 Open Access Article
Open Access ArticleLaser wavelength modulated pulsed laser ablation for selective and efficient production of graphene quantum dots†
Sukhyun Kang‡
ab,
Jeong Ho Ryu‡c,
Byoungsoo Leea,
Kyung Hwan Junga,
Kwang Bo Shimb,
Hyuksu Han *ad and
Kang Min Kim*a
*ad and
Kang Min Kim*a
aKorea Institute of Industrial Technology, Gwahakdanji-ro 137-41, Gangwon-do 25440, Republic of Korea. E-mail: kmkim@kitech.re.kr
bDepartment of Materials Science and Engineering, Hanyang University, 17 Haengdang-dong, Seongdong-gu, Seoul 133-791, Republic of Korea
cDepartment of Materials Science and Engineering, Korea National University of Transportation, 50 Daehak-ro, Chungju-si, Chungbuk 380-702, Republic of Korea
dDepartment of Materials Science and Engineering, Hongik University, Sejong-ro 2639, Sejong, Republic of Korea. E-mail: hhan@hongik.ac.kr
First published on 3rd May 2019
Abstract
Graphene quantum dots (GQDs) and graphene oxide quantum dots (GOQDs) can be used in different applications such as optoelectronic and biomedical applications, respectively. Hence, the selective synthesis of GQDs and GOQDs is highly desirable but challenging. Here, we present GQDs and GOQDs selectively prepared by an easy and simple pulsed laser ablation in liquid (PLAL) method by controlling the laser wavelength. The obtained GQDs and GOQDs showed a significantly different optoelectronic nature mainly due to the existence of surface oxygen-rich functional groups (e.g. carboxyl or hydroxy groups). Also, we described a possible mechanism for the formation of oxygen functional groups during the PLAL process based on the Coulomb explosion model, which can give further insight for designing functional carbon materials.
Graphene quantum dots (GQDs) and graphene oxide quantum dots (GOQDs) are zero-dimensional graphene nanomaterials composed of one or few-layer graphene sheets.1,2 Compared to large size graphene, GQDs or GOQDs exhibit unique optoelectrical properties due to quantum confinement or edge effects related to oxygen-rich functional groups.3–6 Especially, GQDs exhibit a high quantum yield with excellent photostability owing to the intrinsic band structure and physicochemical robustness, which is beneficial for optoelectronic applications.7–10 In addition, although the optical properties of GOQDs are less fascinating compared to those of GQDs, GOQDs have a high potential for biomedical applications, such as drug delivery systems (DDSs) and bioimaging, thanks to excellent biocompatibility with acceptable optical efficiency.11–14
To date, GQDs and GOQDs have been prepared mainly via wet chemical cutting routes. However, chemical reactions in strong acid as well as long-term washing procedure are generally required during a wet chemical process, which limits the practical applications of GQDs and GOQDs.15–19 As an alternative method, pulsed laser ablation in liquid (PLAL) has recently attracted great attention for the synthesis of GQDs and GOQDs. The PLAL method is facile, simple, and environmentally benign because it does not require strong acidic chemical and post-purification steps.20–23
Coulomb explosion model has been proposed to account for the ablation mechanism of carbon materials by PLAL.21,22 Briefly, when the laser pulses inject into the carbon precursor, ionization occurs by multiphoton absorption, resulting in the formation of high temperature and pressure plasma plume. In the plasma plume, Coulomb explosion can take place and subsequently the carbon precursors are ablated to the quantum size leading to the formation of GQDs. Simultaneously, the Coulomb explosion can decompose the solvent during the formation of GQDs or GOQDs.23,24 The decomposed small molecules in the solvent can functionalize the GQDs by inducing chemical bonds with the surface of the GQDs. This can significantly alter the final optoelectronic properties of GQDs. Importantly, laser wavelength has a strong relationship with the decomposition of the solvent given that reaction area and depth between light and liquid matter is mainly determined by the wavelength of laser. Hence, not only natures of solvent but also the laser wavelength can significantly influence the optoelectronic functionalities of GQDs prepared by PLAL method. In our previous report, the effects of chemical properties of solvent on the functionalization of GQDs prepared by PLAL has been studied.25 However, to our best knowledge, relationships between laser wavelength and the functionalization of GQDs during PLAL still remain as unexplored scientific area.
GQDs and GOQDs were fabricated by PLAL methods using MWCNTs as carbon source in high-purity ethanol. Briefly, 50 mg of MWCNTs was dispersed in 500 mL of ethanol by ultrasonication. A Q-switch ND:YAG laser system was employed for PLAL. The MWCNTs suspension, which forms a vertical water column, was ablated by using the horizontal pulsed laser with the wavelength of 355 nm and 532 nm, respectively, at a repetition rate of 10 Hz and ablation energy of 50 mJ. The pulsed laser beam was focused on the center of MWCNTs suspension (see the ESI† for Experimental details) (Fig. 1).
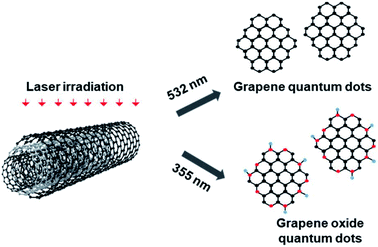 | ||
| Fig. 1 Representative schematic for the possible mechanism of the transform MWCNTs to GQDs and GOQDs using PLAL process (oxygen-rich site are shown as red dots). | ||
The morphology of starting MWCNTs was characterized by transmission electron microscopy (TEM) (Fig. S1†). The TEM images of MWCNTs shows that the wall of CNT has consisted of approximately 25–27 layers of graphene sheets (Fig. S1†). An average diameter of the tube was about 25 nm. Ultrathin morphology of MWCNTs can effectively suppress the pyrolytic phenomenon during PLAL, which is desirable for the formation of homogeneous GQDs or GOQDs colloidal.25 The MWCNTs was transformed to GQDs by PLAL using 532 nm laser source. GQDs with size less than 5 nm was obtained after 10 min of ablation (Fig. 2a). The size distribution of GQDs is fitted by Gaussian curves and shown in Fig. S2a.† An average diameter of 1–5 nm was calculated by counting more than 30 numbers of GQDs. HR-TEM image and corresponding fast Fourier transform (FFT) pattern of GQDs show a highly crystalline structure with a lattice parameter of ∼0.24 nm (Fig. 2b). No crystalline features that correspond to graphite, such as [002] plane, were observed by HR-TEM.26,27 The atomic force microscopy (AFM) data in Fig. 2c and d illustrate the topographic morphology and the height distribution of GQDs. The height line profile in Fig. 2d shows that the thickness of GQDs is less than 1.5 nm corresponding to 3–4 graphene layers. Statistical analysis reveals that more than 85% of the GQDs has a thickness between 0.5 and 1.5 nm, indicating that the produced GQDs has a mono or a few layered structures.
To investigate the effect of laser wavelength on the functionalities of GQDs, we employed the 355 nm laser source (3.4 eV) for PLAL process, which has much higher photon energy than 532 nm laser source (2.33 eV). MWCNTs was ablated in ethanol using the laser source with a wavelength of 355 nm. All the experimental parameters except for laser wavelength were kept identical during PLAL. X-ray photoelectron spectroscopy (XPS) was firstly carried out for the compositional analysis of GQDs ablated by different laser wavelengths, 532 and 355 nm. For the comparison purpose, starting MWCNTs was also studied by XPS. XPS spectra of C 1s for MWCNTs only presents sp2 carbon peak at the binding energy of 284.4 eV (Fig. S3†). Similarly, the GQDs ablated by 532 nm shows a major peak at 284.4 eV, which can be attributed to sp2 carbon peak. The deconvoluted peaks at 286.0 eV and 288.8 eV are associated with hydroxyl or carboxyl functional groups bonded in sp3 orbital structure, respectively (Fig. 3a). According to quantitative analysis of the XPS spectra, fractions of sp2 and sp3 carbon peaks are estimated as 86.55% and 13.45%, respectively. In contrast, the GOQDs ablated by 355 nm show a significantly increased fraction of sp3 carbon peaks from oxygen-rich functional groups (Fig. 3b). Quantitative fractions are calculated as 55.91% and 44.09% for sp2 and sp3 carbon peaks, respectively (Table S1†), indicating that GOQDs ablated by shorter wavelength (355 nm) is literally GOQDs rather than GQDs. These results suggest that the surface functionalization of GQDs by oxygeneous species can be easily achieved by simply changing laser wavelength for PLAL process.
TEM images revealed that the GOQDs have a diameter less than 5 nm (Fig. 2e). The HR-TEM image and the corresponding FFT pattern showed that the crystal structure of the GOQDs has a similar lattice structure with that of GQDs (Fig. 2b). The size distribution of GOQDs was calculated by counting about 45 GOQDs, and the result was fitted by Gaussian curves (Fig. S2b†). An average diameter of 1–5 nm was obtained for the GOQDs. In addition, the height profile for GOQDs was obtained by using AFM, indicating that more than 90% of the GOQDs have topographic height of ∼1.5 nm. Consequently, most of the exfoliated GOQDs mainly exist as a single or few-layered graphene sheets. The size and height of GOQDs were comparable with those of the GQDs produced by low-energy laser source (i.e., longer wavelength). Also, the yield of PLAL process was calculated by dividing the weight of dried GQDs or GOQDs product by the weight of the starting MWCNTs. Based on this method, the yield of GQDs and GOQDs were determined as about 10–12%.
The wavelength of laser can induce multiple effects on PLAL process. Noted that multiphoton absorption is a predominant ablation mechanism when high-frequency pulses such as nanosecond laser is employed for PLAL. The electron density (n0) induced by laser pulse depends on the photon energy E and the number of photons (N).21,28 Laser pulse with high photon energy can lead to more significant multiphoton-ionization and Coulomb explosion, expediting decomposition of molecules.28–30 Hence, the solvent (i.e., ethanol in this study) can be photo-thermally decomposed during PLAL which forms cavitation bubbles, consisted of carbon- or oxygen-based small molecules, on the surface of MWCNTs. The surface functionalization of GQDs by oxygen functional groups during PLAL in ethanol can be described as eqn (1).31
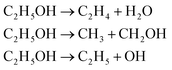 | (1) |
The shorter laser wavelength with higher photon energies can facilitate decomposition of ethanol, correspondingly an easy formation of OH functional groups or defects on the surface of the ablated GQDs or MWCNTs. The OH-enriched solvent can efficiently functionalize the defect-enriched surface of GQDs, and thus the transformation from GOQs to GOQDs likely occurs.
The optical properties of GQDs and GOQDs were investigated using PL (photoluminescence) and PLE (photoluminescence excitation) measurements. Fig. 4a show the PL spectra of the colloidal solutions of GQDs and the GOQDs. The PL intensity of GQDs was about 1.5 times higher than that of GOQDs. The digital images of the GQDs show distinct blue emission (the inset in Fig. 4a, left digital image), while the GOQDs exhibits a mixed emission of blue and green (the inset in Fig. 4a, right digital image). Also, the GQDs shows the excitation independent PL properties at excitation wavelength with 300 to 400 nm, in contrast the GOQDs have excitation dependent PL properties (Fig. S4†). The PLE spectra was investigated at various detection emission wavelengths of PL spectrum (Fig. 4b). Both GQDs and GOQDs exhibit the PLE peaks at about 260 and 360 nm. However, the GOQDs have much broader PLE peak than the GQDs with emission dependent PLE properties. The characteristics of PL and PLE spectra reflect that emissions from the GQDs and GOQDs may have a different mechanism.
 | ||
| Fig. 4 (a) PL spectra of GQDs and GOQDs in ethanol. (b) PLE spectra of GQDs and GOQDs with various emission source. | ||
To investigate PL mechanisms of GQDs and GOQDs, we carried out UV-vis and time-resolved photoluminescence (TRPL) analysis (Fig. 5). The PLE spectra of GOQDs shows excitation dependent PL properties (Fig. S4b†), and also the UV-vis absorbance of GOQDs shows a broad absorption spectrum with a gradual change up to 700 nm (Fig. 5a). These properties of GOQDs are similar to previously reported PLE and UV-vis absorbance results of GOQDs with oxygen-rich functional groups.32,33 Compared to GOQDs, the absorption spectrum of GQDs presents broad absorption peaks with high intensity at about 245 and 310 nm, respectively (Fig. 5b). These absorption peaks correspond to PLE peaks at about 260 and 360 nm, respectively (Fig. 4b). The mixed blue and green emission from GOQDs is originated from intrinsic states and/or extrinsic defect states in the bandgap. Notably, GQDs exhibits distinct blue emission with the strong absorption peak, indicating the intrinsic states in the band structure dominantly affect PL emissions of GQDs.2,7 To clarify possible recombination mechanisms of GQDs and GOQDs, we carried out TRPL analysis (Fig. 5c and d). Table 1 depicts the values obtained by time-correlated single photon counting (TCSPC) characterization. The fluorescence decay curve is fitted with triexponential function (eqn (2)), where fluorescence decay occurs through three different relaxation pathways.
| fit = A + B1e(−t/τ1) + B2e(−t/τ2) + B3e(−t/τ3) | (2) |
| Ex/Emi (nm) | Chi sq. (χ2) | τ1 (ns)/β1 (%) | τ2 (ns)/β2 (%) | τ3 (ns)/β3 (%) | |
|---|---|---|---|---|---|
| GQDs | 370/450 | 1.05 | 1.3/78 | 3.1/17 | 11/5 |
| GOQDs | 370/450 | 1.1 | 0.7/53 | 3/35 | 9/11 |
Conclusions
In conclusion, we successfully demonstrated that the GQDs and GOQDs with controllable oxygen functional groups can be easily prepared by simply changing the laser wavelength for PLAL. The oxygen-rich functional groups can be more easily derived from the solvent (i.e., ethanol) when the shorter wavelength of the laser pulses is employed to trigger photothermal decomposition of the ethanol. According to compositional and structural analysis, selective productions of GQDs and GOQDs can be achieved by modulating the laser wavelength for PLAL process. Chemical bonding of GQDs is mainly composed of pure sp2 carbons with weak oxygen groups, while the various oxygen-rich functional groups were presented on the surface of GOQDs. Furthermore, GQDs and GOQDs showed clearly different optical properties. GOQDs exhibited the blue to green emission, while the emission of GQDs is distinct blue emission. These result demonstrate that by changing the laser wavelength, the amount of oxygen-rich functional groups on the surface of GQDs or GOQDs can be controlled during PLAL process, resulting in modulated optoelectronic properties. Thus, our strategy is remarkably simple and facile way for selectively producing GQDs and GOQDs by PLAL process, which has a hudge potential to be applied in real optoelectonic applications such as optical devices or bioimaging.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Korea Institute of Industrial Technology as “Development of Additively Manufacturing Process Technology of Ni-based Superalloys for Ultra-high efficiency Power Plants” (KITECH EO-19-0016). This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning 2018R1D1A1A02085938.Notes and references
- L. A. Ponomarenko, F. Schedin, M. I. Katsnelson, R. Yang, E. W. Hill, K. S. Novoselove and A. K. Geim, Science, 2008, 320, 356–358 CrossRef CAS PubMed.
- M. Bacon, S. J. Bradley and T. Nann, Part. Part. Syst. Charact., 2014, 31, 415–428 CrossRef CAS.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J. J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- A. Abbas, L. T. Mariana and A. N. Phan, Carbon, 2018, 140, 77–99 CrossRef CAS.
- S. Zhu, Y. Song, J. Wang, H. Wan, Y. Zhang, Y. Ning and B. Yang, Nano Today, 2017, 13, 10–14 CrossRef CAS.
- K. A. Fernando, S. Sahu, Y. Liu, W. K. Lewis, E. A. Guliants, A. Jafariyan, P. Wang, C. E. Bunker and Y. P. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 8363–8376 CrossRef CAS PubMed.
- S. H. Song, M.-H. Jang, J. Chung, S. H. Jin, B. H. Kim, S.-H. Hur, S. Yoo, Y.-H. Cho and S. Jeon, Adv. Opt. Mater., 2014, 2, 1016–1023 CrossRef CAS.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48, 3686–3699 RSC.
- M. Zheng, S. Ruan, S. Liu, T. Sun, D. Qu, H. Zhao, Z. Xie, H. Gao, X. Jing and Z. Sun, ACS Nano, 2015, 9, 11455–11461 CrossRef CAS PubMed.
- Y. Shi, A. Pramanik, C. Tchounwou, F. Pedraza, R. A. Crouch, S. R. Chavva, A. Vangara, S. S. Sinha, S. Jones, D. Sardar, C. Hawker and P. C. Ray, ACS Appl. Mater. Interfaces, 2015, 7, 10935–10943 CrossRef CAS PubMed.
- J. Wen, Y. Xu, H. Li, A. Lu and S. Sun, Chem. Commun., 2015, 51, 11346–11358 RSC.
- X. Tan, Y. Li, X. Li, S. Zhou, L. Fan and S. Yang, Chem. Commun., 2015, 51, 2544–2546 RSC.
- M. Park, H. D. Ha, Y. T. Kim, J. H. Jung, S. H. Kim, D. H. Kim and T. S. Seo, Anal. Chem., 2015, 87, 10969–10975 CrossRef CAS PubMed.
- R. Liu, D. Wu, X. Feng and K. Mullen, J. Am. Chem. Soc., 2011, 133, 15221–15223 CrossRef CAS PubMed.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- Z. Li, W. Zhang, Y. Luo, J. Yang and J. G. Hou, J. Am. Chem. Soc., 2009, 131, 6320–6321 CrossRef CAS PubMed.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS.
- R. Hoffmann, J. Am. Chem. Soc., 1968, 90, 1475 CrossRef CAS.
- J. Yang, T. Ling, W. T. Wu, H. Liu, M. R. Gao, C. Ling, L. Li and X. W. Du, Nat. Commun., 2013, 4, 1695 CrossRef.
- D. Zhang, B. Gokce and S. Barcikowski, Chem. Rev., 2017, 117, 3990–4103 CrossRef CAS PubMed.
- Z. Yan and D. B. Chrisey, J. Photochem. Photobiol., C, 2012, 13, 204–223 CrossRef CAS.
- V. Amendola and M. Meneghetti, Phys. Chem. Chem. Phys., 2013, 15, 3027–3046 RSC.
- S.-L. Hu, K.-Y. Niu, J. Sun, J. Yang, N.-Q. Zhao and X.-W. Du, J. Mater. Chem., 2009, 19, 484–488 RSC.
- S. H. Kang, S. Mhin, H. Han, K. M. Kim, J. L. Jones, J. H. Ryu, J. S. Kang, S. H. Kim and K. B. Shim, Sci. Rep., 2016, 6, 38423 CrossRef CAS.
- L. Wang, Y. Wang, T. Xu, H. Liao, C. Yao, Y. Liu, Z. Li, Z. Chen, D. Pan, L. Sun and M. Wu, Nat. Commun., 2014, 5, 5357 CrossRef CAS PubMed.
- L. Lin and S. Zhang, Chem. Commun., 2012, 48, 10177–10179 RSC.
- E. Arola, IEEE J. Quantum Electron., 2014, 50, 1–12 Search PubMed.
- R. García-Calzada, M. Rodio, K. Bagga, R. Intartaglia, P. Bianchini, V. S. Chirvony and J. P. Martínez-Pastor, RSC Adv., 2015, 5, 50604–50610 RSC.
- E. Boulais, R. Lachaine and M. Meunier, Nano Lett., 2012, 12, 4763–4769 CrossRef CAS PubMed.
- R. Sivaramakrishnan, M. C. Su, J. V. Michael, S. J. Klippenstein, L. B. Harding and B. Ruscic, J. Phys. Chem. A, 2010, 114, 9425–9439 CrossRef CAS PubMed.
- Z. Gan, H. Xu and Y. Hao, Nanoscale, 2016, 8, 7794–7807 RSC.
- Z. Gan, S. Xiong, X. Wu, T. Xu, X. Zhu, X. Gan, J. Guo, J. Shen, L. Sun and P. K. Chu, Adv. Opt. Mater., 2013, 1, 926–932 CrossRef.
- S. Ahirwar, S. Mallick and D. Bahadur, ACS Omega, 2017, 2, 8343–8353 CrossRef CAS.
- F. Liu, M. H. Jang, H. D. Ha, J. H. Kim, Y. H. Cho and T. S. Seo, Adv. Mater., 2013, 25, 3657–3662 CrossRef CAS PubMed.
- S. Zhu, J. Shao, Y. Song, X. Zhao, J. Du, L. Wang, H. Wang, K. Zhang, J. Zhang and B. Yang, Nanoscale, 2015, 7, 7927–7933 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra02087j |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |



