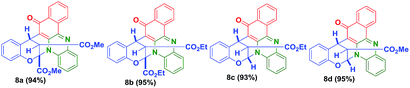
Further, to explore this methodology, we have prepared a variety of vinylogous carbonate derivatives (7a–d) and treated with o-phenylenediamine (1) and 2-hydroxynaphthalene-1,4-dione (2) for 1 h at 180 °C which successfully provided the novel benzo[a]phenazinones fused bicyclic scaffolds compounds (8a–d) in 94–96% yields (Table 3).
This one pot domino reaction is highly stereoselective in nature and was evidenced by single crystal X-ray analyses.17 The stereochemistry of the compounds 4l and 8b is confirmed by X-ray crystallographic analysis (Fig. 3). It can be seen from the crystal structure that the phenyl group and the adjacent ester moiety are in the anti-orientation in benzo[a]phenazinone-fused chromene (4l) which is due to the initial trans geometry of the phenyl group and ester moiety present in the double bond at vicinal position of the compound 4l. Similarly, the ORTEP diagram of compound 8b (Fig. 3) clearly demonstrates that the relative stereochemistry of the ester at the ring junction and the ring junction hydrogen are in syn-orientation with each other.
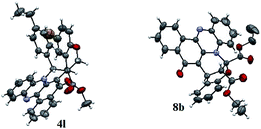 | ||
| Fig. 3 X-ray crystal structure of 4l and 8b.17 | ||
Conclusions
In conclusion, we have successfully developed a novel and efficient method for the synthesis of heptacyclic frameworks containing a benzo[a]phenazinone fused chromene/bicyclic scaffolds via one pot multicomponent domino Knoevenagel intramolecular hetero Diels–Alder (IMHDA) strategy using a Solid State Melt Reaction (SSMR) in a highly diastereoselective and chemoselective fashion. This one pot two-step reaction leads to a novel class of heterocyclic skeleton, which creates five new bonds, three new rings, three contiguous stereogenic centres and one of them being an all carbon quaternary centre. Various advantages of this procedure include solvent and catalyst free synthesis, high yields and short reaction time. Additionally, the only by-product in this reaction is water, which makes this protocol very attractive in green chemistry point of view.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank CSIR (New Delhi) [02(0303)/17/EMR-II] for the financial support. J. S. thanks CSIR, New Delhi for his SRF fellowship.Notes and references
- For some reviews on MC reactions, see: (a) R. M. Armstrong, A. P. Combs, P. A. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123–131 CrossRef CAS; (b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3169–3210 CrossRef; (c) H. Bienaymé, C. Hulme, G. Oddon and P. Schmitt, Chem. –Eur. J., 2000, 6, 3321–3329 CrossRef; (d) A. Dömling, Curr. Opin. Chem. Biol., 2000, 4, 318–323 CrossRef; (e) A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (f) N. Isambert and R. Lavilla, Chem. –Eur. J., 2008, 14, 8444–8454 CrossRef CAS PubMed; (g) B. Ganem, Acc. Chem. Res., 2009, 42, 463–472 CrossRef CAS PubMed; (h) E. Ruijter, R. Scheffelaar and R. V. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234–6246 CrossRef CAS PubMed.
- J. Andraos, Org. Process Res. Dev., 2005, 9, 149–163 CrossRef CAS.
- (a) A. Doemling and I. Ugi, Angew. Chem., Int. Ed. Engl., 2000, 39, 3169–3210 Search PubMed; (b) H. Bienaymé, C. Hulme, G. Oddon and P. Schmitt, Chem.–Eur. J., 2000, 6, 3321–3329 CrossRef.
- Dictionary of Alkaloids, ed., I. W.Southon and J.Buckingham, Chapman & Hall, New York, 1989 Search PubMed.
- (a) J. B. Laursen and J. Nielsen, Chem. Rev., 2004, 104, 1663–1686 CrossRef CAS PubMed; (b) M. Makgatho, R. Anderson, J. O'Sullivan, T. Egan, J. Freese, N. Cornelius and C. Van Rensburg, Drug Dev. Res., 2000, 50, 195–202 CrossRef CAS; (c) V. Andrade-Nieto, M. Goulart, J. F. da Silva, M. J. da Silva, M. Pinto, A. Pinto, M. Zalis, L. Carvalho and A. Krettli, Bioorg. Med. Chem. Lett., 2004, 14, 1145–1149 CrossRef PubMed; (d) C. Neves-Pinto, V. Malta, M. Pinto, R. Santos, S. Castro and A. Pinto, J. Med. Chem., 2002, 45, 740–743 CrossRef PubMed; (e) D. Cartwright, W. Chilton and D. Benson, Appl. Microbiol. Biotechnol., 1995, 43, 211–216 CrossRef CAS; (f) J. Ligon, S. Dwight, P. Hammer, N. Torkewitz, D. Hofmann, H. Kempf and K. Pee, Pest Manage. Sci., 2000, 56, 688–695 CrossRef CAS; (g) M. Muller and T. Sorrell, Prostaglandins, 1995, 50, 301–311 CrossRef CAS PubMed.
- (a) H. Xue, X.-J. Tang, L.-Z. Wu, L.-P. Zhang and C.-H. Tung, J. Org. Chem., 2005, 70, 9727 CrossRef CAS PubMed; (b) M. R. Gill, D. Cecchin, M. G. Walker, R. S. Mulla, G. Battaglia, C. Smythe and J. A. Thomas, Chem. Sci., 2013, 4, 4512 RSC; (c) S. M. Brombosz, A. J. Zucchero, R. L. Phillips, D. Vazquez, A. Wilson and U. H. F. Bunz, Org. Lett., 2007, 9, 4519 CrossRef CAS PubMed; (d) S. Horiuchi, R. Kumai and Y. Tokura, J. Am. Chem. Soc., 2005, 127, 5010 CrossRef CAS PubMed; (e) Q. Miao, Adv. Mater., 2014, 26, 5541 CrossRef CAS PubMed; (f) U. H. F. Bunz, J. U. Engelhart, B. D. Lindner and M. Schaffroth, Angew. Chem., Int. Ed., 2013, 52, 3810 CrossRef CAS PubMed; (g) S. Horiuchi, F. Ishii, R. Kumai, Y. Okimoto, H. Tachibana, N. Nagaosa and Y. Tokura, Nat. Mater., 2005, 4, 163 CrossRef CAS PubMed.
- (a) E. E. Schweizer, N. O. Meeder, and G. P. Ellis in Chromenes, Chromanes, Chromones, Wiley-Interscience, New York, 1977 Search PubMed; (b) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285–2309 CrossRef CAS PubMed; (c) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G. Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939–9953 CrossRef CAS; (d) K. C. Nicolaou, J. A. Pfefferkorn, H. J. Mitchell, A. J. Roecker, S. Barluenga, G. Q. Cao, R. L. Affleck and J. E. Lillig, J. Am. Chem. Soc., 2000, 122, 9954–9967 CrossRef CAS; (e) K. C. Nicolaou, J. A. Pfefferkorn, S. Barluenga, H. J. Mitchell, A. J. Roecker and G. Q. Cao, J. Am. Chem. Soc., 2000, 122, 9968–9976 CrossRef CAS.
- A. Kumar, S. Sharma, R. A. Maurya and J. Sarkar, J. Comb. Chem., 2010, 12, 20–24 CrossRef CAS PubMed.
- (a) J. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato and K. Kikugawa, Mutat. Res., 1997, 395, 47–56 CrossRef PubMed; (b) G. Shanthi and P. T. Perumal, Tetrahedron Lett., 2007, 48, 6785–6789 CrossRef CAS.
- (a) T. A. Engler, K. O. LaTessa, R. Iyengar, W. Chai and K. Agrios, Bioorg. Med. Chem., 1996, 4, 1755–1769 CrossRef CAS; (b) A. Elomri, S. Mitaku, S. Michel, A. L. Skaltsounis, F. Tillequin, M. Koch, A. Pierre, N. Guilbaud, S. Lèonce, L. Kraus, Y. Rolland and G. Atassi, J. Med. Chem., 1996, 39, 4762–4766 CrossRef CAS PubMed; (c) M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295–4298 CrossRef CAS PubMed; (d) C. Tahtaoui, A. Demailly, C. Guidemann, C. Joyeux and P. Schneider, J. Org. Chem., 2010, 75, 3781–3785 CrossRef CAS PubMed; (e) K. Mukai, K. Okabe and H. Hosose, J. Org. Chem., 1989, 54, 557–560 CrossRef CAS; (f) J. Jankun, S. H. Selman and R. Swiercz, Nature, 1997, 387, 561 CrossRef CAS PubMed; (g) S. Paramonov, S. Delbaere, O. Fedorova, Y. Fedorov, V. Lokshin, A. Samat and G. J. Vermeersch, J. Photochem. Photobiol., A, 2010, 209, 111–120 CrossRef CAS; (h) R. A. Evans and G. K. Such, Aust. J. Chem., 2005, 58, 825–830 CrossRef CAS.
- (a) L. F. Tietze and U. Beifuss, Angew. Chem., 1993, 105, 137 CrossRef CAS; (b) L. F. Tietze, G. Brasche, and K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006, p. 160 CrossRef; (c) L. F. Tietze, Chem. Rev., 1996, 96, 115 CrossRef CAS; (d) L. F. Tietze, J. Heterocycl. Chem., 1990, 27, 47 CrossRef CAS; (e) L. F. Tietze in Selectivity–A Goal for Synthetic Efficiency, ed W. Bartmann, and B. M. Trost, VerlagChemie, Weinheim, 1984, p. 299 Search PubMed.
- (a) M. J. Khoshkholgh, S. Balalaie, H. R. Bijanzadeh and J. H. Gross, ARKIVOC, 2009, ix, 114–121 Search PubMed; (b) P. Biswas, J. Ghosh and C. Bandyopadhyay, Tetrahedron Lett., 2014, 55, 6882–6886 CrossRef CAS.
- (a) M. J. Khoshkholgh, M. Lofti, S. Balalaie and F. Rominger, Tetrahedron, 2009, 65, 4228–4234 CrossRef CAS; (b) M. Mollazadeh, M. J. Khoshkholgh, S. Balalaie, F. Rominger and H. R. Bijanzadeh, J. Heterocycl. Chem., 2010, 47, 1200–1208 CrossRef CAS; (c) N. J. Parmar, B. R. Pansuriya, B. M. Labana, T. R. Sutariya, R. Kant and V. K. Gupta, Eur. J. Org. Chem., 2012, 2012, 5953–5964 CrossRef CAS; (d) T. R. Sutariya, B. M. Labana, B. D. Parmar, N. J. Parmar, R. Kantb and V. K. Gupta, RSC Adv., 2015, 5, 23519–23529 RSC; (e) K. C. Majumdar, T. Abu and N. R. Kumar, Tetrahedron, 2012, 68, 5693–5718 CrossRef CAS.
- (a) M. Bakthadoss, D. Kannan and R. Selvakumar, Chem. Commun., 2013, 49, 10947–10949 RSC; (b) M. Bakthadoss, A. Devaraj and D. Kannan, Eur. J. Org. Chem., 2013, 1505–1513 Search PubMed; (c) M. Bakthadoss and G. Sivakumar, Tetrahedron Lett., 2014, 1765–1770 CrossRef CAS; (d) M. Bakthadoss, D. Kannan, J. Srinivasan and V. Vinayagam, Org. Biomol. Chem., 2015, 13, 2870 RSC; (e) M. Bakthadoss and N. Sivakumar, Synlett, 2009, 1014 CrossRef CAS; (f) M. Bakthadoss and G. Murugan, Synth. Commun., 2008, 38, 3406 CrossRef CAS; (g) M. Bakthadoss and R. Selvakumar, J. Org. Chem., 2016, 81, 3391–3399 CrossRef CAS PubMed; (h) M. Bakthadoss, N. Sivakumar and A. Devaraj, Synthesis, 2011, 4, 0611–0618 CrossRef.
- (a) M. Bakthadoss, G. Sivakumar and D. Kannan, Org. Lett., 2009, 11, 4466 CrossRef CAS PubMed; (b) M. Bakthadoss, D. Kannan, N. Sivakumar, P. Malathi and V. Manikandan, Org. Biomol. Chem., 2015, 13, 5597 RSC; (c) M. Bakthadoss and G. Sivakumar, Tetrahedron Lett., 2014, 55, 1765 CrossRef CAS; (d) M. Bakthadoss, R. Selvakumar and J. Srinivasan, Tetrahedron Lett., 2014, 55, 5808 CrossRef CAS; (e) M. Bakthadoss and D. Kannan, RSC Adv., 2014, 4, 11723 RSC.
- (a) S. Paul and Y. R. Lee, Green Chem., 2016, 18, 1488–1494 RSC; (b) S. Vidyacharan, C. Adhikari, V. S. Krishna, R. S. Reshma, D. Sriram and D. S. Sharada, Bioorg. Med. Chem. Lett., 2017, 27(7), 1593–1597 CrossRef CAS.
- CCDC number for the crystal structure 4l and 8b are 1906333 and 1906667.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC [1906333 and 1906667]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra02590a |
| This journal is © The Royal Society of Chemistry 2019 |