Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Factors that determine thione(thiol)–disulfide interconversion in a bis(thiosemicarbazone) copper(II) complex†
Haewon Jeonga,
Yeji Kanga,
Jin Kimb,
Byung-Kwon Kim *a and
Seungwoo Hong
*a and
Seungwoo Hong *a
*a
aDepartment of Chemistry, Sookmyung Women's University, Seoul 04310, Korea. E-mail: kimbk@sookmyung.ac.kr; hsw@sm.ac.kr
bWestern Seoul Centre, Korea Basic Science Institute, Seoul 03759, Republic of Korea
First published on 19th March 2019
Abstract
Solvent-, acidity-, and redox-responsive thione(thiol)–disulfide interconversion were achieved by a dinuclear copper(II) complex bearing a bis(thiosemicarbazone) (bTSC) ligand. The role of copper(II) ion coordination was rationalized by parallel comparison with a bare bTSC ligand and zinc(II) bTSC complexes under identical reaction conditions.
The chalcogen elements such as oxygen, sulfur, and selenium are of substantial biological and technological relevance due to their tremendous potential for industrial and biomimetic applications. In biological systems, thione(thiol)–disulfide exchange reactions play a critical role in a myriad of enzymatic machinery for cellular functions including redox activity, oxidative protein folding, DNA repair/expression, and apoptosis.1 In particular, S-based amino acids such as cysteine and methionine provide a rich redox chemistry by spanning fractional oxidation states up to ten (e.g., from +6 to −2).2 Disulfides are also employed as key compounds in many chemical processes such as a protecting group in organic synthesis, vulcanizing agents for rubber and a sulphenylating agent for enolates and anions.3 Therefore, understanding factors that control the reversible S–S bond cleavage and formation has come to be a prominent challenge in diverse research fields.
Although extensive mechanistic studies on thiol–disulfide exchange have been established for use in dynamic combinatorial chemistry,4 thione–disulfide exchange has been less explored. In this context, the use of thiosemicarbazones (TSCs) that contain a carbothioamide group would be an attractive mechanistic blueprint to provide information regarding thione–disulfide interconversion.
In this work, we explored bis(thiosemicarbazone) (bTSC) as a chelating ligand of copper(II) and zinc(II) ions to probe the thione–disulfide interconversion mechanism. These complexes are observed to control the scission and formation of the S–S bond, promoted by (i) solvent system, (ii) acid–base treatments, and (iii) redox processes with the help of the copper(II) ion coordination (Scheme 1).
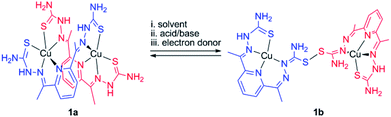 | ||
| Scheme 1 Plausible outline for thione–disulfide interconversion by a dinuclear copper(II) bis(thiosemicarbazone) complex. | ||
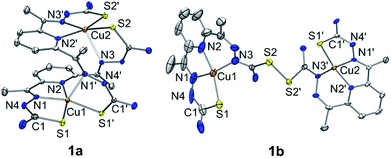
A green dimeric copper(II) complex bearing the bTSC ligand (H2N3S2), [Cu(H2N3S2)2](CH3CN)(CF3SO3)4 (1a), was prepared by following modified literature procedure,5 and characterized by various spectroscopic methods such as UV-vis and electron paramagnetic resonance (EPR) spectroscopies, electrospray ionization mass spectrometry (ESI MS), and X-ray crystallography (details can be found in ESI†). UV-vis spectrum of 1a in CH3CN exhibited an intense charge transfer band at 281 nm (ε = 5.5 × 104 M−1 cm−1) and two broad d–d transition at 580 nm (ε = 450 M−1 cm−1) and 690 nm (ε = 390 M−1 cm−1) (Fig. S1a, ESI†). An ESI MS spectrum of 1a exhibited one prominent peak at m/z of 890.9, whose mass and isotopic distribution patterns correspond to {[Cu2(H2N3S2)2](CF3SO3)}+ (calculated m/z of 891.0) (Fig. S1b, ESI†). An EPR spectrum of 1a clearly showed the presence of a copper(II) ion with g value of 2.02 (Fig. S2a, ESI†). The X-ray crystal structure of 1a revealed a dinuclear copper complex with helical structure due to two rotations around the symmetrical C–C bond adjacent to the pyridine ring (Fig. 1, Table S1 and S4, ESI†). It has been well-documented that the rotational flexibility of bTSC ligand afforded different configurations with multiple binding modes.6 This configuration mode provokes that each copper(II) center is in a distorted square pyramidal N3S2 environment. The average C–S bond length of 1.711(4) Å in bTSC ligand probably retain a partial double bond character (e.g., the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond length of 1.672–1.700 Å in pyridine-2-(1H)-thione vs. the average C–S bond length of 1.8 Å in typical thiol group).7
S bond length of 1.672–1.700 Å in pyridine-2-(1H)-thione vs. the average C–S bond length of 1.8 Å in typical thiol group).7
When an isolated green solid 1a was dissolved in CH3OH, a brown colour solution was obtained. UV-vis spectrum of the new brown complex, denoted as 1b, displayed red-shifted electronic absorption band at 306 nm (ε = 5.8 × 104 M−1 cm−1), and blue-shifted d–d bands 419 nm (ε = 1.1 × 104 M−1 cm−1) and 540 nm (ε = 510 M−1 cm−1) (Fig. S1a, ESI†). An EPR spectrum of 1b clearly showed the presence of a copper(II) ion with g value of 1.98 (Fig. S2b, ESI†). Titration experiment was performed by adding aliquot amount of CH3OH into the CH3CN-solution containing 1a; a clean conversion from 1a to 1b was monitored by UV-vis spectroscopy (Fig. S3, ESI†). Very interestingly, the molecular structure of [Cu(HN3S2)2](CH3OH)(CF3SO3)4 (1b) revealed that two bTSC ligands were bridged through a S–S bond and each copper(II) center exhibited slightly distorted square planar geometry (Fig. 1, Table S1 and S4†). The S–S bond length of 2.069(3) Å is in a good agreement with reported disulfide bond length (Fig. 1 and Table S4†).8 Considering the elongation of C1–S2 bond length (e.g., from 1.712(5) to 1.799(5) Å) along with the shortening of C1–N3 bond (e.g., from 1.361(7) to 1.327(9) Å), the S–S bond formation occurred at the expense of double bond rearrangement from initial C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond to C
S bond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. In addition, when the isolated 1b was dissolved in CH3CN, the spectroscopic feature was identical to 1a (Fig. S1†). In fact, CH3OH might act as a base when 1a was dissolved in CH3OH since CH3OH is amphoteric. The deprotonation of hydrazino moiety of thiosemicarbazone engendered thiolate anion via double bond rearrangement and the nucleophilic attack of thiolate anion on the sulfur atom of another thiosemicarbazone resulted in the disulfide bond formation.4 Therefore, a quantitative solvent-responsive thione–disulfide interconversion was evidenced by changing solvent system.
N bond. In addition, when the isolated 1b was dissolved in CH3CN, the spectroscopic feature was identical to 1a (Fig. S1†). In fact, CH3OH might act as a base when 1a was dissolved in CH3OH since CH3OH is amphoteric. The deprotonation of hydrazino moiety of thiosemicarbazone engendered thiolate anion via double bond rearrangement and the nucleophilic attack of thiolate anion on the sulfur atom of another thiosemicarbazone resulted in the disulfide bond formation.4 Therefore, a quantitative solvent-responsive thione–disulfide interconversion was evidenced by changing solvent system.
Intrigued by the S–S bond scission and formation within bTSC ligand system, we attempted to scrutinize other exogenous factors that directly affect the thione–disulfide interconversion reaction. Given the fact that the disulfide bond formation of 1a occurred along with the double bond rearrangement via a deprotonation of hydrazino moiety in bTSC ligand, the deprotonation of 1a could presumably be a possible synthetic route for the formation of disulfide bond. Indeed, it is well-established that in thione–thiol tautomerism,9 thione form prevails in neutral and acidic media while the equilibrium shifts toward thiol in alkaline medium. Taken together, thione containing ligands might be susceptible to undergo the disulfide bond formation unless they could shift the equilibrium toward thiol via proton transfer (e.g., pKa of thione ligands) and double bond rearrangement.
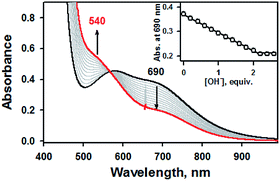
Addition of 2.2 equiv. of KOH to the reaction solution of 1a in CH3CN resulted in the immediate solution colour changes from green to brown, indicating the immediate conversion of 1a to 1b. (Fig. S4, ESI†). Titration experiment clearly suggested the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 deprotonation of 1a (Fig. 2). Remarkably, further addition of 3.0 equiv. of HClO4 to the reaction solution containing 1b led to a quantitative recover of 1a (Fig. S4, ESI†). This interconversion cycle could be repeated several times. Thus, 1a and 1b can be readily interconverted through the acid–base chemistry in plausible consequence of thione–thiol equilibrium shift.
2 deprotonation of 1a (Fig. 2). Remarkably, further addition of 3.0 equiv. of HClO4 to the reaction solution containing 1b led to a quantitative recover of 1a (Fig. S4, ESI†). This interconversion cycle could be repeated several times. Thus, 1a and 1b can be readily interconverted through the acid–base chemistry in plausible consequence of thione–thiol equilibrium shift.
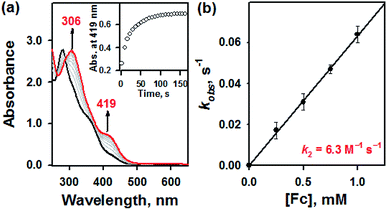
Furthermore, it has been well-documented that the redox processes are involved in the thiol–disulfide exchange in biological system.10 For instance, oxidative protein folding due to the transformation of glutathione (GSH) to glutathione disulfide (GSSG) has extensively examined by in vivo studies.10 To examine this redox-induced thiol(thione)–disulfide exchange, we examined the electrochemical properties of 1a and 1b. In electron transfer reaction, 1a disappeared with a first-order rate profile with a concomitant formation of 1b upon addition of ferrocene (Fc, Eox = 0.37 V vs. SCE); a second-order rate constant (k2) of 6.3 M−1 s−1 was obtained at 10 °C (Fig. 3). This result indicated that one-electron reduction potential of 1a is between above 0.37 V vs. SCE. This was also confirmed by cyclic voltammetry, providing that the Ered of 1a is 0.52 V vs. SCE (Fig. S5, ESI†). Reasonably, the presence of electron source might facilitate the thiol formation over the thione within bTSC ligand. Taken together, by virtue of the copper(II) ion coordination combined with three exogenous factors including solvent, acid/base, and electron donor, thione was converted to thiol prior to the S–S bond formation. Indeed, it has been reported that chalcogenone ligands could be converted to the corresponding dichalcogenide ligands in the presence of copper(II) ion.11 This phenomenon is greatly relevant to the co-existence of copper(II) ion and GSH in the biological system since it has been proposed that the tight control of the copper(II)–GSH complex and the depletion of GSH level could prevent the copper-dependent DNA damage and certain diseases such as Wilson's disease and rheumatoid arthritis.12
To scrutinize the role of copper, we explored the thione–disulfide exchange reactions by using a bare bTSC ligand and a zinc(II) complex bearing the bTSC ligand serving as a redox-inactive metal coordinating complex. When a bare bTSC ligand was dissolved in different solvents such as CH3CN and CH3OH, we did not observe any distinct features (Fig. S6a, ESI†). Furthermore. the thione–disulfide exchange did not upon neither direct treatment of an acid/base or an electron donor into a solution containing the bTSC ligand (Fig. S6b and S6c, ESI†). Therefore, the sole bTSC ligand without the copper(II) ion coordination could not the reversible thione–disulfide interconversion.
The zinc(II) complexes bearing the bTSC ligand were prepared in both CH3CN and CH3OH, denoted as 2a and 2b, respectively (details can be found in ESI†). UV-vis spectra of 2a and 2b displayed characteristic bands at 272 nm (ε = 2.7 × 104 M−1 cm−1) in CH3CN and 278 nm (ε = 2.7 × 104 M−1 cm−1) in CH3OH, respectively (Fig. S7, ESI†). The solid-state X-ray structures of 2a and 2b revealed completely different geometries; while 2a presented a sulfur-bridged dinuclear zinc(II) complex in a distorted pentagonal geometry, 2b was pentagonal bipyramidal geometry (Fig. 4, Table S3 and S5 ESI†). When an isolated solid 2a was dissolved in CH3OH, the spectroscopic feature was identical to 2b, and vice versa (Fig. S7, ESI†). Thus, a solvent-responsive thione–disulfide exchange reaction did not occur when a zinc(II) ion was coordinated to bTSC ligand.
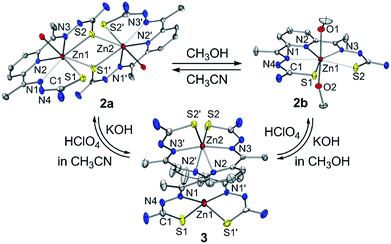 | ||
| Fig. 4 X-ray crystal structures of solvent- and acidity-dependent zinc(II) bis(thiosemicarbazone) complexes with thermal ellipsoids showing 50% probability. | ||
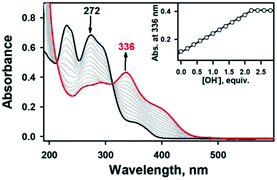
To aid comparison, acidity-, and redox-responsive thione–disulfide exchange reaction in the identical reaction conditions were also reassessed. Addition of 2.2 equiv. of KOH to either yellow solutions of 2a or 2b afforded an identical deep yellow complex. The deprotonated complex, denoted as 3, displayed characteristic absorption bands at 336 nm (ε = 3.5 × 104 M−1 cm−1) and a shoulder at 400 nm (ε = 1.5 × 104 M−1 cm−1) (Fig. S8, ESI†). Titration experiment clearly suggested the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 deprotonation of 2a (Fig. 5). While 1H NMR spectrum of 2a showed a clear signal at 10.3 ppm, which is indicative of NH at hydrazino moiety within bTSC ligand, 3 did not show any NH signal indicating the deprotonation at hydrazino moiety (Fig. S9, ESI†). Furthermore, the molecular structure of 3 revealed a dimeric complex with the elongation of C–S bonds length from 1.695(4) to 1.740(3) Å due to the double bond rearrangement (Fig. 4, Table S3 and S5, ESI†); this is consistent with the dimeric zinc(II) complex having the C–S single bond character (e.g., bis(thiosemicarbazonato) ligand). Interestingly, upon addition of 3.0 equiv. of HClO4, 3 rapidly reverts back to 2a in CH3CN or to 2b in CH3OH, suggesting that (i) 3 and 2 can be readily interconverted through the acid–base reaction and (ii) the acid–base equilibria are also solvent dependent (Fig. 4 and S8, ESI†). We further attempted to introduce an electron donor into the solution of 2a and 3 in order to promote S–S bond formation, however, thione–disulfide exchange did not take place (Fig. S10, ESI†). On the basis of these series of control experiments by using the bTSC ligand and a zinc(II) complex bearing bTSC ligand, a copper(II) ion coordination might be preceded by other exogenous factors such as solvent, acidity of media and redox process.
2 deprotonation of 2a (Fig. 5). While 1H NMR spectrum of 2a showed a clear signal at 10.3 ppm, which is indicative of NH at hydrazino moiety within bTSC ligand, 3 did not show any NH signal indicating the deprotonation at hydrazino moiety (Fig. S9, ESI†). Furthermore, the molecular structure of 3 revealed a dimeric complex with the elongation of C–S bonds length from 1.695(4) to 1.740(3) Å due to the double bond rearrangement (Fig. 4, Table S3 and S5, ESI†); this is consistent with the dimeric zinc(II) complex having the C–S single bond character (e.g., bis(thiosemicarbazonato) ligand). Interestingly, upon addition of 3.0 equiv. of HClO4, 3 rapidly reverts back to 2a in CH3CN or to 2b in CH3OH, suggesting that (i) 3 and 2 can be readily interconverted through the acid–base reaction and (ii) the acid–base equilibria are also solvent dependent (Fig. 4 and S8, ESI†). We further attempted to introduce an electron donor into the solution of 2a and 3 in order to promote S–S bond formation, however, thione–disulfide exchange did not take place (Fig. S10, ESI†). On the basis of these series of control experiments by using the bTSC ligand and a zinc(II) complex bearing bTSC ligand, a copper(II) ion coordination might be preceded by other exogenous factors such as solvent, acidity of media and redox process.
To summarize, we have successfully synthesized and characterized copper(II) and zinc(II) complexes bearing bTSC ligand. Given the presence of thione group on the bTSC ligand, this synthetic approach facilitates direct investigations on the thione–disulfide interconversion among bare bTSC ligand, copper(II), and zinc(II) complexes bearing bTSC ligand as a function of exogenous factors such as solvent, acid/base, and electron donor. As demonstrated above, thione–disulfide interconversion can be modulated by several exogenous factors accompanied by the copper(II) ion coordination.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NRF of Korea through MSIP (NRF-2017R1C1B2002037 to S. H. and NRF-2018R1C1B6008668 to B. K.).Notes and references
- A. Pompella, A. Bahegyi and M. Wellman-Rousseau, Thiol Metabolism and Redox Regulation of Cellular Functions, IOS Press, Amsterdam, 2002, pp. 1–348 CrossRef CAS PubMed; E. M. Allen and J. J. Mieyal, Antioxid. Redox Signaling, 2012, 17, 1748 CrossRef CAS PubMed; L. E. S. Netto, M. A. de Oliveira, C. A. Tairum and J. F. da Silva Neto, Free Radical Res., 2016, 50, 206 CrossRef PubMed; A. A. Ukuwela, A. I. Bush, A. G. Wedd and Z. Xiao, Chem. Sci., 2018, 9, 1173 RSC.
- Y. M. Torchinsky, Sulfur in Proteins, Pergamon, Oxford, 1981, pp. 1–277 CrossRef CAS PubMed; H. Beinert, R. H. Holm and E. Münck, Science, 1997, 277, 653 CrossRef CAS PubMed.
- S. Oae, Organic Sulfur Chemistry: Structure and Mechanism, Boca Raton, Florida, 1991, pp. 1–433 CrossRef CAS; R. J. Cremlyn, An Introduction to Organosulfur Chemistry, New York, Wiley and Sons, 1996, pp. 1–262 CrossRef CAS; L. Bischoff, C. David, L. Martin, H. Meudal, B.-P. Roques and M.-C. Fournié-Zaluski, J. Org. Chem., 1997, 62, 4848 CrossRef CAS; A. M. Joseph, B. George, K. N. Madhusoodanan and R. Alex, Rubber Sci., 2015, 28, 82 Search PubMed.
- W. J. Lees and G. M. Whitesides, J. Org. Chem., 1993, 58, 642 CrossRef CAS; S. Otto, R. L. E. Furlan and J. K. M. Sanders, Science, 2002, 297, 590 CrossRef PubMed; A. R. Stefankiewicz and J. K. M. Sanders, Chem. Commun., 2013, 49, 5820 RSC; S. P. Black, J. K. M. Sanders and A. R. Stefankiewicz, Chem. Soc. Rev., 2014, 43, 1861 RSC; W. Drożdż, M. Kolodziejski, G. Markiewicz, A. Jenczak and A. R. Stefankiewicz, Int. J. Mol. Sci., 2015, 16, 16300 CrossRef PubMed; M. Mondal and A. K. Hirsch, Chem. Soc. Rev., 2015, 44, 2455 RSC; Y. Altay, M. Tezcan and S. Otto, J. Am. Chem. Soc., 2017, 139, 13612 CrossRef PubMed; S. Sobczak, W. Drożdż, G. Lampronti, A. Belenguer, A. Katrusiak and A. R. Stefankiewicz, Chem.–Eur. J., 2018, 24, 8769 CrossRef PubMed; P. Frei, R. Hevey and B. Ernst, Chem.–Eur. J., 2019, 25, 60 Search PubMed.
- A. I. Matesanz, I. Cuadrado, C. Pastor and P. Souza, Z. Anorg. Allg. Chem., 2005, 631, 780 CrossRef CAS; D. Dayal, D. Palanimuthu, S. V. Shinde, K. Somasundaram and A. G. Samuelson, J. Biol. Inorg Chem., 2011, 16, 621 CrossRef PubMed.
- R. Pedrido, M. R. Bermejo, M. J. Romero, M. Vázquez, A. M. González-Noya, M. Maneiro, M. J. Rodríguez and M. I. Fernández, Dalton Trans., 2005, 572 RSC; S. A. Hosseini-Yazdi, S. Hosseinpour, A. A. Khandar, W. S. Kassel and N. A. Piro, Inorg. Chim. Acta, 2015, 427, 124 CrossRef CAS.
- P. Souza, A. Arquero, A. Garcia-Onrubia, V. Fernández, A. M. Leiva and U. Müller, Z. Naturforsch., 1989, 44b, 946 Search PubMed; T. S. Lobana, P. K. Bhatia and E. R. T. Tiekink, J. Chem. Soc., Dalton Trans., 1989, 749 RSC.
- M. M. Kimani, H. C. Wang and J. L. Brumaghim, Dalton Trans., 2012, 41, 5248 RSC; F. Jiang, M. A. Siegler, X. Sun, L. Jiang, C. F. Guerra and E. Bouwman, Inorg. Chem., 2018, 57, 8796 CrossRef CAS PubMed.
- S. Stoyanov, I. Petkov, L. Antonov, T. Stoyanova, P. Karagiannidis and P. Aslanidis, Can. J. Chem., 1990, 68, 1482 CrossRef CAS.
- E. E. Reid, Organic Chemistry of Bivalent Sulfur, Chemical Publication Co., New York, vol. III, 1960 CrossRef CAS; C. Hwang, A. J. Sinskey and H. F. Lodish, Science, 1992, 257, 1496 CrossRef CAS; W. J. Lees, Curr. Opin. Chem. Biol., 2008, 12, 740 CrossRef PubMed; P. I. Merksamer, A. Trusina and F. R. Papa, Cell, 2008, 135, 933 CrossRef PubMed.
- M. M. Kimani, H. C. Wang and J. L. Brumaghim, Dalton Trans., 2012, 41, 5248 RSC.
- J. M. Walshe, Am. J. Med., 1956, 21, 487 CrossRef CAS PubMed; A. Deiss, G. R. Lee and G. E. Cartwright, Ann. Intern. Med., 1970, 73, 413 CrossRef PubMed; I. A. Jaffe, Arthritis Rheum., 1970, 13, 436 CrossRef PubMed; R. Osterberg, R. Ligaarden and D. Persson, J. Inorg. Biochem., 1979, 10, 341 CrossRef; M. M. Jones, Crit. Rev. Toxicol., 1991, 21, 209 CrossRef PubMed; P. M. Hanna and R. P. Mason, Arch. Biochem. Biophys., 1992, 295, 205 CrossRef; L. Milne, P. Nicotera, S. Orrenius and M. J. Burkitt, Arch. Biochem. Biophys., 1993, 304, 102 CrossRef PubMed; N. Spear and S. D. Aust, Arch. Biochem. Biophys., 1995, 317, 142 CrossRef PubMed; I. Jiménez and H. Speisky, J. Trace Elem. Med. Biol., 2000, 14, 161 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and X-ray crystallographic data. CCDC 1895981–1895985. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra01115c |
| This journal is © The Royal Society of Chemistry 2019 |