Open Access Article
Open Access ArticleNano-kaolin/Ti4+/Fe3O4: a magnetic reusable nano-catalyst for the synthesis of pyrimido[2,1-b]benzothiazoles†
Bi Bi Fatemeh Mirjalili * and
Roya Soltani
* and
Roya Soltani
Department of Chemistry, College of Science, Yazd University, Yazd, P.O.Box 89195-741, Iran. E-mail: fmirjalili@yazd.ac.ir; Fax: +98 3538210644; Tel: +98 3531232672
First published on 14th June 2019
Abstract
Herein, nano-kaolin/Ti4+/Fe3O4 as a new magnetic nano-catalyst was synthesized, and its structural properties were characterized using various techniques such as Fourier transform infrared (FTIR) spectroscopy, field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), a vibrating sample magnetometer (VSM), thermogravimetric analysis (TGA) and energy-dispersive X-ray spectroscopy (EDX). This catalyst was used for the synthesis of pyrimido[2,1-b]benzothiazoles via the one-pot condensation of 2-aminobenzothiazole, an aldehyde and β-keto ester under solvent-free conditions at 100 °C. This simple protocol has many advantages such as easy workup, high product yields, short reaction times and reusability of the catalyst.
Introduction
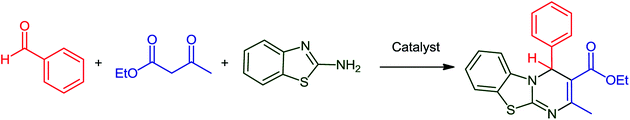
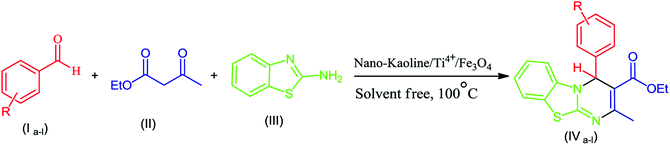
Pyrimido[2,1-b]benzothiazoles are an important category of fused heterocycles, with the benzothiazole ring having diverse pharmaceutical and industrial properties.1 These compounds have shown various biological activities such as anti-bacterial,2 anti-tumor,3,4 anti-inflammatory,5,6 antiviral,7 anticonvulsant,8 anti-cancer9 and antifungal activities.10 These compounds have been synthesized via the condensation reaction of 2-amino benzothiazole, β-keto ester and aldehydes. Previously, various catalysts, such as thiamine hydrochloride (VB1),11 Fe3O4@nano-cellulose-TiCl,12 nano-TiCl2/cellulose,13 nanocellulose/BF3/Fe3O4,14 nano-Fe3O4@SiO2–TiCl3,15 kaolin,16 and tetrabutylammonium hydrogen sulfate (TBAHS),17 have been applied for the preparation of these compounds. However, the drawback of these protocols is the high cost of the catalyst.Kaolin or hydrated aluminum silicate (Al2O3·2SiO2·2H2O)18 has been used in numerous fields such as in the medicine, paint, ceramic, rubber, paper, petroleum and glass industries.19 Moreover, one of the most valuable application of kaolin is as promoter in chemical industry;20 in recent years, magnetic nanoparticles (Fe3O4) have been used due to their advantages such as high stability, low toxicity and easy separation from reaction media.21–23 On the other hand, single atoms,24–27 such as transition metal atoms and their ions,28,29 have been widely studied and used for the promotion of organic reactions. In this study, we report the synthesis and characterization of nano-kaolin/Ti4+/Fe3O4 for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles via the condensation reaction of ethyl acetoacetate, aromatic aldehydes, and 2-amino benzothiazole.
Results and discussion
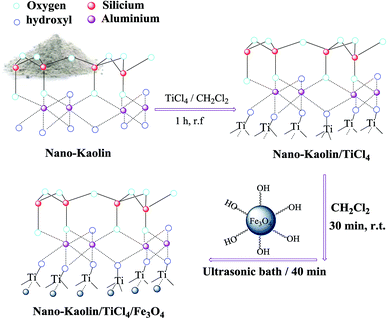
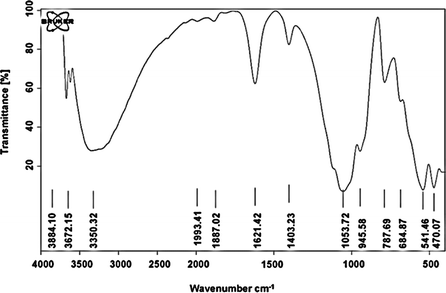
Herein, nano-kaolin/Ti4+/Fe3O4 was prepared as a new catalyst in two-steps. At first, TiCl4 was added to the mixture of nano-kaolin and CH2Cl2. The resulting white powder (nano-kaolin/Ti4+) was added to the suspension of nano Fe3O4 in CH2Cl2 under the ultrasonic condition for the formation of nano-kaolin/Ti4+/Fe3O4 as a brown magnetic powder (Scheme 1). The morphology and structure of kaolin/Ti4+/Fe3O4 were studied by various techniques such as FTIR, FE-SEM, TEM, XRD, VSM, TGA and EDX. The FTIR spectra of kaolin, Fe3O4, nano-kaolin/Ti4+ and nano-kaolin/Ti4+/Fe3O4 are shown in Fig. 1. The absorption bands at 3342 and 3620 cm−1 correspond to the stretching vibrations of the O–H bond. The band at 538 cm−1 is attributed to Fe/O in the Fe3O4 nanoparticles. The Al–O and Si–O stretching vibration bands are visible at 420–550 and 1050–1100 cm−1, respectively, in the FTIR spectrum of nano-kaolin/Ti4+/Fe3O4.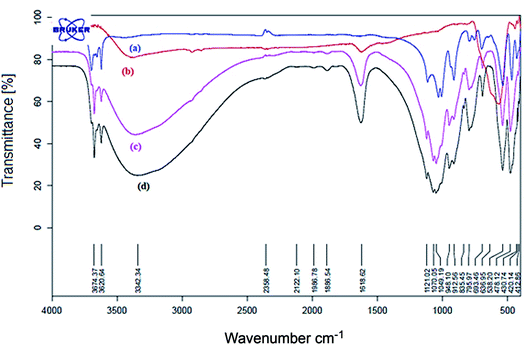 | ||
| Fig. 1 FTIR spectra of (a) nano-kaolin, (b) Fe3O4, (c) nano-kaolin/Ti4+, and (d) nano-kaolin/Ti4+/Fe3O4. | ||
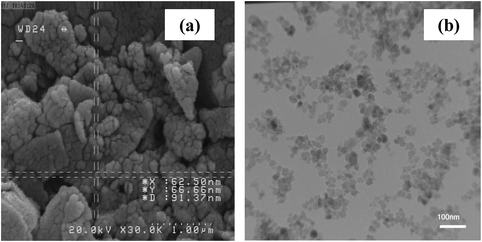
The particle size of nano-kaolin/Ti4+/Fe3O4 was studied using field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) and found to be less than 100 nm (Fig. 2).
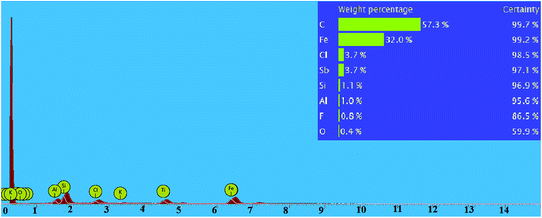
Energy-dispersive X-ray spectroscopy (EDS) was used to determine the percentage of elements in nano-kaolin/Ti4+/Fe3O4 (Fig. 3). The percentage of Fe, Si, Al, Ti, Cl, O and K in nano-kaolin/Ti4+/Fe3O4 was 37.4, 25.5, 12.6, 11.0, 8.2, 4.2 and 1.2, respectively.
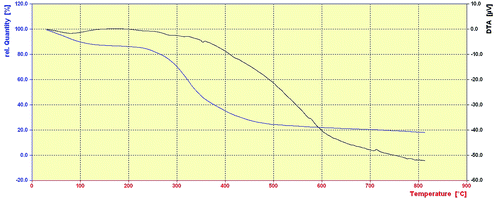
The thermal stability (TG-DTA) of nano-kaolin/Ti4+/Fe3O4 was studied by thermogravimetric analysis (TGA) in the temperature range of 50–800 °C (Fig. 4). According to Fig. 4, at 100–250 °C, the catalyst weight was reduced by 4%; this could be related to the removal of moisture and bonded water. Moreover, the catalyst lost 80% of its weight in the temperature range of 250–600 °C probably due to the collapse of the kaolin network. According to the TGA curve, this catalyst is stable up to 230 °C and suitable for reactions that are carried out at temperatures below 230 °C.
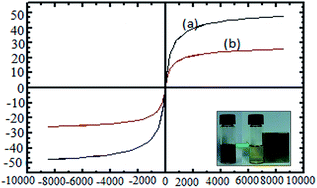
The vibrating sample magnetometer (VSM) pattern of the catalyst at room temperature shows that the coercivity value is zero, and there is no hysteresis loop and remanence; this confirms the catalytic superparamagnetic property (Fig. 5). The saturation magnetization (Ms) values of Fe3O4 and nano-kaolin/Ti4+/Fe3O4 were 50 and 23 emu g−1, respectively. Although the magnetization of the catalyst is lower than that of Fe3O4, the proposed catalyst can be easily separated from the solution using an external magnet.
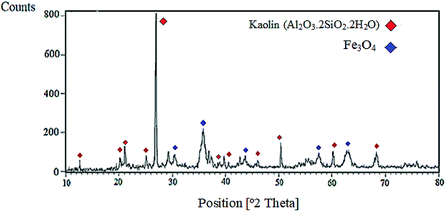
The X-ray diffraction (XRD) pattern of nano-kaolin/Ti4+/Fe3O4 is shown in Fig. 6. According to the XRD pattern, the signals at 2θ = 13°, 20°, 25°, 41°, 46°, 61° and 68° indicate the presence of kaolin. The four signals at 2θ = 21°, 27°, 39° and 50° indicate the existence of SiO2. Moreover, the signals at 2θ = 31°, 36°, 44°, 58° and 63° are related to Fe3O4. Presumably, three other peaks at the 2θ value of 37°, 43° and 55° revealed that Ti was bonded to kaolin and Fe3O4.
The catalytic activity of the catalyst was investigated for the synthesis of 4H-pyrimido[2,1-b]benzothiazole using the three-component reaction of β-keto ester, aromatic aldehydes and 2-aminobenzothiazole. To select optimum conditions, the reaction of ethyl acetoacetate, benzaldehyde and 2-amino benzothiazole was studied as a model reaction under different conditions. According to Table 1, the best conditions for the synthesis of 4H-pyrimido[2,1-b]benzothiazole under solvent-free conditions correspond to the utilization of 0.03 g of catalyst at 100 °C.
| Entry | Catalyst (g) | Solvent/condition | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a The amount ratios of 2-aminobenzothiazole (mmol), benzaldehyde (mmol) and ethyl acetoacetate (mmol) equals to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.b Isolated yield.c Nano-kaolin/Ti4+/Fe3O4. 1.b Isolated yield.c Nano-kaolin/Ti4+/Fe3O4. |
||||
| 1 | Fe3O4 (0.03) | —/100 °C | 1.5 | 52 |
| 2 | Kaolin (0.03) | —/100 °C | 1.5 | 72 |
| 3 | Nano-kaolin/Ti4+ (0.03) | —/100 °C | 1.5 | 78 |
| 4 | Catalystc (0.03) | H2O/reflux | 3 | 50 |
| 5 | Catalystc (0.03) | C2H5OH/reflux | 4 | 30 |
| 6 | Catalystc (0.03) | —/80 °C | 2 | 82 |
| 7 | Catalystc (0.03) | —/90 °C | 2 | 75 |
| 8 | Catalystc (0.03) | —/110 °C | 1.5 | 95 |
| 9 | Catalystc (0.025) | —/100 °C | 1.5 | 65 |
| 10 | Catalystc (0.03) | —/100 °C | 1.5 | 95 |
| 11 | Catalystc (0.04) | —/100 °C | 1.5 | 78 |
According to the conditions optimized for the model reaction, 4H-pyrimido[2,1-b]benzothiazole derivatives were synthesized by the reaction of various aromatic aldehydes, 2-aminobenzothiazole and ethylacetoacetate in the presence of the nano-kaolin/Ti4+/Fe3O4 catalyst. Based on the results tabulated in Table 2, the effects of the electron and the nature of the substituent on aldehyde significantly affected the time and yield of the reaction. The presence of electron-withdrawing groups in aryl aldehyde rings increased the activity of the aldehyde group and their yields were compared with that of electron-donating groups; the structure of the product was identified using melting point, FTIR, and 1H-NMR spectral results.
| Entry | R | Product | Time (h) | Yieldb (%) | Melting point | Ref. | |
|---|---|---|---|---|---|---|---|
| Observed | Reported | ||||||
| a One mmol of 2-aminobenzothiazole, aldehyde and ethyl acetoacetate was used.b Isolated yield. | |||||||
| 1 | H | IVa | 1.5 | 95 | 178–180 | 178–180 | 23 |
| 2 | 4-NO2 | IVb | 0.5 | 98 | 172–173 | 170–172 | 30 |
| 3 | 4-Cl | IVc | 2.2 | 95 | 141–143 | 140–142 | 15 |
| 4 | 4-Br | IVd | 1.6 | 92 | 111–113 | 110–114 | 31 |
| 5 | 4-OH | IVe | 3 | 90 | 210–214 | 110–112 | 30 |
| 6 | 2-NO2 | IVf | 1 | 50 | 120–124 | 123–125 | 14 |
| 7 | 2-Cl | IVg | 2.2 | 78 | 131–133 | 130–132 | 17 |
| 8 | 3-NO2 | IVh | 0.5 | 80 | 222–224 | 222–224 | 23 |
| 9 | 3-OH | IVi | 1.25 | 94 | 259–261 | 260–263 | 12 |
| 10 | 2,4-(Cl)2 | IVj | 1.4 | 70 | 133–135 | 133–135 | 13 |
| 11 | 2-OEt | IVk | 1.1 | 85 | 170–172 | 171–173 | 12 |
| 12 | 3,4-(OH)2 | IVl | 0.9 | 60 | 227–229 | 225–227 | 15 |
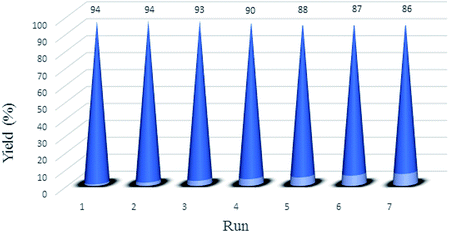
To investigate the recovery and reuse function of nano-kaolin/Ti4+/Fe3O4, the catalyst was used for seven times in the model reaction under identical conditions. After using the catalyst in the model reaction, it was isolated by an external magnet, washed with ethanol and then dried at room temperature. The results indicate that the catalyst nano-kaolin/Ti4+/Fe3O4 can be reused without any significant loss of its catalytic activity (Fig. 7). The FTIR spectrum of the reused catalyst shows that there is no change in the catalyst structure during the recovery process (Fig. 8).
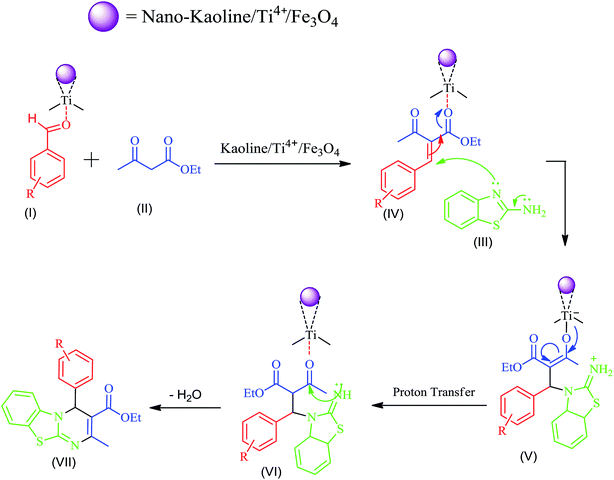
The proposed mechanism for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles is shown in Scheme 2. In this reaction, the Ti4+ cation in the catalyst acts as a Lewis acid and activates the carbonyl groups in the substrates. At first, the aldehyde (I) as an electrophile and β-keto esters (II) as active methylene compounds produce the alkene (IV) via the Knoevenagel reaction. Then, the 2-aminobenzothiazole (III) reacts with the alkene (IV) via a Michael addition reaction, and an iminium ion (V) is formed. Subsequently, using proton transfer and intramolecular cyclization, the 4H-pyrimido[2,1-b]benzothiazole derivative(VII) is formed.
To investigate the performance of the nano-kaolin/Ti4+/Fe3O4 catalyst in synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives, condensation of benzaldehydes, 2-aminobenzothiazole and ethyl acetoacetate was employed as the model reaction, and the results were compared with those obtained using other reported catalysts. The results of this study are shown in Table 3. According to the obtained results, the nano-kaolin/Ti4+/Fe3O4 is one of the best catalyst for this purpose.
| Entry | Catalyst | Solvent/condition | Time (h) | Yieldb (%) (ref.) |
|---|---|---|---|---|
| a One mmol of any substrate (2-aminobenzothiazole, benzaldehyde and ethyl acetoacetate) was used.b Isolated yield.c 1,1,3,3-N,N,N′,N′-Tetramethylguanidinium trifluoroacetate.d Tetrabutylammonium hydrogen sulfate. | ||||
| 1 | TMGTc (0.08 g) | —/100 °C | 5 | 66 (31) |
| 2 | TBAHSd (10 mol%) | Ethylene glycol/120 °C | 2 | 83 (17) |
| 3 | Acetic acid (10 mol%) | Methanol/reflux | 12 | 65 (32) |
| 4 | AlCl3 (10 mol%) | —/60 °C | 1.2 | 79 (30) |
| 5 | FeF3 (10 mol%) | —/80 °C | 2 | 85 (33) |
| 6 | Nano-cellulose/BF3/Fe3O4 (0.06 g) | —/100 °C | 1 | 80 (14) |
| 7 | Nano-kaolin/TiCl4/Fe3O4 (0.03 g) | —/100 °C | 1.5 | 95 (this work) |
Conclusion
Due to the importance of green chemistry, we have introduced the preparation and characterization of nano-kaolin/Ti4+/Fe3O4 as a novel, eco-friendly and magnetically recyclable heterogeneous catalyst. This catalyst was used to synthesize 4H-pyrimido[2,1-b]benzothiazole derivatives via the one-pot three-component reaction of 2-aminobenzothiazole, aldehydes and ethyl acetoacetate under solvent-free conditions at 100 °C. Some of the important advantages of this protocol include short reaction times, high yields, easy work-up procedure and easy separation of the catalyst with reusability.Experimental
General remarks
A Bruker (DRX-400 Avance) NMR spectrometer was used to obtain the NMR spectra. FTIR spectra were obtained using the Bruker, Equinox 55 spectrometer. Melting points were determined by the Buchi melting point B-540 B.V.CHI apparatus. Field emission scanning electron microscopy (FE-SEM) was carried out via Mira 3-XMU, and transmission electron microscopy (TEM) was conducted using Philips CM120 with the LaB6 cathode and the accelerating voltage of 120 kV. The X-ray diffraction (XRD) pattern was obtained by the Philips X'pert MPD diffractometer equipped with a Cu Kα anode (k = 1.54 Å) in the 2θ range from 10° to 80°. The VSM measurements were performed using a vibrating sample magnetometer (Meghnatis Daghigh Kavir Co. Kashan, Iran). The thermogravimetric analysis (TGA) was conducted by NETZSCH TG 209 F1 Iris. Energy-dispersive X-ray spectroscopy (EDS) of the catalyst was conducted by the EDS instrument Phenom pro X.Preparation of the Fe3O4 NPs
A mixture of FeCl3·6H2O (2.7 g, 10 mmol) and FeCl2·4H2O (1 g, 5 mmol) in deionized water (25 ml) was heated until 80 °C. Then, 17 ml of NH3 (30%) was added slowly. The mixture was stirred using a mechanical stirrer for 30 minutes. Then, black magnetic nanoparticles were deposited using an external magnet and washed three times with deionized water. Finally, the Fe3O4 NPs were dried at 80 °C for 4 hours.Preparation of nano-kaolin/Ti4+
In a beaker, 1 ml of TiCl4 was added dropwise to a mixture of nano-kaolin (2 g) in 10 ml of dichloromethane and stirred using a mechanical stirrer for 1 h at room temperature. The obtained suspension was filtered, washed with dichloromethane and dried at room temperature.Preparation of nano-kaolin/Ti4+/Fe3O4
At first, a mixture of nano-kaolin/Ti4+ (2 g) and dichloromethane (10 mL) was placed in an ultrasonic bath for 30 minutes. Then, nano-Fe3O4 (1 g) was added to the mixture and placed in the ultrasonic bath for 40 minutes to disperse the particles. The resulting suspension was obtained by an external magnet, washed with dichloromethane, and dried at room temperature.General procedure for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives
In a sand bath, a mixture of aldehyde (1 mmol), ethyl acetoacetate (1 mmol), 2-aminobenzothiazole (1 mmol) and nano-kaolin/Ti4+/Fe3O4 (0.03 g) was heated to 100 °C. The right time for the completion of the reactions is shown in Table 2. After completion of the reaction, the reaction mixture was dissolved in ethanol, and the catalyst was separated by an external magnet. Finally, water was added to the residue, and the product appeared as a pure solid. For the recovery of the catalyst, the magnetically resolved catalyst was washed with ethanol at least three times and then dried at room temperature.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Research Council of Yazd University is gratefully acknowledged for the financial support for this work.References
- Z. Y. Yu, Q. S. Fang, J. Zhou and Z. B. Song, Res. Chem. Intermed., 2016, 42, 2035 CrossRef CAS.
- M. S. Chaitanya, G. Nagendrappa and V. P. Vaidya, J. Chem. Pharm. Res., 2010, 2, 206 CAS.
- A. Y. Hassan, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2856 CrossRef CAS.
- M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary and M. M. El-Kerdawy, Eur. J. Med. Chem., 2014, 85, 576 CrossRef CAS PubMed.
- D. V. Kashinath, Y. M. Rajmani and C. S. Ravindra, J. Pharma Res., 2013, 6, 574 Search PubMed.
- V. K. Deshmukh, P. Raviprasad, P. A. Kulkarni and S. V. Kuberkar, Int. J. ChemTech Res., 2011, 3, 136 CAS.
- M. A. El-Sherbeny, Arzneim.-Forsch./Drug Res., 2000, 50, 848 CAS.
- M. M. M. Gineinah, Sci. Pharm., 2001, 69, 53 CrossRef CAS.
- M. Yadav, V. K. Deshmukh and S. R. Chaudhari, Int. J. Pharm. Sci. Rev. Res., 2013, 22, 41 CAS.
- S. Maddila, S. Gorle, N. Seshadri, P. Lavanya and S. B. Jonnalagadda, Arabian J. Chem., 2016, 9, 681 CrossRef CAS.
- S. R. Vaidya and J. J. Chamergore, Chem. Biol. Interface, 2016, 6, 47 CAS.
- S. Azad and B. F. Mirjalili, RSC Adv., 2016, 6, 96928 RSC.
- S. Azad and B. F. Mirjalili, Res. Chem. Intermed., 2017, 43, 1723 CrossRef CAS.
- B. F. Mirjalili and F. Aref, Res. Chem. Intermed., 2018, 44, 4519 CrossRef CAS.
- S. A. Fazeli-Attar and B. F. Mirjalili, Res. Chem. Intermed., 2018, 44, 6419 CrossRef CAS.
- P. K. Sahu, P. K. Sahu and D. D. Agarwal, RSC Adv., 2013, 3, 9854 RSC.
- L. Nagarapu, H. K. Gaikwad, J. D. Palem, R. Venkatesh, R. Bantu and B. Sridhar, Synth. Commun., 2013, 43, 93 CrossRef CAS.
- Q. Zhang, W. Tongamp and F. Saito, Powder Technol., 2011, 212, 354 CrossRef.
- H. H. Murray, Appl. Clay Sci., 2000, 17, 207 CrossRef CAS.
- A. M. Doyle, T. M. Albayati, A. S. Abbas and Z. T. Alismaeel, Renewable Energy, 2016, 97, 19 CrossRef CAS.
- A. H. Lu, E. E. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed.
- X. Batlle and A. Labarta, J. Phys. D: Appl. Phys., 2002, 35, R15 CrossRef CAS.
- R. Narayanan, Ch. Tabor and M. A. El-Sayed, Top. Catal., 2008, 48, 60 CrossRef CAS.
- L. Xu, L.-M. Yang and E. Ganz, Theor. Chem. Acc., 2018, 137, 98 Search PubMed.
- J.-H. Liu, L.-M. Yang and E. Ganz, J. Mater. Chem. A, 2019, 7, 11944 RSC.
- J.-H. Liu, L.-M. Yang and E. Ganz, J. Mater. Chem. A, 2019, 7, 3805 RSC.
- J.-H. Liu, L.-M. Yang and E. Ganz, ACS Sustainable Chem. Eng., 2018, 6, 15494 CrossRef CAS.
- S. Azad and B. F. Mirjalili, Mol. Diversity, 2019, 23, 413 CrossRef CAS PubMed.
- B. F. Mirjalili, A. Bamoniri and L. Asadollah Salmanpoor, J. Nanostruct., 2018, 8, 276 CAS.
- P. K. Sahu, P. K. Sahu, J. Lal, D. Thavaselvam and D. D. Agarwal, Med. Chem. Res., 2012, 21, 3826 CrossRef CAS.
- A. Shaabani and A. Maleki, Appl. Catal., A, 2007, 331, 149 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, Y. Sharma and D. D. Agarwal, J. Heterocycl. Chem., 2014, 51, 1193 CrossRef CAS.
- A. B. Atar, Y. S. Jeong and Y. T. Jeong, Tetrahedron, 2014, 70, 5207 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01767d |
| This journal is © The Royal Society of Chemistry 2019 |