Open Access Article
Open Access ArticleLayered photochromic films stacked from spiropyran-modified montmorillonite nanosheets†
Mengke Gan‡
a,
Tianliang Xiao‡a,
Zhaoyue Liu *a and
Yao Wang*b
*a and
Yao Wang*b
aKey Laboratory of Bio-Inspired Smart Interfacial Science and Technology of Ministry of Education, School of Chemistry, Beihang University, Beijing 100191, P. R. China. E-mail: liuzy@buaa.edu.cn
bGuangdong Provincial Key Laboratory of Optical Information Materials and Technology, Institute of Electronic Paper Displays, South China Academy of Advanced Optoelectronics, South China Normal University, Guangzhou 510006, P. R. China. E-mail: wangyao@m.scnu.edu.cn
First published on 23rd April 2019
Abstract
Two-dimensional (2D) nanosheets are a class of fascinating host material that demonstrates a high specific surface area for the immobilization of functional molecules. Herein, we describe a layered photochromic film using montmorillonite 2D nanosheets immobilized with spiropyran units, which demonstrates a remarkable and reversible photochromic behavior. The synthesis of the layered photochromic film includes the intercalation and exfoliation of montmorillonite powders into 2D nanosheets using a spiropyran-modified surfactant and a subsequent vacuum filtration. The photochromic units of spiropyran-modified quaternary ammonium groups are immobilized on the surface of montmorillonite 2D nanosheets through an electrostatic interaction after exchanging with the native cations in montmorillonite during the intercalation and exfoliation. The photoisomerization of the spiropyran units between closed-ring spiropyran and open-ring merocyanine upon visible/UV irradiation contributes to the photochromic behavior of the layered film. The color contrast between the coloration and decoloration states of photochromic film is optimized by increasing the amount of spiropyran-modified cationic surfactant during the intercalation and exfoliation process. Our layered films with a visual photochromic behavior may promote their applications for optical data storage, optical switching and chemical sensing.
1. Introduction
Photochromic molecules which can undergo reversible color change upon the external stimulus of light have attracted much attention for potential applications in optical data storage, optical switching and chemical sensing.1–6 Spiropyran is one of the most studied photochromic molecules because of its fast and reproducible color switching resulting from the photoisomerization between the colorless (light yellow) closed-ring spiropyran (SP) form and deeply colored open-ring merocyanine (MC) form.7–10 Photochromic films are strongly desired for further applications, and are generally derived from the dispersion of the spiropyran molecules into a polymer matrix or the polymerisation of spiropyran-based monomers.7,11–13 The development of an alternative strategy towards novel photochromic films is essentially important.Two-dimensional (2D) nanosheets are an emerging class of nanomaterials that demonstrate an ultrahigh specific surface area because of their large lateral size and ultrathin thickness.14–19 These unique surface characteristics make 2D nanosheets act as fascinating nanosized building blocks to immobilize functional molecules. Furthermore, the solution-based processability of 2D nanosheets is favorable for the fabrication of high-quality layered thin film by a simple method such as vacuum filtration.14,20,21 Montmorillonite is one of extensively studied layered clay minerals. The layer spacing of Al–O octahedral sheet sandwiched by two Si–O tetrahedral sheets can be intercalated with functional molecules by exchanging their native cations with organic cations.22–25 Several examples have reported that the adsorption of cationic spiropyrans into montmorillonite interlayers through ion exchange for photochromic application.9,26–31 However, only a few works focused on the fabrication of photochromic films using spiropyrans-intercalated montmorillonite. Saso et al. fabricated ultrathin spiropyran-montmorillonite hybrid film by the Langmuir–Blodgett method.26 Kinashi et al. prepared photochromic films by casting a suspension containing cationic spiropyran-intercalated montmorillonites on glass substrate.28 In spite of these works, the development of a stable, uniform and large-area photochromic films with a visual color switch derived from spiropyran-intercalated montmorillonite still remains a challenge.
Herein, we reported a novel photochromic layered film using montmorillonite 2D nanosheets immobilized with spiropyran units. Two kinds of surfactants containing quaternary ammonium cations were used to intercalate and exfoliate montmorillonite powders into high-quality 2D nanosheets. The spiropyran-modified quaternary ammonium cations were immobilized on the surface of 2D nanosheets during the intercalation and exfoliation, which acted as the photochromic units. The exfoliated montmorillonite nanosheets were subsequently stacked into a stable photochromic layered film by a vacuum filtration, which demonstrated a remarkable and reversible photochromic behavior.
2. Results and discussion
2.1 Photochromic properties of synthesized spiropyran-modified cationic surfactants
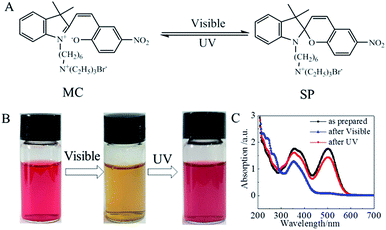
The solid-stated spiropyran-modified cationic surfactant, 1′(6-triethylammoniohexyl)-3′,3′-dimethyl-6-nitrospiro(2H-1-benzopyran-2,2′-indoline)bromide (SP-6) was in closed-ring SP state (Fig. 1A). When SP-6 was dissolved into water, an open-ring merocyanine (MC) state was formed spontaneously through the cleavage of the Cspiro–O bond by the high polarity of water.7,32 The photochromic properties of SP-6 in water were then investigated. As shown in Fig. 1B, the aqueous solution of SP-6 (1 mM) exhibited a pink color because of the spontaneous formation of MC form. The maximal absorption peak in visible region was observed at 503 nm due to the extended π-conjugation between the indoline and the benzopyran moieties in MC state (Fig. 1C).7 The irradiation of visible light changed the color of solution to be light yellow because of the photoisomerization of MC to SP state. Correspondingly, the absorption peak at 503 nm almost disappeared. When the SP solution was irradiated by UV light, the characteristic absorption peak at 503 nm increased gradually following the irradiation time (Fig. S1†). Subsequently, the color of the solution recovered to be pink color. These results indicated that the aqueous solution of our synthesized spiropyran-modified surfactant demonstrated a clear photochromic property.2.2 Characterization of montmorillonite 2D nanosheets immobilized with spiropyran
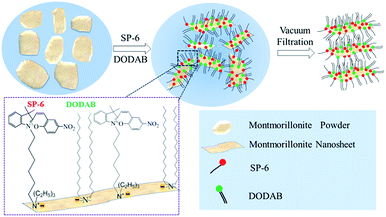
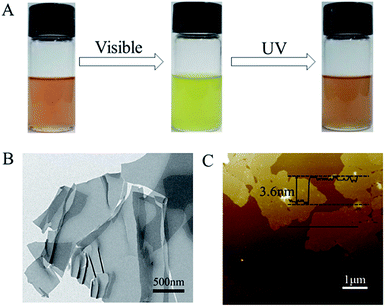
It has been well-recognized that montmorillonite powders can be intercalated and exfoliated into 2D nanosheets through the exchange of its native cations with organic cations in the surfactants,33,34 or through the thorough stirring in water.35 In our work, two kinds of surfactants were used to intercalate and exfoliate montmorillonite powders. One surfactant is a synthesized SP-6 cationic surfactant. The other one is a commercially available dioctadecyldimethylammonium bromide (DODAB). The quaternary ammonium cations having spiropyran groups in SP-6 acted as the photochromic units. The double octadecyl chains in DODAB were used to improve the dispersion of exfoliated 2D nanosheets in chloroform and enhance the mechanical stability of the photochromic film. As shown in Fig. 2, when montmorillonite powders were stirred with SP-6 and DODAB in water at 70 °C, the quaternary ammonium cations in the surfactants of SP-6 and DODAB exchanged with native cations of Na+, Ca2+ and K+ of montmorillonite.24,25 The electrostatic interaction between the quaternary ammonium cations and the negatively charged surface of montmorillonite resulted in the immobilization of spiropyran groups in SP-6 and the double octadecyl chains in DODAB on the surface of montmorillonite 2D nanosheets (Fig. 2). The hydrophobic double octadecyl chains made the dispersion of exfoliated 2D nanosheets in chloroform to form a stable colloidal suspension, which demonstrated a similar photochromism from light pink to light yellow resulting from the photoisomerization between MC and SP state (Fig. 3A).The visible irradiation resulted in the decoloration of 2D nanosheets dispersion solution and the UV illumination recovered its color. In a subsequent process, the stable dispersion of 2D nanosheets immobilized with SP-6 in chloroform was used to fabricate a photochromic film by a vacuum filtration through a nylon filter.
The structure of montmorillonite 2D nanosheets exfoliated by SP-6 and DODAB was characterized by TEM measurement. The TEM image (Fig. 3B) indicated that the exfoliated montmorillonite showed a clear 2D nanosheet structure with a lateral size of several hundred of nanometers, which could be used as the building blocks for the formation of a layered film. The AFM measurement also revealed the structure of nanosheets with a similar lateral dimension (Fig. 3C). The height profile indicated that the thickness of montmorillonite 2D nanosheets was ∼3.6 nm, which was much larger than that in native montmorillonite34 due to the immobilization of quaternary ammonium ions containing spiropyran groups and the double octadecyl chains.36 Supposed that the thickness of a pure single-layer montmorillonite nanosheet was ∼0.96 nm,37 the thickness of quaternary ammonium ions on the two sides of 2D nanosheets was determined to be ∼2.64 nm.
2.3 Photochromic behaviors of layered films
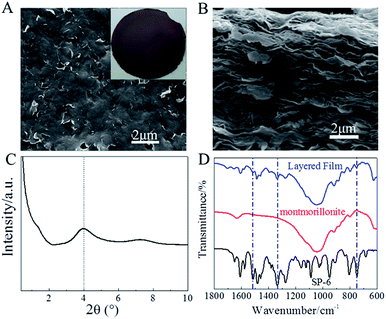
The montmorillonite 2D nanosheets in chloroform could be stacked into dark-pink, paper-like layered film by a vacuum filtration of the suspension solution through a nylon filter membrane (inset in Fig. 4A). The surfactant of DODAB was essentially necessary for the intercalation and exfoliation of montmorillonite powers into 2D nanosheets because the hydrophobic double octadecyl chains could improve the dispersion of exfoliated 2D nanosheets in chloroform. Otherwise, a high-quality layered film could not be obtained. Note that the layered film derived from only DODAB-exfoliated montmorillonite showed a color of light yellow, which was the native color of montmorillonite nanosheets (Fig. S2†). The self-standing layered film demonstrated a smooth surface without obvious defects (Fig. 4A). The wrinkles on the surface indicated the layered film was composed of nanosheets structure. The cross-sectional SEM image (Fig. 4B) verified the dense stacking of nanosheets into an oriented layered structure. The calculated d001 spacing in layered film was ∼2.2 nm determined from the XRD diffraction peak at 2θ = 4.0° based on the Bragg's equation (nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) (Fig. 4C). Note that the very weak XRD diffraction peak at 2θ = 7.2° was derived from the small amount of unexfoliated montmorillonite.34 The bimolecular layer with a length of ∼5.0 nm formed by DODAB molecules increased the d001 spacing of layered film.33 The Fourier transform infrared (FTIR) spectra were used to further clarify the existence of spiropyran units in the layered film. In comparison with pure montmorillonite (Fig. 4D), the FTIR spectrum of layered film displayed the typical characteristic peaks for the stretching vibration of nitro group in SP-6 at 1515 cm−1 and 1335 cm−1, and for the bending vibration of –CH in phenyl groups of SP-6 at 747 cm−1,32 indicating the successful immobilization of spiropyran units in the layered film.
θ) (Fig. 4C). Note that the very weak XRD diffraction peak at 2θ = 7.2° was derived from the small amount of unexfoliated montmorillonite.34 The bimolecular layer with a length of ∼5.0 nm formed by DODAB molecules increased the d001 spacing of layered film.33 The Fourier transform infrared (FTIR) spectra were used to further clarify the existence of spiropyran units in the layered film. In comparison with pure montmorillonite (Fig. 4D), the FTIR spectrum of layered film displayed the typical characteristic peaks for the stretching vibration of nitro group in SP-6 at 1515 cm−1 and 1335 cm−1, and for the bending vibration of –CH in phenyl groups of SP-6 at 747 cm−1,32 indicating the successful immobilization of spiropyran units in the layered film.
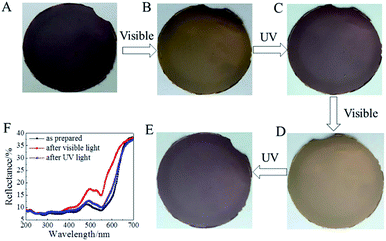
The self-standing layered film demonstrated a remarkable photochromism at room temperature and ambient pressure. In this case, the weight percentage of SP-6 in the total surfactants during the intercalation and exfoliation process of montmorillonite was 30%. Note that not all the SP-6 molecules were intercalated into montmorillonite. As shown in Fig. 5A–E, the as-prepared self-standing layered film demonstrated a color of dark purple with a strong absorption in visible region due to the MC state of SP-6 (Fig. 5F). The MC state was formed during the intercalation and exfoliation process of montmorillonite by DODAB and SP-6 due to the high polarity of water. The red shift of the characteristic peak of MC from 503 nm in aqueous solution to 550 nm in stacked layered film was ascribed to the aggregation of MC in the interlayer space of montmorillonite 2D nanosheets. Following the visible irradiation, the isomerization of MC to SP reduced the visible absorption of the layered film, which decolorized the film to be dark yellow. The UV irradiation was able to recover the color of decolorized film to be dark purple following the recovery of the characteristic peak of MC at 550 nm. Furthermore, the photochromism of layered film was reversible, as evidenced by the four cycles of coloration and decoloration states (Fig. 5A–E).
2.4 Enhancement of the visual photochromic contrast of layered films
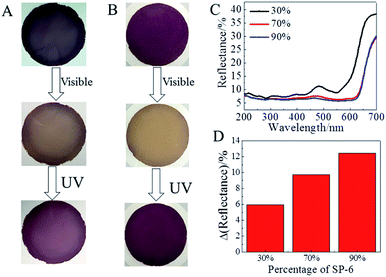
Generally, a high spiropyran quantity in the film is strongly desired to enhance the photochromic performance. The quantity of spiropyran in our layered film could be facilely increased by increasing the weight percentage of SP-6 during the intercalation and exfoliation of montmorillonite into 2D nanosheets. As shown in Fig. 6A and B, when the weight percentage of SP-6 in the total surfactants during the intercalation and exfoliation process increased from 30% to 70% and 90%, the color the as-prepared layered film became deeper because of the increased spiropyran quantity in the layered film, which was evidenced by the reduced visible reflectance of MC in the diffuse reflection spectra (Fig. 6C). Note that the layered film prepared with 90% SP-6 was difficult to be peeled off from the nylon filter. More importantly, the high spiropyran quantity magnified the visual color contrast between the coloration and decoloration states of the layered film as shown in Fig. 6A and B. Following the increase of SP-6 weight percentage in the total surfactants from 30% to 70% and 90% during the intercalation and exfoliation process, the difference between the reflectance of coloration and decoloration states at 550 nm increased from 5.9% to 10% and 12.4%. This magnified visual photochromic contrast might promote the potential application of photochromic films.3. Experimental
3.1 Synthesis of spiropyran-modified cationic surfactant
The spiropyran-modified cationic surfactant, 1′(6-triethylammoniohexyl)-3′,3′-dimethyl-6-nitrospiro(2H-1-benzopyran-2,2′-indoline)bromide (SP-6, C30H42N3O3Br), was synthesized according to the ref. 32 and 38. The chemical structure was shown in Fig. 1A. The final product was characterized by 1H NMR and mass spectroscopy (Fig. S3 and S4†). 1H NMR (CDCl3) δ: 1.10 (3H, s,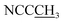 ), 1.19 (3H, s,
), 1.19 (3H, s, 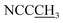 ), 1.28 (13H, t,
), 1.28 (13H, t, 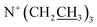 and
and 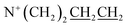 ), 1.57–1.64 (2H, m,
), 1.57–1.64 (2H, m, 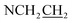 ), 1.76 (2H, s,
), 1.76 (2H, s, 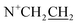 ), 3.04–3.13 (2H, m,
), 3.04–3.13 (2H, m, 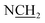 ), 3.18–3.24 (2H, m,
), 3.18–3.24 (2H, m, 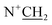 ), 3.36–3.43 (6H, m,
), 3.36–3.43 (6H, m, 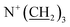 ), 5.81 (1H, d, H3), 6.48 (1H, d, H4′), 6.64 (1H, d, H8), 6.77 (1H, t, H6′), 6.91 (1H, d, H4), 6.99 (1H, d, H7′), 7.09 (1H, t, H5′), 7.88–7.93 (2H, m, H5, H7) ppm. Mass spectrum: m/z: 492.3 (C30H42N3O3+).
), 5.81 (1H, d, H3), 6.48 (1H, d, H4′), 6.64 (1H, d, H8), 6.77 (1H, t, H6′), 6.91 (1H, d, H4), 6.99 (1H, d, H7′), 7.09 (1H, t, H5′), 7.88–7.93 (2H, m, H5, H7) ppm. Mass spectrum: m/z: 492.3 (C30H42N3O3+).
3.2 Synthesis of the photochromic layered film
The montmorillonite nanosheets were prepared by the intercalation and exfoliation of montmorillonites powders with an ion exchange capacity of 120 mequiv./100 g (Nanocor, USA, specific surface area: 15–20 m2 g−1) using our synthesized SP-6 and commercially available dioctadecyldimethylammonium bromide (DODAB, Aldrich) in water.33,34 The quaternary ammonium cations having spiropyran groups in SP-6 acted as the photochromic units. The double octadecyl chains in DODAB were used to improve the dispersion of exfoliated 2D nanosheets in chloroform and enhance the mechanical stability of the photochromic film. Typically, 0.2 g of montmorillonite powders, 51 mg of SP-6 and 119 mg of DODAB were stirred in 10 mL of water at 70 °C for 1 h. The weight percentage of SP-6 in the total surfactants during the intercalation and exfoliation process of montmorillonite was 30%. After cooling to room temperature, the suspension was centrifuged at 2000 rpm. The obtained precipitate was washed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform/methanol, followed by being dispersed into chloroform to form a suspension. The photochromic layered film was obtained by a vacuum filtration of the suspension solution through a nylon filter membrane (50 mm diameter and 0.45 μm pore size, Shanghai Xinya Company, China). Without specific explanation, a self-standing photochromic film was obtained by peeling off it from the nylon filter after vacuum filtration.
1 chloroform/methanol, followed by being dispersed into chloroform to form a suspension. The photochromic layered film was obtained by a vacuum filtration of the suspension solution through a nylon filter membrane (50 mm diameter and 0.45 μm pore size, Shanghai Xinya Company, China). Without specific explanation, a self-standing photochromic film was obtained by peeling off it from the nylon filter after vacuum filtration.
3.3 Characterization
1H NMR was recorded on an Agilent 400 MHz NMR spectrometer. The mass spectrum was obtained with an Agilent-1100 LC/MSD mass spectrometer. The morphology of exfoliated montmorillonite 2D nanosheets was observed by a FEI JEM-1200EX transmission electron microscope (TEM). The thickness of 2D nanosheets was measured using Bruker Dimension Icon atomic force microscope (AFM). The surface and cross-sectional morphology of the stacked layered films were observed using a FEI Quanta FEG 250 environmental scanning electron microscope (SEM). An Ultima IV X-ray diffraction meter (Rigaku Corporation, Japan) was used to determine the interlayer spacing of layered film. The UV-visible absorption and diffuse reflection spectra were recorded using a UV-3600 spectrometer (Shimadzu, Japan). The Fourier transform infrared (FTIR) spectra were measured on a Nicolet 6700 spectrometer (Thermo).3.4 Photochromic characterization
The photochromism of the SP-6 aqueous solution and the layered film from the photoisomerization between the deeply colored merocyanine (MC) and light yellow spiropyran (SP) states was carried out by irradiation of visible light with an irradiance of 100 mW cm−2 from a Xe lamp (Beijing Perfectlight Technology Co. Ltd, China). The recovery to MC form was achieved by irradiation of 365 nm ultraviolet (UV) light with an irradiance of 10 mW cm−2 from a super-high pressure mercury lamp (CHF-XM500, Beijing Trusttech Co. Ltd, China). The corresponding absorption changes were characterized by the UV-visible absorption and diffuse reflection spectra.4. Conclusions
In summary, we have successfully fabricated a novel layered photochromic film using montmorillonite 2D nanosheets immobilized with spiropyran units. The nanosheets were prepared by the intercalation and exfoliation of montmorillonite using a synthesized spiropyran-modified cationic surfactant and a commercial cationic surfactant with double octadecyl chains in water. The spiropyran-modified quaternary ammonium groups were immobilized on the surface of 2D nanosheets by the cationic exchange during the intercalation and exfoliation, which acted as the photochromic units. The 2D nanosheets were stacked into a photochromic layered film by a vacuum filtration, which demonstrated a remarkable and reversible photochromic behavior.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Key Research and development Program of China (2017YFA0206902, 2017YFA0206900), Beijing Municipal Science & Technology Commission (No. Z181100004418013), National Natural Science Foundation of China (21571011 and 51673007), the Fundamental Research Funds for the Central Universities (YWF-19-BJ-J-115) and the Startup Foundation from SCNU (No. 8S0134).Notes and references
- M. Zhu, L. Zhu, J. J. Han, W. Wu, J. K. Hurst and A. D. Q. Li, J. Am. Chem. Soc., 2006, 128, 4303–4309 CrossRef CAS PubMed
.
- S. Kawata and Y. Kawata, Chem. Rev., 2000, 100, 1777–1788 CrossRef CAS
.
- H. Tian and S. Yang, Chem. Soc. Rev., 2004, 33, 85–97 RSC
.
- J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378–399 CrossRef CAS PubMed
.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS
.
- S. Coiai, E. Passaglia, A. Pucci and G. Ruggeri, Materials, 2015, 8, 3377–3427 CrossRef CAS
.
- R. Klajn, Chem. Soc. Rev., 2014, 43, 148–184 RSC
.
- Y. Funasako, A. Takaki, M. Inokuchi and T. Mochida, Chem. Lett., 2016, 45, 1397–1399 CrossRef CAS
.
- K. Takagi, T. Kurematsu and Y. Sawaki, J. Chem. Soc., Perkin Trans. 2, 1991, 1517–1522 RSC
.
- T. Yamaguchi and M. Ogawa, Chem. Lett., 2018, 47, 189–191 CrossRef CAS
.
- K. Uchida, A. Takata, M. Saito, A. Murakami, S. Nakamura and M. Irie, Adv. Mater., 2003, 15, 785–788 CrossRef CAS
.
- M. Levitus and P. F. Aramendía, J. Phys. Chem. B, 1999, 103, 1864–1870 CrossRef CAS
.
- G. Berkovic, V. Krongauz and V. Weiss, Chem. Rev., 2000, 100, 1741–1753 CrossRef CAS PubMed
.
- C. Tan, X. Cao, X. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han, G. Nam, M. Sindoro and H. Zhang, Chem. Rev., 2017, 117, 6225–6331 CrossRef CAS PubMed
.
- Y. Chen, Z. Fan, Z. Zhang, W. Niu, C. Li, N. Yang, B. Chen and H. Zhang, Chem. Rev., 2018, 118, 6409–6455 CrossRef CAS PubMed
.
- C. Tan, Z. Lai and H. Zhang, Adv. Mater., 2017, 29, 1701392 CrossRef PubMed
.
- H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng and S. Qiao, Chem. Rev., 2018, 118, 6337–6408 CrossRef CAS PubMed
.
- J. Wan, S. D. Lacey, J. Dai, W. Bao, M. S. Fuhrer and L. Hu, Chem. Soc. Rev., 2016, 45, 6742–6765 RSC
.
- D. Chimene, D. L. Alge and A. K. Gaharwar, Adv. Mater., 2015, 27, 7261–7284 CrossRef CAS PubMed
.
- J. Kang, V. K. Sangwan, J. D. Wood and M. C. Hersam, Acc. Chem. Res., 2017, 50, 943–951 CrossRef CAS PubMed
.
- K. Shehzad, Y. Xu, C. Gao and X. Duan, Chem. Soc. Rev., 2016, 45, 5541–5588 RSC
.
- B. N. Narayanan, R. Koodathil, T. Gangadharan, Z. Yaakob, F. K. Saidu and S. Chandralayam, Mater. Sci. Eng., B, 2010, 168, 242–244 CrossRef CAS
.
- J. Shi, H. Liu, Z. Lou, Y. Zhang, Y. Meng, Q. Zeng and M. Yang, Comput.
Mater. Sci., 2013, 69, 95–99 CrossRef CAS
.
- Y. Xi, R. L. Frost and H. He, J. Colloid Interface Sci., 2007, 305, 150–158 CrossRef CAS PubMed
.
- W. Xie, Z. Gao, W. Pan, D. Hunter, A. Singh and R. Vaia, Chem. Mater., 2001, 13, 2979–2990 CrossRef CAS
.
- N. Saso, T. Yamamoto, Y. Umemura and Y. Einaga, Colloids Surf., A, 2008, 317, 309–315 CrossRef CAS
.
- K. Takagi, T. Kurematsu and Y. Sawaki, J. Chem. Soc., Perkin Trans. 2, 1995, 1667–1671 RSC
.
- K. Kinashi, H. Kita, M. Misaki, Y. Koshiba, K. Ishida, Y. Ueda and M. Ishihara, Thin Solid Films, 2009, 518, 651–655 CrossRef CAS
.
- T. Seki and K. Ichimura, Macromolecules, 1990, 23, 31–35 CrossRef CAS
.
- H. Tomioka and T. ltoh, J. Chem. Soc., Chem. Commun., 1991, 532–5533 RSC
.
- H. Nishikiori, R. Sasai, K. Takagi and T. Fujii, Langmuir, 2006, 22, 3376–3380 CrossRef CAS PubMed
.
- C. Sun, K. Arimitsu, K. Abe, T. Ohkubo, T. Yamashita, H. Sakai and M. Abe, Mater. Technol., 2004, 22, 229–237 CAS
.
- Y. Okahata and A. Shimizu, Langmuir, 1989, 5, 954–959 CrossRef CAS
.
- T. Xiao, Q. Liu, Q. Zhang, Z. Liu and J. Zhai, J. Phys. Chem. C, 2017, 121, 18954–18961 CrossRef CAS
.
- H. Yao, Z. Tan, H. Fang and S. Yu, Angew. Chem., Int. Ed., 2010, 49, 10127–10131 CrossRef CAS PubMed
.
- H. He, Y. Ma, J. Zhu, P. Yuan and Y. Qing, Appl. Clay Sci., 2010, 48, 67–72 CrossRef CAS
.
- H. Sun, J. Zhang, L. Li, J. Xu and D. Sun, Colloids Surf., A, 2013, 426, 26–32 CrossRef CAS
.
- H. Sakai, H. Ebana, K. Sakai, K. Tsuchiya, T. Ohkubo and M. Abe, J. Colloid Interface Sci., 2007, 316, 1027–1030 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Photochromism of SP-6 aqueous solution; layered montmorillonite nanosheets; 1H NMR and mass spectrum of SP-6. See DOI: 10.1039/c9ra01480b |
| ‡ Mengke Gan and Tianliang Xiao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |