Open Access Article
Open Access ArticleA new way to improve the light-fastness of azo reactive dyes through the introduction of benzene sulfonamide derivatives into the triazine ring
Xiong Wei,
Ma Wei and
Zhang Shufen *
*
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116012, P. R. China. E-mail: zhangshf@dlut.edu.cn; Fax: +86 411 84986264; Tel: +86 411 84986265
First published on 17th June 2019
Abstract
Herein, a new kind of hetero-bifunctional reactive dyes with high light-fastness was designed and synthesized by introducing benzene sulfonamide and its derivatives into the triazine ring. Benzene sulfonamide or its derivatives and 2-amino-5-naphthol-7-sulfonic acid (J-acid) were condensed with cyanuric chloride to synthesize coupling components, which were then coupled with the diazo salt of 4-(β-sulfatoethylsulfonyl)aniline. The dyes were characterized by IR spectroscopy and MS. The color fastness test proved that the light-fastness of the dyes could be improved by 1 grade via the introduction of benzene sulfonamide derivatives into the triazine ring when compared with the case of the control dyes. Fluorescence spectra demonstrated that after the introduction of benzene sulfonamide derivatives, the dye molecule could return to the ground state from the excited state and emit fluorescence; in addition, the introduced benzene sulfonamide derivatives helped to deteriorate the adverse effect of UV light on the dye. Moreover, the dyeing results showed that the dyes containing the sulfonamide groups had equal dyeing properties when compared with that of the control dyes.
Introduction
Reactive dyes exhibit good wet-fastness because they form chemical bonds with the textile fibre.1,2 Since the average fixation of mono-functional reactive dyes is below 65%,3,4 reactive dyes are usually designed as bi-functional or multi-functional structures to increase their utilization.5 Hetero-bifunctional reactive dyes are widely used due to their good wet-fastness under both acidic and alkali conditions.6,7Due to its brilliant color, abundant hues, simple synthesis procedures, excellent structure diversity and high molar extinction coefficients, the azo chromophore has become the most widely used chromophore. More than 80% of chromophores used in reactive dyes are azo chromophores.8 However, under natural conditions, the azo group will slowly decompose in the presence of moisture, UV light, ozone, etc.9–12 With the decomposition of the azo group, the light-fastness of the azo dyes rapidly decreases. Therefore, the development of a strategy to improve the light-fastness of azo dyes is of great importance and current interest. Generally, there are two ways to improve the light-fastness of azo dyes: first, the use of chromophores, such as anthraquinone and metal azo complexes, with high light-fastness,13–15 and second, the incorporation of UV absorbers into the dye molecules or the treatment of textiles with UV absorbers before or after dyeing.16–22 UV absorbers are usually introduced into a dye molecule by linkage with cyanuric chloride,17 and the latter also acts as a reactive group in a reactive dye.
The photofading process of azo dyes occurs when the dye molecule is excited from the ground state to the excited state via the absorption of a photon, and then, the dye molecule in the excited state can react with oxygen,23–25 the return of the dye molecule to the ground state from the excited state is favourable for the light-fastness of azo dyes. The dye molecule can return to the ground state from the excited state by emitting light; this phenomenon is known as fluorescence.
To date, no studies have been reported on the introduction of a sulfonamide group directly into the s-triazine ring in the dye industry to improve the light-fastness of azo dyes. Moreover, significant attention has been focused on the incorporation of β-sulfatoethylsulfonyl aniline into dyes to improve the affinity and fixation of dyes;26,27 in previous studies, the sulfonamide group has also been introduced into dyes as a linkage group to replace benzidine since benzidine is highly carcinogenic and mutagenic.28–32
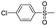
Due to their simplest structure containing a sulfonamide group, herein, benzene sulfonamide and its derivatives were directly incorporated into the s-triazine ring with the aim to obtain a new kind of reactive dyes with high light-fastness. The new kinds of dyes were synthesized using 4-(β-sulfatoethylsulfonyl) aniline as the diazo component. The coupling component was synthesized by condensing cyanuric chloride with benzene sulfonamide or its derivatives and 2-amino-5-naphthol-7-sulfonic acid (J-acid). The influence of benzene sulfonamide and its derivatives on the light-fastness has been discussed, and the dyeing properties have also been tested.
Experiment
Materials and instruments
2-Amino-5-naphthol-7-sulfonic acid (J-acid) and 4-(β-sulfatoethylsulfonyl) aniline (para ester) were obtained from Zhejiang Shunlong Chemical Co. Cyanuric chloride and benzene sulfonamide and its derivatives were purchased from Aladdin Industrial Corp. Aniline and sulfanilic acid were bought from Tianjin Guangfu Chemical Reagent Co. All the other chemicals involved in this study were of chemical grade. All the materials were used without further purification.The FTIR spectra were obtained via the JASCO 430 spectrometer (JASCO, Japan) using KBr pellets. The API-ES-Mass spectra were obtained using the HP1100 mass spectrometer (Hewlett-Packard, USA). UV-Vis spectra were obtained using the HP-8453 UV-Visible spectrometer (Hewlett-Packard, USA). Cotton dyeing was operated using an XW-PDR (Jinjiang Xinwang Dyeing & Finishing Machinery Factory). Fluorescence spectra were acquired using the F-7000 fluorescence spectrometer (HITACHI, Japan).
Synthesis of dyes
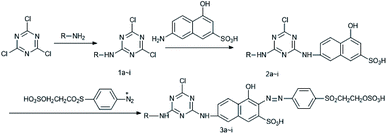
The synthesis of the mono-azo reactive dyes 3a–i based on J-acid with the substitution of benzene sulfonamide or its derivatives is depicted in Scheme 1. The synthetic procedure of the dye 3a is described as below.3a: yield: 83.1%. IR (KBr, cm−1): 3448 (N–H, O–H), 2975 (C–H), 1594 (N–H), 1545 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1498 (triazine), 1390, 1137 (SO2–NH), 1195, 1083 (–SO3H). M/S (ES-API): m/z = 798.9, found 798.0 ([M − H]−), 398.5 ([M − 2H]2−/2).
N), 1498 (triazine), 1390, 1137 (SO2–NH), 1195, 1083 (–SO3H). M/S (ES-API): m/z = 798.9, found 798.0 ([M − H]−), 398.5 ([M − 2H]2−/2).
The dyes 3b–g were synthesized via the same way by simply changing the condensation reactant.
Dye 3b was synthesized by replacing benzene sulfonamide with 4-sulfamoyl benzoic acid. Yield: 103.4%. IR (KBr, cm−1): 3429 (N–H, O–H), 2975 (C–H), 1596 (N–H), 1561 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1499 (triazine), 1453 (O–H), 1385, 1137 (SO2–NH), 1257, 1051 (–SO3H). M/S (ES-API): m/z = 842.98, found 842.0 ([M − H]−), 420.6 ([M − 2H]2−/2).
N), 1499 (triazine), 1453 (O–H), 1385, 1137 (SO2–NH), 1257, 1051 (–SO3H). M/S (ES-API): m/z = 842.98, found 842.0 ([M − H]−), 420.6 ([M − 2H]2−/2).
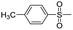
Dye 3c was obtained by replacing benzene sulfonamide with 4-toluene sulfonamide. Yield: 88.6%. IR (KBr, cm−1): 3463 (N–H, O–H), 2978 (C–H), 1595 (N–H), 1556 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1498 (triazine), 1451, 1390 (C–H), 1320, 1137 (SO2–NH), 1221, 1052 (–SO3H). M/S (ES-API): m/z = 813.0, found 811.8 ([M − H]−), 405.5 ([M − 2H]2−/2).
N), 1498 (triazine), 1451, 1390 (C–H), 1320, 1137 (SO2–NH), 1221, 1052 (–SO3H). M/S (ES-API): m/z = 813.0, found 811.8 ([M − H]−), 405.5 ([M − 2H]2−/2).
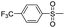
Dye 3d was synthesized by replacing benzene sulfonamide with 4-trifluoromethyl benzene sulfonamide. Yield: 72.2%. IR (KBr, cm−1): 3444 (N–H, O–H), 2976 (C–H), 1595 (N–H), 1543 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1499 (triazine), 1324, 1189, 1168 (Ar–CF3), 1390, 1136 (SO2–NH), 1225, 1051 (–SO3H). M/S (ES-API): m/z = 866.9, found 865.9 ([M − H]−), 432.5 ([M − 2H]2−/2).
N), 1499 (triazine), 1324, 1189, 1168 (Ar–CF3), 1390, 1136 (SO2–NH), 1225, 1051 (–SO3H). M/S (ES-API): m/z = 866.9, found 865.9 ([M − H]−), 432.5 ([M − 2H]2−/2).
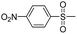
Dye 3e was obtained by replacing benzene sulfonamide with 4-nitrobenzene sulfonamide. Yield: 77.5%. IR (KBr, cm−1): 3442 (N–H, O–H), 2975 (C–H), 1594 (N–H), 1564 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1529, 1355 (–NO2), 1498 (triazine), 1385, 1138 (SO2–NH), 1226, 1052 (–SO3H). M/S (ES-API): m/z = 843.9, found 842.9 ([M − H]−), 421.0 ([M − 2H]2−/2).
N), 1529, 1355 (–NO2), 1498 (triazine), 1385, 1138 (SO2–NH), 1226, 1052 (–SO3H). M/S (ES-API): m/z = 843.9, found 842.9 ([M − H]−), 421.0 ([M − 2H]2−/2).
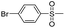
Dye 3f was synthesized by replacing benzene sulfonamide with 4-chlorobenzene sulfonamide. Yield: 89.6%. IR (KBr, cm−1): 3406 (N–H, O–H), 2978 (C–H), 1595 (N–H), 1542 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1498 (triazine), 1386, 1136 (SO2–NH), 1257, 1050 (–SO3H), 1087 (Ar–Cl). M/S (ES-API): m/z = 832.9, found 831.8 ([M − H]−), 415.5 ([M − 2H]2−/2).
N), 1498 (triazine), 1386, 1136 (SO2–NH), 1257, 1050 (–SO3H), 1087 (Ar–Cl). M/S (ES-API): m/z = 832.9, found 831.8 ([M − H]−), 415.5 ([M − 2H]2−/2).
Dye 3g was obtained by replacing benzene sulfonamide with 4-bromobenzene sulfonamide. Yield: 99.4%. IR (KBr, cm−1): 3422 (N–H, O–H), 2975 (C–H), 1595 (N–H), 1564 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1498 (triazine), 1388, 1137 (SO2–NH), 1221, 1051 (–SO3H), 1078 (Ar–Br). M/S (ES-API): m/z = 876.9, found 437.5 ([M − 2H]2−/2).
N), 1498 (triazine), 1388, 1137 (SO2–NH), 1221, 1051 (–SO3H), 1078 (Ar–Br). M/S (ES-API): m/z = 876.9, found 437.5 ([M − 2H]2−/2).
Synthesis of control dyes 3h–i
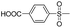
The synthesis procedure for control dye 3h is described below.Cyanuric chloride (1.92 g, 0.0105 mol) and 20 g of ice cubes were stirred for 30 minutes at 0–5 °C. The first condensation reaction was started by adding aniline (0.93 g, 0.01 mol) at the temperature of 0–5 °C for 2 hours. Then, the condensation mixture was added to J-acid (2.39 g, 0.01 mol) at the temperature of 30 °C and pH of 6–7. The reaction was monitored by TLC (eluent: n-butanol/isopropanol/ethyl acetate/H2O = 2/4/1/3; v/v). The diazotization and coupling process were the same as those of dye 3a.
3h: yield: 84.4%. IR (KBr, cm−1): 3397 (N–H, O–H), 2976 (C–H), 1599 (N–H), 1575 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1496 (triazine), 1298 (C–N), 1255 (C–O), 1227, 1049 (–SO3H). M/S (ES-API): m/z = 735.03, found 734.0 ([M − H]−), 366.5 ([M − 2H]2−/2).
N), 1496 (triazine), 1298 (C–N), 1255 (C–O), 1227, 1049 (–SO3H). M/S (ES-API): m/z = 735.03, found 734.0 ([M − H]−), 366.5 ([M − 2H]2−/2).
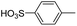
3i was synthesized using sulfanilic acid instead of aniline. Yield: 82.0%. IR (KBr, cm−1): 3443 (N–H, O–H), 2973 (C–H), 1596 (N–H), 1557 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1495 (triazine), 1370 (C–N), 1222, 1050 (–SO3H). M/S (ES-API): m/z = 814.98, found 406.5 ([M − 2H]2−/2).
N), 1495 (triazine), 1370 (C–N), 1222, 1050 (–SO3H). M/S (ES-API): m/z = 814.98, found 406.5 ([M − 2H]2−/2).
Dyeing process
The dyes 3a–i were applied to the dyeing cotton at 1–2% owf color shade to achieve dyeing cotton with a similar K/S value at Na2SO4 of 100 g L−1 (added in three times) and Na2CO3 of 10 g L−1 under the fixation of 75 °C. The liquor-to-goods ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
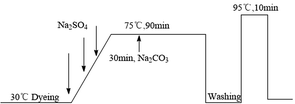
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After dyeing, the colored cotton was washed thoroughly; then, the dyeing cotton was soaped in a 2 g L−1 OP-10 solution at 95 °C for 10 min and finally washed and dried. The dyeing process was conducted as descripted in Fig. 1.
1. After dyeing, the colored cotton was washed thoroughly; then, the dyeing cotton was soaped in a 2 g L−1 OP-10 solution at 95 °C for 10 min and finally washed and dried. The dyeing process was conducted as descripted in Fig. 1.
The exhaustion, fixation and reactivity of the dyes were calculated using eqn (1)–(3), and the absorbance was determined using the HP 8453 UV-Vis spectrophotometer at the λmax of each dye.
| E = (A0 − A1)/A0 × 100% | (1) |
| F = (A0 − A1 − A2)/A0 × 100% | (2) |
| R = E/F × 100% | (3) |
Color fastness measurement
The wash fastness was assessed using a 5 g L−1 standard soap solution at 40 °C for 30 min according to GB/T 3921-2008. The rub fastness was tested according to GB/T 3920-2008 using a Y(B)571-II crockmeter (Darong Standard Textile Apparatus Co. Ltd., Wenzhou). The light-fastness was tested according to GB/T 8427-2008 using YG(B)611-V light-fastness tester (Darong Standard Textile Apparatus Co. Ltd. Wenzhou). The K/S value was measured by using a Ultrascan XE Color Measuring and Matching Meter (Hunter Co. UK).Results and discussion
Dye synthesis
In this study, dyes based on J-acid were synthesized, as depicted in Scheme 1. Benzene sulfonamide derivatives were condensed with cyanuric chloride to obtain a new kind of reactive dyes with high light-fastness.Synthesis of the reactive dyes 3a–i
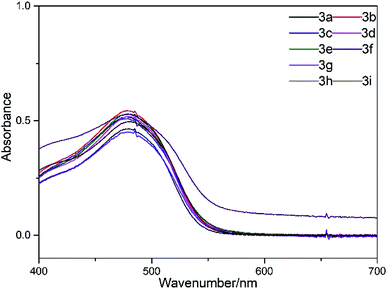
The reactants R–NH2 and J-acid were condensed with cyanuric chloride, and then, the obtained product was coupled with a para ester diazo salt to synthesize the dyes 3a–i, as depicted in Scheme 1. Benzene sulfonamide and its derivatives were used to synthesize the dyes 3a–g containing sulfonamide groups. Dyes substituted with the anilino and 4-sulfonato phenyl groups were used to synthesize the control dyes 3h–i.The visible spectra of the dyes 3a–i are shown in Fig. 2. As shown in Fig. 2, the dyes 3a–i exhibit same absorption feature in the visible light region due to the presence of same chromophore based on J-acid. All the dyes showed a broad absorption band from 400 to 550 nm, with the maximum absorption wavelength of about 480 nm.
The structural and UV-Vis absorption information of 3a–i are listed in Table 1. The molar absorption coefficients range from 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 L mol−1 cm−1 to 27
103 L mol−1 cm−1 to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 079 L mol−1 cm−1. Dyes substituted with benzene sulfonamide and its derivatives have higher molar absorption coefficients than the control dyes 3h–i. When different substitution groups were incorporated into the dye molecules, the solubility of the dyes 3a–i significantly differed. The dye 3h substituted with aniline exhibited the lowest solubility of 20.20 g L−1. When another water-soluble group, carboxyl group, was substituted into the dye molecule, 3b exhibited the highest solubility of 226.94 g L−1 among all the dyes.
079 L mol−1 cm−1. Dyes substituted with benzene sulfonamide and its derivatives have higher molar absorption coefficients than the control dyes 3h–i. When different substitution groups were incorporated into the dye molecules, the solubility of the dyes 3a–i significantly differed. The dye 3h substituted with aniline exhibited the lowest solubility of 20.20 g L−1. When another water-soluble group, carboxyl group, was substituted into the dye molecule, 3b exhibited the highest solubility of 226.94 g L−1 among all the dyes.
The incorporation of benzene sulfonamide and its derivatives had a positive effect on the dye solubility; the improved solubility most probably originated due to the increase in molecular polarity, which could help reduce the formation of dye clusters; on the other hand, the increased molecular polarity originated due to the deterioration of molecular planarity, which resulted from the introduction of the sulfonamide group.
Dyeing properties
The dyeing process was conducted as shown in Fig. 1. The dyes 3a–i were applied to the dyeing cotton at 1–2% omf to achieve the close K/S values of about 15.5–16.5. To reduce the hydrolysis of vinyl sulfonate and enhance the reactivity of the monochlorotriazine reactive group, the fixation temperature was set at 75 °C.Dyeing results
The dyeing results for 3a–i are provided in Table 2. To compare the influence of the sulfonamide group on the dyeing properties of different dyes, the control dyes 3h and 3i without the substitution of the sulfonamide group were designed and compared with the dyes 3a–g substituted with benzene sulfonamide and its derivatives. Moreover, Fig. 3 displays the images of the dyeing cotton. As shown in Fig. 3, the dyes 3a–i provide the same orange color, and all the dyeing cotton samples show good color uniformity.| Dye | E% | F% | R% |
|---|---|---|---|
| 3a | 98.7 | 86.4 | 87.6 |
| 3b | 86.7 | 72.0 | 83.1 |
| 3c | 96.0 | 82.9 | 86.3 |
| 3d | 98.1 | 84.2 | 85.8 |
| 3e | 93.9 | 79.9 | 85.0 |
| 3f | 96.5 | 79.7 | 82.6 |
| 3g | 98.4 | 86.1 | 87.5 |
| 3h | 95.7 | 87.7 | 91.7 |
| 3i | 95.1 | 87.3 | 91.8 |
In Table 2, we can see that the exhaustion of the dyes 3a and 3h was 98.7% and 95.7%, and the fixation was 86.4% and 87.7%, respectively. The exhaustion and fixation of the dyes 3a and 3h were almost the same. The difference in the structures of the dyes 3a and 3i originated due to the substitution of the sulfonamide group; however, similar dyeing properties obtained at 75 °C indicated that the introduction of the sulfonamide group had a slight influence on the dyeing properties. Using another soluble group in the dye molecule, the solubility of the dyes 3b and 3i was found to be significantly higher than that of those without the soluble group. Owing to the high solubility of dye 3b (up to 226.94 g L−1), its exhaustion was only 86.7%, which was lower than that of the other dyes. Moreover, the presence of the carboxyl group in 3b in the form of –COO− would increase the electrostatic repulsion between the dyes and the cotton fibers. Although it was substituted with another water-soluble group, the dye 3i still maintained the high exhaustion of 95.1%, which was attributed to its low solubility and high substantivity. The fixation of the dye 3b was 72.0%, which was lower than that of the dye 3i (87.3%). The fixation difference between 3b and 3i was due to the increase in steric hindrance during the fixation reaction caused by the introduction of the sulfonamide group.
For the dyes 3c–g with different substitution groups on the benzene sulfonamide residue, the solubility differed from 66.98 to 222.58 g L−1. However, no obvious difference in their exhaustion and fixation was observed; this implied that the substitution groups on the benzene sulfonamide had no essential influence on the dye exhaustion and fixation. The steric hindrance caused by the incorporation of sulfonamide made the reaction between the reactive dyes and cotton difficult to occur; this led to a decrease in the fixation of the dyes 3c–g.
Color fastness of the dyes
The color fastness of the dyes with similar K/S values on cotton was tested and is presented in Table 3. Since the covalent bonds formed between the dyes and cotton are the same, the rub-fastness and wash-fastness of the dyes involved in this study should be the same. However, because of higher water solubility of dyes due to the introduction of benzene sulfonamide or its derivatives, the color fastness test showed that the rub-fastness and wash-fastness of the dyes 3a–g containing benzene sulfonamide and its derivatives were slightly better than those of the control dyes 3h and 3i. The high water-solubility provided the dyes with a good wash-off property, which was favourable to improve the wet-fastness and rub-fastness. Moreover, the light-fastness values of the dyes 3b–g containing benzene sulfonamide derivatives were 4–5 grade, which were 1 grade higher than those of the dye 3a and the control dyes 3h and 3i. The difference in the light-fastness could be explained by the difference in their fluorescence spectra.| Entry | K/S | Light-fastness | Rub-fastness | Wet-fastness | |||
|---|---|---|---|---|---|---|---|
| Dry | Wet | Change | Cotton | Wool | |||
| 3a | 15.66 | 3–4 | 4–5 | 4 | 4–5 | 4 | 4–5 |
| 3b | 16.56 | 4–5 | 4 | 4 | 4 | 4 | 4–5 |
| 3c | 16.61 | 4–5 | 4 | 4 | 4 | 4 | 4–5 |
| 3d | 16.65 | 4–5 | 4–5 | 4 | 4 | 4–5 | 4–5 |
| 3e | 15.61 | 4–5 | 4–5 | 4 | 4–5 | 4 | 4–5 |
| 3f | 16.18 | 4–5 | 4–5 | 4 | 4–5 | 4 | 4–5 |
| 3g | 16.11 | 4–5 | 4–5 | 4 | 4–5 | 4 | 4–5 |
| 3h | 15.58 | 3–4 | 4 | 3–4 | 4–5 | 4 | 4–5 |
| 3i | 15.98 | 3–4 | 4–5 | 4 | 4 | 4 | 4–5 |
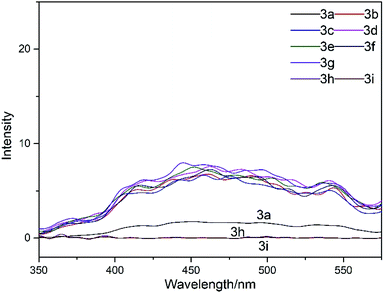
The fluorescence emission spectra of the dyes 3a–g and control dyes 3h and 3i were obtained, as displayed in Fig. 4. All the dyes have been tested at the concentration of 2 g L−1, which is about the dyeing concentration. Moreover, the fluorescence intensity of the system has been determined from 350 to 575 nm with the excitation wavelength of 300 nm since all the dyes show their maxima absorption around 300 nm in the UV region.
From Fig. 4, we can conclude that after the substitution of the benzene sulfonamide derivatives, the dyes 3b–g show weak fluorescence emission characteristics, which can help the dye molecule in the excited state to return to the ground state by emitting fluorescence; in addition, the dye 3a shows weaker fluorescence than the dyes 3b–g; this weakens the effective energy transfer of the dye 3a. Moreover, no fluorescence was observed for the dyes 3h and 3i; this illustrated that the dyes 3h and 3i could not return to the ground state from the excited state by emitting fluorescence. Therefore, the differences in the light-fastness of the dyes 3a–i were most possibly caused by the differences in their fluorescence spectra.
Conclusions
Herein, benzene sulfonamide and its derivatives were condensed with cyanuric chloride to synthesize a new series of hetero-bifunctional azo dyes; this new kind of dyes was synthesized using 4-(β-sulfatoethylsulfonyl) aniline as the diazo component, and the coupling component was synthesized by condensing cyanuric chloride with benzene sulfonamide or its derivatives and J-acid. According to the fastness test, dyes substituted with benzene sulfonamide derivatives displayed better light fastness than the control dyes, which was about 1 grade higher than that of the control dyes. Moreover, the fluorescence spectra showed that after the introduction of the sulfonamide group, the dyes showed weak fluorescence, which could help transfer the UV light. Via the transfer of the absorbed UV light, the destructiveness of the UV light to azo dyes was weakened. Moreover, the dyeing results showed that the incorporation of the sulfonamide group had a little influence on the exhaustion of the dyes; however, the fixation of the dyes decreased as the reaction between the reactive dyes and cotton became difficult to occur due to the increase in the steric hindrance caused by the introduction of sulfonamide groups.Conflicts of interest
All authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFB0307401), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (Grant No. 21421005), Ministry of Education (the Ministry of Education Innovation Team, IRT-13R06) and Dalian University of Technology (Dalian University of Technology Innovative Research Team, DUT2013TB07).References
- D. M. Lewis, Color. Technol., 2014, 130, 382–412 CAS.
- Y. Li, S. Zhang, J. Yang, S. Jiang and Q. Li, Dyes Pigm., 2008, 76, 508–514 CrossRef CAS.
- J. A. Taylor, Rev. Prog. Color. Relat. Top., 2000, 30, 93–107 CrossRef CAS.
- P. Kelshaw, J. Soc. Dyers Colour., 1998, 114, 35–36 CrossRef CAS.
- V. R. Kanetkar, G. S. Shankarling and S. Patil, Colourage, 2000, 47, 35–46 CAS.
- M. Matsui, U. Meyer and H. Zollinger, J. Soc. Dyers Colour., 1988, 104, 425–431 CrossRef CAS.
- A. A. Mousa, Dyes Pigm., 2007, 75, 747–752 CrossRef CAS.
- A. Bafana, S. S. Devi and T. Chakrabarti, Environ. Rev., 2011, 19, 350–370 CrossRef CAS.
- K. Bredereck and C. Schumacher, Dyes Pigm., 1993, 21, 23–43 CrossRef CAS.
- K. Bredereck and C. Schumacher, Dyes Pigm., 1993, 23, 121–133 CrossRef CAS.
- K. Bredereck and C. Schumacher, Dyes Pigm., 1993, 23, 135–147 CrossRef CAS.
- S. N. Batchelor, D. Carr, C. E. Coleman, L. Fairclough and A. Jarvis, Dyes Pigm., 2003, 59, 269–275 CrossRef CAS.
- A. P. Manian, R. Paul and T. Bechtold, Color. Technol., 2016, 132, 107–113 CAS.
- B. Shan, X. Tong, W. Xiong, W. Qiu, B. Tang, R. Lu, W. Ma, Y. Luo and S. Zhang, Dyes Pigm., 2015, 123, 44–54 CrossRef CAS.
- A. D. Towns, Dyes Pigm., 1999, 42, 3–28 CrossRef CAS.
- M. L. Gulrajani, Advances in the dyeing and finishing of technical textiles, 2013, pp. 48–50 Search PubMed.
- R. Rajagopal and S. Seshadri, Dyes Pigm., 1988, 9, 233–241 CrossRef CAS.
- G. Shabir, A. Saeed, M. Arshad and P. A. Channar, J. Chem. Soc. Pak., 2017, 39, 1017–1028 CAS.
- E. Yousif and R. Haddad, SpringerPlus, 2013, 2, 398 CrossRef PubMed.
- J. C. Paya, P. Diaz-Garcia, I. Montava, P. Miro-Martinez and M. Bonet, J. Ind. Text., 2016, 45, 2–12 Search PubMed.
- Y. Tang, P. Gao, M. Wang, J. Zhu and X. Wan, RSC Adv., 2014, 4, 22617–22620 RSC.
- J.-W. Joo and J. Jang, Fibers Polym., 2018, 19(4), 782–789 CrossRef CAS.
- C. Chen, B. Zhou, D. Lu and G. Xu, J. Photochem. Photobiol., A, 1995, 89, 25–29 CrossRef CAS.
- X. Chen, X. Peng, A. Cui, B. Wang, L. Wang and R. Zhang, J. Photochem. Photobiol., A, 2006, 181, 79–85 CrossRef CAS.
- S. N. Batchelor, D. Carr, C. E. Coleman, L. Fairclough and A. Jarvis, Dyes Pigm., 2003, 59, 269–275 CrossRef CAS.
- D. M. Lewis and A. A. Siddique, Color. Technol., 2006, 122, 217–226 CAS.
- D. M. Lewis, A. H. Renfrew and A. A. Siddique, Dyes Pigm., 2000, 47, 151–167 CrossRef CAS.
- W. Li-Min, Chin. J. Struct. Chem., 2007, 26, 6 Search PubMed.
- F. Wang, L. Wang, X. Cai, Y. Sun, L. Su, L. Zhang, Z. Han, G. Zhang and G. Wang, Color. Technol., 2012, 128, 425–433 CAS.
- L. Wang, X. Pan, F. Wang, L. Yang and L. Liu, Dyes Pigm., 2008, 76, 636–645 CrossRef CAS.
- S. Zhang, Dyes Pigm., 1999, 43, 5 Search PubMed.
- W. Zu-Wang, Chem. Res. Chin. Univ., 2001, 27, 10 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |