Open Access Article
Open Access ArticleCO2-philic moderate selective layer mixed matrix membranes containing surface functionalized NaX towards highly-efficient CO2 capture†
Mohammad Salehi Maleh and
Ahmadreza Raisi *
*
Department of Chemical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Hafez Ave., P. O. Box 15875-4413, Tehran, Iran. E-mail: raisia@aut.ac.ir; Fax: +98 21 66405847; Tel: +98 21 64543125
First published on 17th May 2019
Abstract
A functional moderate selective layer mixed matrix membrane (F-MSL-MMM) is a promising candidate to obtain superior separation of industrial gases, compared to commonly mixed matrix membranes. In this work, highly permeable and selective F-MSL-MMMs consisting of a moderate Pebax layer filled with functionalized NaX nano-zeolite over a microstructure PES support layer with the desired operating stability were prepared to investigate the effect of –COOH surface functionalization on the NaX nano-zeolites and to achieve the efficient separation of CO2/N2, CO2/CH4 and CH4/N2 gas mixtures. It was found that modification of the nano-zeolite had significant effects on the morphology and gas separation performance of the F-MSL-MMMs. The incorporation of NaX–COOH nano-zeolites into the polymeric matrix showed superior dispersion, capabilities and interactions over unmodified zeolite without any defects. In addition, based on gas permeation results at various filler loadings and feed pressures, different trends were observed for the permeability and selectivity of the F-MSL-MMMs. Combination of NaX up to 1.5% into the moderate selective layer enhanced the CO2 permeability (187.76 barrer) and CO2/N2 (288.86), CO2/CH4 (57.41) and N2/CH4 (5.03) selectivities at 6 bar.
Introduction
During the past few decades, the use of polymeric membranes has expanded in gas separation applications, such as CO2 separation from natural gas and flue gas.1 Later, a lot of research was performed to improve the properties of polymeric membranes, and as a result, in order to use the advantages of polymeric and inorganic materials, mixed matrix membranes (MMMs) containing a dispersed inorganic phase in a continuous polymer phase were introduced.2,3 Zeolites are a promising inorganic phase for use in MMMs for gas separation due to their high porosity, superior chemical and thermal stability, uniform pores with molecular sieving property and adsorption selectivity. Likewise, zeolites are particularly effective in CO2 capture with an absorption-based separation property.4In addition, conventional MMMs have a number of defects that challenge the performance of these membranes. Incompatibility, weak adhesion or repulsion between the polymer and inorganic filler, the hardening of a layer of polymer around the filler, partial pore blockage of porous fillers and particle accumulation result in the creation of various interfacial defects and a non-ideal morphology in the MMMs. In order to fabricate defect-free MMMs, various methods have been widely used by researchers, among which the use of copolymers such as polyether-block-amide is one effective solution.5–7 Polyether-block-amide is a rubbery copolymer with the commercial name of Pebax, which includes a rigid polyamide (PA6 or PA12) segment and a soft polyethylene oxide (PEO) segment, the hard-soft structure improving adhesion between the polymer phase and the particles. In addition, the hard polyamide parts provide the mechanical strength of the membranes and the soft polyethylene oxide parts due to their CO2-philic structure, cause an increase in permeability and selectivity for CO2 separation from natural gas and flue gas.2,8
The surface chemical modification of the filler particles is another effective solution to prevent undesirable spaces near the polymer–particle interface and to avoid the accumulation of fillers, which improves the gas separation performance of MMMs. The filler particles are usually modified by amine, hydroxylic, alkylic, sulfonic acid, carboxylic acid or imidazole groups.9 Up to now, various filler particles, such as SiO2, CNTs, MCM-41, ZIF-8, MOFs or GO,2,3,8,10–18 have been modified and used as a dispersant phase in a polymeric matrix to improve the membrane separation properties.
Among the various functional groups, the effect of modification of nano-zeolites with carboxylic functional groups in a moderate selective layer has not been studied, while this functional group can have very favorable effects on the performance of functional moderate selective layer mixed matrix membranes (F-MSL-MMMs). The existence of carboxylic functional groups in F-MSL-MMMs materials has also been confirmed to enhance their gas separation performance due to a reversible reaction with CO2.19 The surface chemical modification of the fillers enables them to react reversibly with CO2, and, therefore, they are expected to act as carriers for CO2. The surface modification of fillers with carboxylic groups develops not only a filler–polymer interface compatibility but also improves the selectivity and permeability of CO2. The F-MSL-MMMs including modified fillers are expected to overcome Robeson's upper limit and have greater stability and mechanical strength. Consequently, the main aim of this study is to investigate the effect of chemical modification on improving the morphology of the Pebax–NaX nano-zeolite interface, avoiding the accumulation of nanoparticles, as well as improving permeability and selectivity. In addition, a further goal is a comparison of the performance of MSL-MMMs containing NaX and NaX–COOH nanoparticles.
In this work, MSL-MMMs containing pure and modified NaX nanoparticles were fabricated separately in four loadings in a Pebax-1657 polymeric matrix on polyethersulfone (PES) sublayer membranes. The MSL-MMMs were characterized by SEM, FTIR, DSC and contact angle (CA) analyses. The NaX nano-zeolites were synthesized by a hydrothermal method, and then functionalized with carboxylic groups by the reflux method. The crystallinity structure, crystal size, particle size, particle shape and chemical properties of the nanoparticles were characterized by XRD, FESEM, FTIR, BET and DLS analyses. The effect of filler surface modification, loading of filler and operating conditions, such as feed pressure and long-term stability, on the permeability of pure CO2, CH4, and N2 gases, as well as the CO2/N2, CO2/CH4 and CH4/N2 gas selectivities of MSL-MMMs, were studied.
Experimental
Materials
Ethanol (EtOH), methanol (MeOH), N,N-dimethyl-formamide (DMF), sodium hydroxide (NaOH) and chloroacetic acid (ACS, reagent) were purchased from Merck (Germany). Sodium aluminate (NaAlO2) and fumed silica (SiO2, 7 nm) were purchased from Sigma-Aldrich (USA). Pebax® MH 1657 (a copolymer with a combination of PA6 and PEO oxide with a weight ratio of 40/60) and PES (MW = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Ultrason E6020P) were purchased from Arkema Inc. (France) and BASF (Germany), respectively. In all experiments, deionized water (DI) was used. All chemicals with a purity higher than 99% were used without further purification. Furthermore, CO2, CH4 and N2 gases with a purity > 99.999% were used in the permeation experiments.
000 g mol−1, Ultrason E6020P) were purchased from Arkema Inc. (France) and BASF (Germany), respectively. In all experiments, deionized water (DI) was used. All chemicals with a purity higher than 99% were used without further purification. Furthermore, CO2, CH4 and N2 gases with a purity > 99.999% were used in the permeation experiments.
Synthesis and functionalization of NaX nano-zeolite
The synthesis of NaX nano-zeolites was performed by the hydrothermal heating method using a microwave oven, according to the procedure previously presented by Ansari et al.20 In this process, first, 5.34 g of NaOH was added to a polypropylene bottle containing 50 g of DI water, and when it was completely dissolved, the solution temperature increased. After cooling the solution to the ambient temperature and adding 2.42 g of NaAlO2, the solution became milky and then became colorless again after a few moments of continuous stirring. Then, 3.43 g of fumed silica was slowly added to the solution. Finally, to obtain a homogeneous alumina silicate gel, the solution was continuously stirred at 25 °C for 24 h. Then, for the hydrothermal heating, the prepared gel was placed in a microwave oven (CE1110C, Samsung, Korea, 900 W output power, wavelength of 2.45 GHz) at 90 °C for 3 h until crystals of NaX zeolite were formed. Then, for the separation and purification of the synthesized NaX nano-zeolite, the resulting solution was initially washed several times using a centrifuge with DI water to remove the unreacted agents and for the pH to be normalized (usually below 8) and then dried in an oven at 80 °C for 24 h.The NaX nano-zeolites were functionalized by carboxylic groups based on the method provided by Ingole et al.21 First, 1.5 g of NaX was added to 225 mL of DI water and stirred at 25 °C and sonicated for 2 h and 1 h, respectively. Then, 1.925 g of chloroacetic acid was slowly added to the NaX suspension under stirring on a magnetic stirrer. After reflux at around 100 °C for 8 h, the suspension mixture was first cooled to the ambient temperature and then washed using a centrifuge for 10 min with DI water until it reached normal pH. After washing 4 times, it was placed in an oven to dry at 80 °C for 24 h. A schematic of the steps for the synthesis and functionalization of NaX is shown in Fig. S1 in the ESI.†
Fabrication of MSL-MMMs
The PES support sublayer of MSL-MMM was fabricated by the non-solvent phase inversion method.22 First, in order to prepare a clear homogeneous PES solution, 16 g of the dried PES granules were dissolved in 84 g of DMF solvent and then stirred for 24 h. Finally, the solution was placed under vacuum for 2 h to remove all the air bubbles. Then, using a casting knife with a certain gap (200 μm), a layer of PES polymeric solution was cast on a flat glass plate and immediately placed in a non-solvent bath (DI water at 25 °C) and allowed to exchange the solvent/non-solvent. After enough time, the phase inversion process was complete and the formed PES membrane was immersed in another DI water bath for 24 h, during which the remaining solvent in the membrane was absolutely removed. Then, the PES membrane was dried at ambient temperature for 24 h. Finally, a PES support membrane with a thickness of ∼60 μm was obtained.The MSL-MMMs were prepared by casting of an MSL-MMMs doped solution on the PES sublayer membrane by the solution casting-solvent evaporation method. The casting doping solution included Pebax-1657 polymer, water/ethanol solvent and nano-zeolite particles. Initially, in order to remove humidity, the nanoparticles and copolymer granules were dried in an oven at 60 °C for 5 h. The Pebax-1657 was dissolved in water/ethanol (30/70% by weight) solvent under reflux and stirring at 70 °C for 24 h to obtain a homogeneous Pebax solution. Then, a certain amount of homogenous suspension of nanoparticles in the water/ethanol (30/70% by weight) solvent was prepared and sonicated for 30 min. In order to prepare the doping solution containing nanoparticles, 1/4 of the prepared homogeneous polymer solution was added in 4 steps, each time, to the homogenous suspension, stirred for 30 min and sonicated for 15 min. The final solution was stirred vigorously for 60 min and placed in an ultrasonic bath for 30 min. The weight percentage of each component of the doping solution for the preparation of different membranes is listed in Table 1. To fabricate an MSL-MMM, the PES sublayer was first fixed on a piece of flat glass via removable tape and then using a casting-knife with a certain gap (350 μm), a layer of doping solution was cast onto the PES substrate layer. For the solvent evaporation, the prepared films were placed in an oven at 35 °C for 24 h, and to eliminate the remaining solvent, they were placed in an oven at 60 °C for 12 h. Finally, the thickness of the selective layer was ∼15 μm on the PES support layer. A schematic of the procedure for preparing the PES substrate membrane, the doping solution containing nano-zeolite particles and MSL-MMMs is illustrated in Fig. S2.†
| Sample name | Solvent (% wt) | Polymer (% wt) | Nanoparticles (% wt) | Membrane |
|---|---|---|---|---|
| PP | 90.0 | 10 | 0.0 | PES/Pebax |
| PPN0.5 | 89.5 | 10 | 0.5 | PES/Pebax–NaX |
| PPN1 | 89.0 | 10 | 1.0 | PES/Pebax–NaX |
| PPN1.5 | 88.5 | 10 | 1.5 | PES/Pebax–NaX |
| PPN2 | 88.0 | 10 | 2.0 | PES/Pebax–NaX |
| PPNC0.5 | 89.5 | 10 | 0.5 | PES/Pebax–NaX–COOH |
| PPNC1 | 89.0 | 10 | 1.0 | PES/Pebax–NaX–COOH |
| PPNC1.5 | 88.5 | 10 | 1.5 | PES/Pebax–NaX–COOH |
| PPNC2 | 88.0 | 10 | 2.0 | PES/Pebax–NaX–COOH |
Characterization analyses
The crystalline structures of the NaX nano-zeolites were investigated using an X-ray diffraction (XRD) instrument (Equinox 3000, Inel, France). Likewise, the crystallinity degree of the membranes was calculated using the XRD analysis. The XRD tests were carried out using CuKα radiation of λ = 1.5419 Å at 45 kV, 40 mA, 25 °C and angle diffraction (2θ) from 5 to 40° with 0.03° min−1 scanning speed. The average crystal size of the nano-zeolites was calculated using Scherrer's equation (eqn (1)) at major peaks with 2θ of 6 (111), 16 (331) and 27° (642).
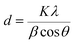 | (1) |
In this equation, d is the average crystal size, K is the shape factor, λ is the X-ray wavelength, β is the line broadening from full width at half maximum (FWHM) and θ is the Bragg angle.
The BET analysis areas of the NaX and NaX–COOH nano-fillers were obtained using nitrogen adsorption–desorption isotherms at 77 K on a Quantachrome Autosorb-1 analyzer (USA). Before testing, the nano-zeolite particles were degassed under vacuum at 120 °C and 10−4 Pa for 12 h.
Dynamic light scattering (DLS) analysis was applied to measure the particle size distribution of NaX and NaX–COOH nano-fillers using a Malvern Zetasizer Nano (Malvern Instruments, Worcestershire, England) at 632.8 nm wavelength and 173° scattering angle. The samples were dispersed in a water/ethanol mixture by sonication at 20 °C for 1 h before carrying out the DLS analysis.
The presence of functional groups on the surface of NaX and NaX–COOH zeolite nanoparticles, neat Pebax-1657 and the interaction between polymer and particles of MSL-MMMs were characterized using Fourier transform infrared (FTIR) analysis. The FTIR tests were accomplished with a Nicolet Nexus 670 spectrometer (Nicolet Instrument Co., Madison, WI, USA) in the wave number range 400 cm−1 to 4000 cm−1 under ambient conditions and with a spectral resolution of 4 cm−1. A mean of 20 scans from each membrane (each sample: 2 × 4 cm) and each nanoparticle (each sample: about 0.5–1 g) was taken.
The morphology and particle size range of the synthesized zeolite nanoparticles and also the surface and cross-sectional morphologies of the fabricated neat and MSL-MMMs were studied using FESEM (Hitachi S-4700, NJ, USA) and SEM devices (AIS2300C, Seron Technology's, South Korea), respectively. To prepare the cross-section of the membranes, the samples were broken down in liquid nitrogen. Finally, before taking images with the SEM device, all of the samples (nanoparticles, cross-section and surface of the membranes) were coated with a layer of gold using sputtering.
Investigations of the glass transition temperature (Tg) and crystallinity degree of the membranes were performed with differential scanning calorimeter (DSC) analysis. The DSC analysis was conducted with a Mettler-Toledo instrument (Mettler-Toledo Inc., Switzerland). The DSC measurements were operated in the temperature range from −100 to 220 °C using a standard heating–cooling–heating method at a rate of 10 °C min−1 under a pure argon atmosphere. The crystallinity degree of the fabricated membranes was calculated using eqn (2).
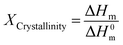 | (2) |
In this equation ΔHm is the melting enthalpy of each segment of polymer (the area of the melting peak in the DSC curves) and ΔH0m is the melting enthalpy of fully crystalline sections of the polymer (ΔH0m,PEO = 166.4 J g−1 and ΔH0m,PA = 230 J g−1).17,23 The total crystallinity of the fabricated membranes was estimated by considering 60% PEO crystallinity and 40% PA crystallinity.24
Gas permeability
An examination of the gas permeability of the fabricated membranes was conducted with a gas permeability apparatus that controlled the temperature and pressure. The gas permeability experiments were operated using a constant volume and variable pressure method. In all single gas permeability measurements, pure gases, including N2, CH4 and CO2, were investigated at temperatures of 25 °C and various pressures of 2, 4 and 6 bar. A cross-flow flat plate membrane module with an effective surface area of 12.5 cm2 was utilized in the gas permeability apparatus and the permeate pressure downstream of the membrane was measured every 0.3 s by a pressure transmitter. After transmitting data to the computer and plotting the variation in measured pressure over time, the pressure-time gradient (dp/dt) was calculated and by inserting it in eqn (3), the permeability of the gas was obtained. Before each test, the membrane and downstream volume of the membrane were vacuumed by a vacuum pump.
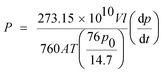 | (3) |
In this equation, P, V, l, A, T, p0 and dp/dt represent the gas permeability (barrer, 1 barrer = 10−10 cm3 (STP) cm cm−2 s−1 cm Hg−1), the downstream volume (cm3), the thickness of the membrane selective layer (μm), the active surface area of the membrane (cm2), the operating temperature (K), the feed pressure (psia) and the steady-state rate of increasing downstream pressure, respectively.
As shown in eqn (4), the ideal selectivity of a pair of gases (A relative to B) was calculated by dividing the pure gas permeability of A by the pure gas permeability of B.
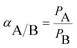 | (4) |
A schematic of the gas permeability set-up is presented in Fig. S3.†
Results and discussion
Characterization of NaX nano-zeolites
The XRD patterns of the synthesized NaX and NaX–COOH nano-zeolites are demonstrated in Fig. 1a and evaluated with the standard XRD pattern. As shown in Fig. 1a, the obtained XRD patterns are consistent with the standard XRD pattern and obviously indicate that the synthesized crystals of the zeolites are pure.20 A mean crystal size of 16 nm was obtained using Scherrer's equation (eqn (1)).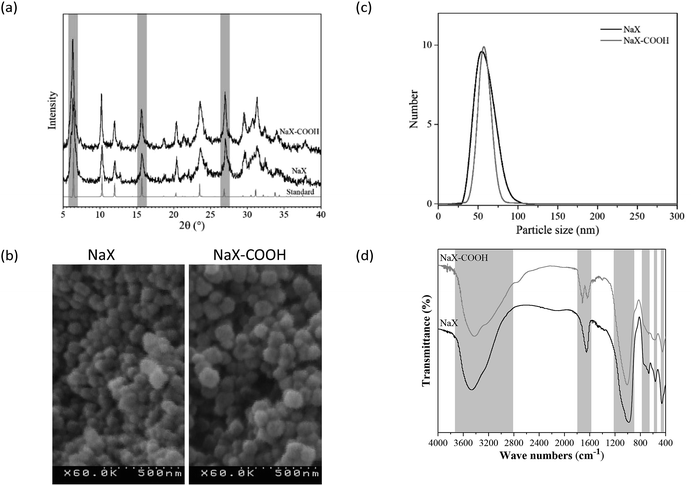 | ||
| Fig. 1 The XRD patterns (a), FESEM image (b), DLS results (c) and FTIR spectra (d) of the synthesized NaX and NaX–COOH nano-zeolites. | ||
An FESEM image of the synthesized zeolites is presented in Fig. 1b. The FESEM images clearly reveal that the zeolite particles are morphologically alike and the particle size of the synthesized samples is about 40 to 90 nm.
Fig. 1c shows the DLS analysis to determine the particle size distribution of the NaX and NaX–COOH nano-zeolites. As indicated in this figure, the particle size distribution of NaX and NaX–COOH nano-zeolites is in the range of 40 to 90 nm, and the means are 55 and 57 nm, respectively. In addition, the results of the DLS test are in good agreement with the FESEM and XRD results. The crystalline structures of unmodified and modified particles have a uniform distribution.
The FTIR spectra of the NaX and NaX–COOH nano-zeolites are displayed in Fig. 1d. The band at 462 cm−1 is attributed to the internal vibrations of TO4 (T = Si or Al) tetrahedral (S4R symmetric bending), whereas the band at 563 cm−1 is related to the double-ring vibration (D6R T–O–T symmetric stretching). The peak at 671 cm−1 detects the internal vibration of T–O symmetric stretching, while the peak at 755 cm−1 demonstrates vibrations corresponding to external linkages between tetrahedra (S4R T–O–T symmetric stretching). The 986 cm−1 band is assigned to the Si–O–Al asymmetric stretching vibration mode of the T–O bonds. The peaks in the wave number range 2500–3800 cm−1 and 1647 cm−1 indicate O–H stretching of absorbed water in the cavities and channels of NaX zeolite and the O–H bending vibration of water molecules, respectively.25–27 As can be seen, in the spectrum of the modified zeolite, in addition to the major peaks of the crystal structure, the carboxylic group peaks appear. The peak appearing at 1716 cm−1 corresponds to the free carboxylic acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch. The wide peak of the O–H band is also shifted to lower wave numbers, which indicates the O–H bond in the carboxylic acid group.
O stretch. The wide peak of the O–H band is also shifted to lower wave numbers, which indicates the O–H bond in the carboxylic acid group.
Moreover, the physical properties of nano-zeolites obtained from BET analysis are presented in Table 2. The BET surface area, total pore volume and mean pore size of the NaX nano-zeolite were higher than those of the functionalized zeolite. This reduction is due to the imposition of functional groups into the vacuity of the NaX zeolite micropores, which results in partial blocking of the micropores.28
| Sample name | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | Mean pore diameter (nm) |
|---|---|---|---|
| NaX | 573 | 0.350 | 0.81 |
| NaX–COOH | 520 | 0.322 | 0.77 |
Characterization of membranes
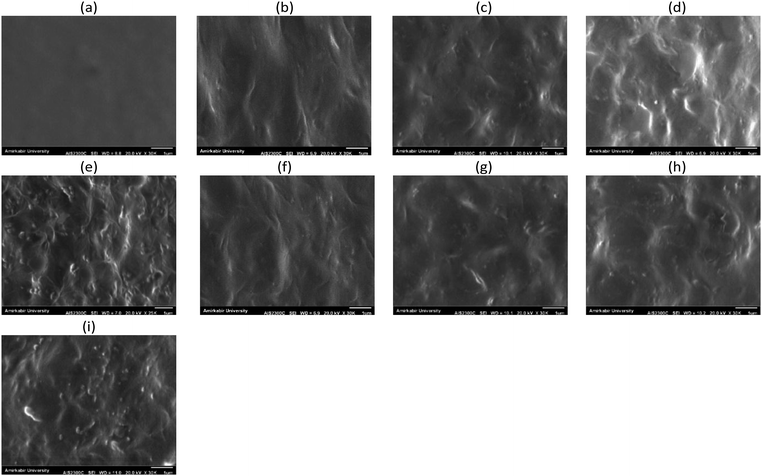 | ||
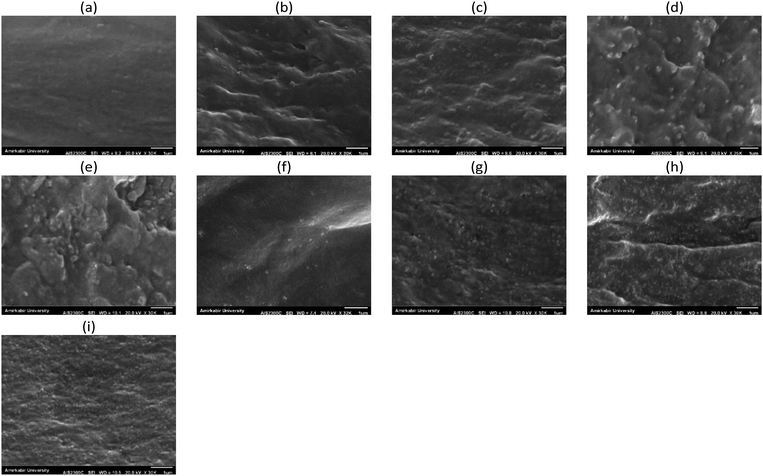
| Fig. 2 The SEM surface images of the active layer of PP (a), PPN0.5 (b), PPN1 (c), PPN1.5 (d), PPN2 (e), PPNC0.5 (f), PPNC1 (g), PPNC1.5 (h) and PPNC2 (i). | ||
 | ||
| Fig. 3 The SEM cross sectional images of the active layer of PP (a), PPN0.5 (b), PPN1 (c), PPN1.5 (d), PPN2 (e), PPNC0.5 (f), PPNC1 (g), PPNC1.5 (h) and PPNC2 (i). | ||
Also, MSL-MMMs containing 0.5 to 1.5% wt NaX and NaX–COOH zeolites reveal that zeolite nanoparticles in the polymeric matrix are uniformly dispersed without any accumulation or interfacial voids, while the modified zeolites had much better distribution and very suitable interfacial interactions in the matrix. The suitable dispersion is due to the rubbery nature of the polymer phase and the presence of soft segments of PEO in Pebax-1657, while the zeolite particles stick well to the polymer.
At a zeolite loading of 2% wt, the NaX nano-zeolites are approximately agglomerated, while the NaX–COOH nano-zeolites are free of any defects such as non-selective voids at the interface, an agglomeration or sedimentation of particles due to the presence of the carboxylic functional groups and also due to the strong interaction and increase in the stress concentration at the interfaces of the polymer and filler.16
| Sample name | Tg (°C) | ΔHm (J g−1) | Xcrystallinity (%) | |||
|---|---|---|---|---|---|---|
| PEO | PA | PEO | PA | Total | ||
| PP | −55.35 | 14.43 | 23.07 | 14.45 | 25.08 | 18.7 |
| PPN0.5 | −52.99 | 16.32 | 23.35 | 16.35 | 25.38 | 19.96 |
| PPN1 | −51.45 | 18.25 | 23.87 | 18.28 | 25.95 | 21.35 |
| PPN1.5 | −49.38 | 19.54 | 24.68 | 19.57 | 26.83 | 22.47 |
| PPN2 | −48.52 | 20.93 | 25.71 | 20.96 | 27.95 | 23.76 |
| PPNC0.5 | −52.66 | 17.11 | 23.41 | 17.14 | 25.45 | 20.46 |
| PPNC1 | −49.41 | 19.34 | 25.13 | 19.37 | 27.32 | 22.55 |
| PPNC1.5 | −47.04 | 20.87 | 26.91 | 20.9 | 29.25 | 24.24 |
| PPNC2 | −46.13 | 22.41 | 27.73 | 22.44 | 30.14 | 25.52 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–C
O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H bonds in the hard PA6 segment of Pebax-1657.29
O and N–H bonds in the hard PA6 segment of Pebax-1657.29
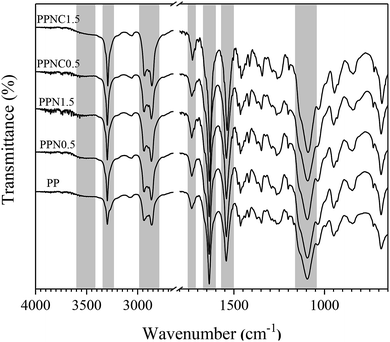
As indicated in the spectra of the zeolite-filled MSL-MMMs, the intensity of the characteristic peaks at 800–1200, 1500–1800 and 2800–3500 cm−1 changed with an increase in the NaX and NaX–COOH zeolite loading of the membranes. The peak at 800–1200 cm−1 is associated with an improvement in the interaction of zeolites and the C–O–C group of the soft PEO segment30,31 and the peaks at 1500–1800 and 2800–3500 cm−1 are attributed to the interaction of zeolites and N–H, H–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the hard PA6 segment. The broad band appearing at a wave number of 3500 cm−1 is due to the formation of hydrogen bonding between the zeolites and the polymer matrix.32,33 These results are in good agreement with the observations reported in other investigations30,32 and the results of the DSC analysis.
O groups of the hard PA6 segment. The broad band appearing at a wave number of 3500 cm−1 is due to the formation of hydrogen bonding between the zeolites and the polymer matrix.32,33 These results are in good agreement with the observations reported in other investigations30,32 and the results of the DSC analysis.
| Membrane sample | Contact angle (°) |
|---|---|
| PP | 64 |
| PPN0.5 | 63 |
| PPN1 | 61 |
| PPN1.5 | 59 |
| PPN2 | 55 |
| PPNC0.5 | 62 |
| PPNC1 | 59 |
| PPNC1.5 | 57 |
| PPNC2 | 54 |
On the other hand, the presence of carboxylate groups in the membrane leads to an increase in membrane hydrophilicity.19 Therefore, at a lower loading, the carboxylic group increases the hydrophilicity of the MSL-MMMs containing modified zeolite compared to membranes containing the unmodified zeolite. The contact angle results were in good agreement with the contact angle data in previous studies.30,31
Gas permeation of membranes
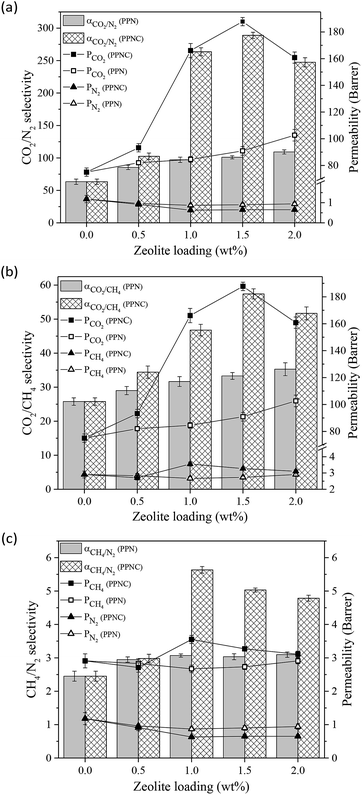 | ||
| Fig. 5 The effect of nano-zeolite loading on the gas permeability and selectivity of the CO2 and N2 mixture (a), CO2 and CH4 mixture (b) and CH4 and N2 mixture (c) at 6 bar and 25 °C. | ||
In MSL-MMMs, the solubility of gases can be affected by the condensability of the gases (the critical temperature of gases), the interactions between the gases and polymer chains, and interactions between the gases and the functional groups of the nanoparticles. Likewise, the diffusivity of the gases can be affected by the kinetic diameter of the gases, the partial fractional free volume of the polymer, interactions between polymer and gases, polymer chain flexibility, nanoparticle pore size, pore blocking of nanoparticles, compatibility of the nanoparticles and the polymer, and agglomeration of nanoparticles. The presence of nanoparticles in the polymeric matrix has a variety of effects on the morphology and performance of MSL-MMMs. The solubility of the polar gases and the molecular sieving of the small kinetic diameter gases increase. The tortuousness of the permeation pathway increases for non-polar penetrant molecules and for gases larger than the filler pores. The strong compatibility and interaction between the polymer and filler lead to a hardening of the polymer chains around the polymer/filler interface and, occasionally, to partial pore blocking of the particles. The placement of the fillers between the chains leads to a reduction in movement and flexibility of the chains and fractional free volume.
As shown in Fig. 5, for all the fabricated membranes, the CO2 permeability was significantly higher than for CH4 or N2 gases. The much higher critical temperature and the smaller kinetic diameter of CO2 than for the other gases caused greater solubility and diffusivity. In addition, CO2 polar gas has the highest solubility due to the strong tendency towards the polar segment of Pebax-1657 compared to the non-polar gases (CH4 and N2). Zeolites with a low tendency to absorb CH4 and N2 gases also lead to prolongation of the total path of their penetration into the membrane. It should be noted that the pore size of the zeolites is larger than the molecular size of all the tested gases, but the zeolites actually separate gases by selective sorption, and the pore flow through the zeolite cavities has a smaller effect on separation. Therefore, the higher solubility and higher diffusion of the CO2 gas resulted in a higher permeability of this gas compared to other gases through the neat membrane and MSL-MMMs.
As shown in Fig. 5, the combination of NaX and NaX–COOH porous nano-fillers significantly affected the permeability and selectivity. By increasing the amount of nanoparticle loading up to 1.5% wt, the permeability of CO2 gas increased, but the permeability of other gases was reduced. Likewise, the selectivity of all pairs of gases increased. The SEM surface images of the MSL-MMMs in Fig. 2 showed that by increasing the loading amount of nano-zeolites, the number of these fillers increases on the membrane surface. Therefore, due to the high sorption capacity of the zeolite filler for polar gases and the high chemical tendency of CO2 with the NaX nano-zeolites, the interaction between the gas and membrane surface increased and led to an increase in CO2 permeability. On the other hand, the gradual decrease in the permeability of the other gases could be due to the formation of a hard layer around the nano-fillers, a decrease in the fractional free volume, partial pore blocking of the nanoparticles and, most importantly, the weak tendency of zeolites towards non-polar gases.4,9
In the MSL-MMMs comprising NaX–COOH compared to the MSL-MMMs containing NaX, the permeability of CO2 was much higher and the permeability of CH4 and N2 gases was lower. The –COOH functional group of the modified zeolites can effectively enhance the surface affinity toward CO2 molecules,34 and as a result, the CO2 gas permeability and subsequently the CO2/CH4 and CO2/N2 selectivities increase. In addition, the results of the BET analysis indicated that the smaller pore size of the NaX–COOH compared to the NaX nano-zeolites hinders the permeation of molecules with a high kinetic diameter that would have an effect of improving the CO2 permeability compared to N2 and CH4.16,28 The merging of nano-zeolite into the Pebax-1657 matrix limits the movement of the polymer chains through the formation of hydrogen bonds between the hydroxyl groups of the filler and the Pebax-1657 polymer chains, as illustrated by the FTIR analysis in Fig. 4. This limitation is greater for the modified zeolites due to the formation of stronger bonds, but the solubility of polar gases is also significantly increased. Based on the results of the DSC test in Table 3, the addition of nano-zeolites made the polymeric chains harder. Thus MSL-MMMs containing NaX–COOH and NaX have a significantly higher Tg than the neat membranes.
With a loading of 2% wt of unmodified NaX zeolite, the nanoparticle aggregation tendency increased, as shown in Fig. 5. The weak interactions and the lack of appropriate adhesion between the filler and the polymer caused the accumulation of nanoparticles. However, the CO2 permeability decreased dramatically for the MSL-MMM with the maximum modified zeolite loading (2% wt). Reducing the CO2 permeability at the highest loading amount of NaX–COOH can be attributed to the increased interaction between the polymer and the functionalized filler. Thus, the filler affects the crystallinity of polyamide and polyethylene oxide segments. The presence of NaX–COOH in the PEO soft section leads to a hardening of the chains and thus reduces the CO2 gas permeability; although in the polyamide sector, it leads to the breakdown of the amide chain and a slight increase in the free volume, as proven by the FTIR and DSC analyses.
Fig. 5 shows the ideal selectivity of the pairs of gases for the neat membrane and MSL-MMMs. For the neat membrane, the CO2/N2, CO2/CH4 and CH4/N2 ideal selectivities are 63.85, 28.21 and 2.26, respectively. While the addition of NaX and NaX–COOH nano-zeolites resulted in a significant improvement in the ideal selectivity of all pairs of gases. By increasing the loading amount of nano-zeolites to 1.5% wt, the selectivities of CO2/N2, CO2/CH4 and CH4/N2 for the MSL-MMM containing NaX nano-zeolite increased to 101.03, 33.31 and 3.03, respectively, and for the MSL-MMM containing NaX–COOH nano-zeolite they increased to 288.86, 57.42 and 5.03. This trend for gas-pair selectivity can be attributed to their permeability ratio, and the reasons for the permeability behaviour are expressed above.
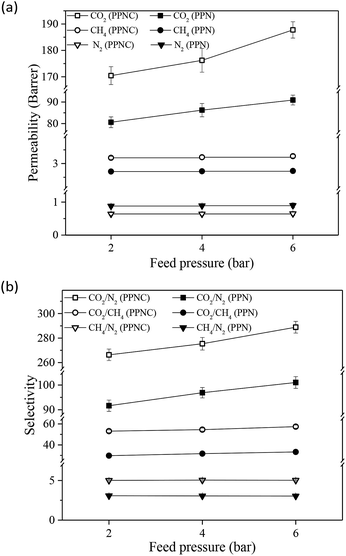 | ||
| Fig. 6 The effect of operating pressure on the gas permeability (a) and selectivity (b) of the PPN1.5 and PPNC1.5 at 25 °C. | ||
As shown in Fig. 6a, the permeability of CH4 and N2 gases appears to be constant with the application of higher pressure. This behavior implies that the fabricated membranes are not compacted with increasing operating pressure. The prepared membranes based on Pebax-1657 have a semi-crystalline structure, in which the crystalline parts, such as rigid segments and physical crosslinking in the membrane structure, can provide the mechanical strength. Thus, resistance versus compaction and reducing the movement of the polymer chains are observed with an increase in feed pressure. Additionally, incorporation of nanoparticles increases the mechanical strength of MSL-MMMs. The effect of modified nanoparticles was greater than that of the unmodified nanoparticles in increasing the mechanical strength. Ultimately, with an increase in the operating pressure, the mass transfer driving force increases and causes an increase in the gas diffusivity. Therefore, the permeability of gases increases very slightly, with no compaction occurring in the membranes and an increase in gas diffusivity. The gas permeability increase in interacting and polar gases like CO2 in the MSL-MMMs including modified nano-zeolites was more significant due to the presence of carboxylic functional groups. Furthermore, the CO2/N2 and CO2/CH4 selectivities were slightly enhanced as the operating pressure increased, while the CH4/N2 selectivity was almost constant, as shown in Fig. 6b. These trends can be attributed to the effect of operating pressure on the gas permeabilities of the prepared MSL-MMMs. Similar observations have been reported in previous research.2,16,30
| Filler type | Filler loadinga (% wt) | P (bar) | T (°C) | Permeability (barrer) | Selectivity | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | CH4 | N2 | CO2/N2 | CO2/CH4 | CH4/N2 | |||||
| a Based on the polymer weight. | ||||||||||
| NaX–COOH | 15 | 6 | 25 | 187.76 | 3.27 | 0.65 | 288.86 | 57.41 | 5.03 | This work |
| SiO2–COOH | 8 | 2 | 25 | 133.01 | 2.96 | 1.08 | 123.16 | 45 | 2.74 | 10 |
| MWNTs-NH2 | 9 | 7 | 35 | 200 | 12.5 | 3.84 | 52 | 16 | 3.26 | 14 |
| GO-DA-Zn2+ | 1 | 2 | 30 | 137.9 | 4.79 | — | — | 28.8 | — | 38 |
| UiO-66-NH2 | 10 | 3 | 25 | 87 | — | 1.32 | 66 | — | — | 12 |
| ImGO | 0.8 | 8 | 25 | 76.20 | 2.5 | 0.72 | 105.50 | 30.5 | 3.47 | 2 |
| UiO-66-NH2 | 15 | 7 | 25 | 393.4 | 9.9 | — | — | 39.8 | — | 16 |
| TiO2-CMC | 3 | 20 | 25 | 194.6 | — | 2.36 | 82.4 | — | — | 39 |
| TiO2-AS | 3 | 20 | 25 | 188.6 | — | 2.22 | 84.9 | — | — | 39 |
| GO-IL-NH2 | 10 | 4 | 25 | 143 | 6.25 | 1.8 | 79.44 | 22.88 | 3.47 | 40 |
| MIL-53-NH2 | 10 | 10 | 35 | 149.1 | 7.3 | 2.7 | 55.22 | 20.42 | 2.7 | 41 |
| GO-APTS | 0.7 | 2 | 35 | 172 | 10.1 | 3.8 | 45.26 | 17.03 | 2.65 | 42 |
| ZIF-8-ZN/Ni | 10 | 5 | 25 | 408 | 7.87 | — | 51.8 | — | — | 43 |
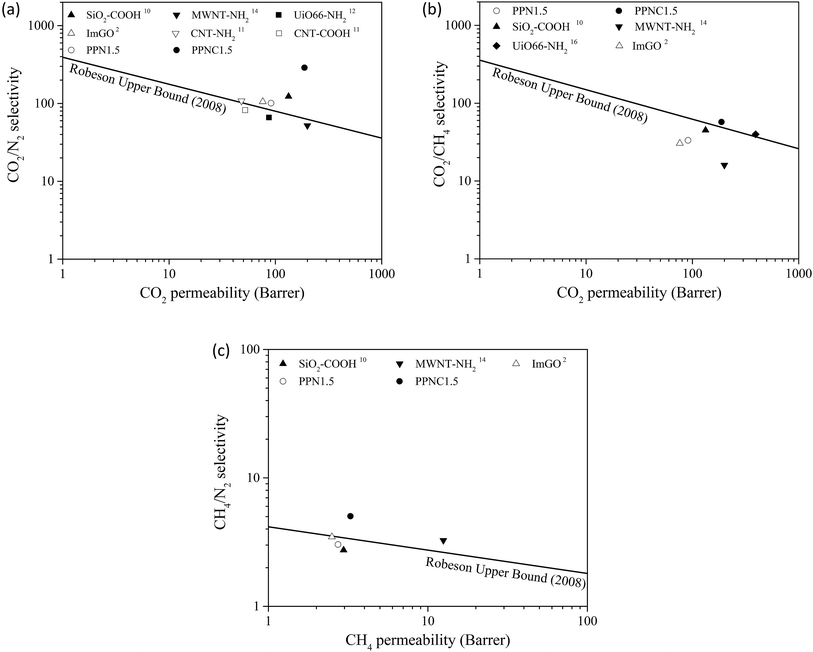
Finally, the permselectivities of the MSL-MMMs were compared with Robeson's upper limit and the results are shown in Fig. 7. As can be seen, the PPN1.5 and PPNC1.5 membranes overcome Robeson's upper bound, which proves that the efficiency of separation is much higher than that of conventional MMMs in other research.
Conclusions
A highly permeable and highly selective membrane including the zeolites combined with a Pebax-1657 moderate selective layer on a highly permeable PES support layer was developed for gas separation applications. Functionalized NaX nano-zeolites were employed to comprehend the effect of chemical surface modification on the gas separation performance compared to conventional MMMs. Loading the NaX and NaX–COOH nano-zeolites into a Pebax polymeric matrix effectively promoted the CO2 permeability and CO2/N2, CO2/CH4 and N2/CH4 selectivities due to the better CO2 sorption, good compatibility and strong interfacial interaction of the filler/polymer chains. In addition, the permeability and selectivity of MSL-MMMs were amplified by changing the operating pressure from 2 to 6 bar. The results confirmed that MSL-MMMs containing carboxyl modified NaX zeolite had superior separation performance compared to MSL-MMMs containing neat NaX zeolite. Moreover, SEM images indicated that the modification of NaX nano-zeolite led to good filler/polymer interaction and filler dispersion. The MSL-MMMs were also explored by performing DSC, FTIR and long-term permeability tests. The results revealed that the addition of nano-zeolites rigidified the polymer matrix chains by forming strong hydrogen bonds, ultimately preventing the occurrence of compaction and plasticization of MSL-MMMs. MSL-MMMs containing 1.5% wt of carboxyl functionalized NaX nanoparticles passed the Robeson's upper bound limit for the separation of CO2/N2, CO2/CH4 and CH4/N2. Finally, it can be deduced that loading functionalized NaX into the Pebax-1657 moderate selective layer is feasible for developing MSL-MMMs with high separation performance in the cases of CO2 in flue and natural gas.Conflicts of interest
There are no conflicts to declare.References
- G. Dong, H. Li and V. Chen, J. Mater. Chem. A, 2013, 1, 4610 RSC.
- Y. Dai, X. Ruan, Z. Yan, K. Yang, M. Yu, H. Li, W. Zhao and G. He, Sep. Purif. Technol., 2016, 166, 171–180 CrossRef CAS.
- H. Wu, X. Li, Y. Li, S. Wang, R. Guo, Z. Jiang, C. Wu, Q. Xin and X. Lu, J. Membr. Sci., 2014, 465, 78–90 CrossRef CAS.
- N. Kosinov, J. Gascon, F. Kapteijn and E. J. J. J. o. M. S. Hensen, J. Membr. Sci., 2016, 499, 65–79 CrossRef CAS.
- M. A. Aroon, A. F. Ismail, T. Matsuura and M. M. Montazer-Rahmati, Sep. Purif. Technol., 2010, 75, 229–242 CrossRef CAS.
- T.-S. Chung, L. Y. Jiang, Y. Li and S. Kulprathipanja, Prog. Polym. Sci., 2007, 32, 483–507 CrossRef CAS.
- P. S. Goh, A. F. Ismail, S. M. Sanip, B. C. Ng and M. Aziz, Sep. Purif. Technol., 2011, 81, 243–264 CrossRef CAS.
- D. Zhao, J. Ren, Y. Wang, Y. Qiu, H. Li, K. Hua, X. Li, J. Ji and M. Deng, J. Membr. Sci., 2017, 521, 104–113 CrossRef CAS.
- Y. Cheng, Z. Wang and D. Zhao, Ind. Eng. Chem. Res., 2018, 57, 4139–4169 CrossRef CAS.
- A. Ghadimi, T. Mohammadi and N. Kasiri, Int. J. Hydrogen Energy, 2015, 40, 9723–9732 CrossRef CAS.
- S. A. Habibiannejad, A. Aroujalian and A. Raisi, RSC Adv., 2016, 6, 79563–79577 RSC.
- J. Shen, G. Liu, K. Huang, Q. Li, K. Guan, Y. Li and W. Jin, J. Membr. Sci., 2016, 513, 155–165 CrossRef CAS.
- N. C. Su, H. G. Buss, B. D. McCloskey and J. J. Urban, ACS Macro Lett., 2015, 4, 1239–1243 CrossRef CAS.
- D. Zhao, J. Ren, H. Li, X. Li and M. Deng, J. Membr. Sci., 2014, 467, 41–47 CrossRef CAS.
- H. R. Amedi and M. Aghajani, Microporous Mesoporous Mater., 2017, 247, 124–135 CrossRef CAS.
- M. Mozafari, R. Abedini and A. Rahimpour, J. Mater. Chem. A, 2018, 6, 12380–12392 RSC.
- P. D. Sutrisna, J. Hou, M. Y. Zulkifli, H. Li, Y. Zhang, W. Liang, D. M. D'Alessandro and V. Chen, J. Mater. Chem. A, 2018, 6, 918–931 RSC.
- X. Jiang, S. Li, S. He, Y. Bai and L. Shao, J. Mater. Chem. A, 2018, 6, 15064–15073 RSC.
- J. Shen, L. Wu, D. Wang and C. Gao, Desalination, 2008, 223, 425–437 CrossRef CAS.
- M. Ansari, A. Aroujalian, A. Raisi, B. Dabir and M. Fathizadeh, Adv. Powder Technol., 2014, 25, 722–727 CrossRef CAS.
- P. G. Ingole, M. I. Baig, W. K. Choi and H. K. Lee, J. Mater. Chem. A, 2016, 4, 5592–5604 RSC.
- I. Sadeghi, A. Aroujalian, A. Raisi, B. Dabir and M. Fathizadeh, J. Membr. Sci., 2013, 430, 24–36 CrossRef CAS.
- A. Ghadimi, M. Amirilargani, T. Mohammadi, N. Kasiri and B. Sadatnia, J. Membr. Sci., 2014, 458, 14–26 CrossRef CAS.
- H. Rabiee, A. Ghadimi and T. Mohammadi, J. Membr. Sci., 2015, 476, 286–302 CrossRef CAS.
- J. Coates, Encyclopedia of analytical chemistry, 2000 Search PubMed.
- B. M. Abu-Zied, Microporous Mesoporous Mater., 2011, 139, 59–66 CrossRef CAS.
- A. Zainal Abidin, N. H. H. Abu Bakar, E. P. Ng and W. L. Tan, J. Taibah Univ. Sci., 2017, 11, 1070–1079 CrossRef.
- X. Wang, H. Li and X.-J. Hou, J. Phys. Chem. C, 2012, 116, 19814–19821 CrossRef CAS.
- G. Dong, J. Hou, J. Wang, Y. Zhang, V. Chen and J. Liu, J. Membr. Sci., 2016, 520, 860–868 CrossRef CAS.
- K. Zarshenas, A. Raisi and A. Aroujalian, J. Membr. Sci., 2016, 510, 270–283 CrossRef CAS.
- M. Fathizadeh, A. Aroujalian and A. Raisi, J. Membr. Sci., 2011, 375, 88–95 CrossRef CAS.
- R. Surya Murali, A. F. Ismail, M. A. Rahman and S. Sridhar, Sep. Purif. Technol., 2014, 129, 1–8 CrossRef CAS.
- L. Xiang, Y. Pan, G. Zeng, J. Jiang, J. Chen and C. Wang, J. Membr. Sci., 2016, 500, 66–75 CrossRef CAS.
- B. Liu, H. Li, X. Ma, R. Chen, S. Wang and L. Li, RSC Adv., 2018, 8, 38965–38973 RSC.
- Y. Naito, Y. Kamiya, K. Terada, K. Mizoguchi and J. S. Wang, J. Appl. Polym. Sci., 1996, 61, 945–950 CrossRef CAS.
- H. Lin and B. D. Freeman, J. Membr. Sci., 2004, 239, 105–117 CrossRef CAS.
- S. Semenova, S. Smirnov and H. Ohya, J. Membr. Sci., 2000, 172, 75–89 CrossRef CAS.
- D. Peng, S. Wang, Z. Tian, X. Wu, Y. Wu, H. Wu, Q. Xin, J. Chen, X. Cao and Z. Jiang, J. Membr. Sci., 2017, 522, 351–362 CrossRef CAS.
- A. A. Shamsabadi, F. Seidi, E. Salehi, M. Nozari, A. Rahimpour and M. Soroush, J. Mater. Chem. A, 2017, 5, 4011–4025 RSC.
- G. Huang, A. P. Isfahani, A. Muchtar, K. Sakurai, B. B. Shrestha, D. Qin, D. Yamaguchi, E. Sivaniah and B. Ghalei, J. Membr. Sci., 2018, 565, 370–379 CrossRef CAS.
- S. Meshkat, S. Kaliaguine and D. J. S. Rodrigue, Sep. Purif. Technol., 2018, 200, 177–190 CrossRef CAS.
- J. Zhang, Q. Xin, X. Li, M. Yun, R. Xu, S. Wang, Y. Li, L. Lin, X. Ding and H. Ye, J. Membr. Sci., 2019, 570, 343–354 CrossRef.
- X. Zhang, T. Zhang, Y. Wang, J. Li, C. Liu, N. Li and J. Liao, J. Membr. Sci., 2018, 560, 38–46 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01654f |
| This journal is © The Royal Society of Chemistry 2019 |