Open Access Article
Open Access ArticleRoom-temperature solution synthesis of ZnMn2O4 nanoparticles for advanced electrochemical lithium storage†
Chunhui Wang‡
a,
Chunxian Zhou‡a,
Bao Zhanga,
Xing Ou *a,
Liang Caoa,
Chunli Pengb and
Jiafeng Zhanga
*a,
Liang Caoa,
Chunli Pengb and
Jiafeng Zhanga
aSchool of Metallurgy and Environment, Central South University, Changsha 410083, PR China. E-mail: ouxing@csu.edu.cn
bSchool of Energy Science and Engineering, Central South University, Changsha 410083, PR China
First published on 19th March 2019
Abstract
Taking advantage of synergistic effects, the ternary metal oxides have attracted tremendous interest. Herein, ZnMn2O4 nano-particles have been fabricated via a facile one-step approach at room temperature, that of simply mixing ZnO and MnO in KOH aqueous solution without templates. When used as an anode for lithium ion batteries, it delivers the excellent structure stability (1028.9 mA h g−1 at 1.0 A g−1 after 400 cycles). Surprisingly, the low-cost and eco-friendly route provides a novel strategy to synthesize the mixed transition metal oxide electrodes with readily scaled-up production.
Presently, the energy density of lithium-ion batteries (LIBs) needs urgently to be improved to meet the market development, which is principally restricted by the current commercial graphite anode materials (≈372 mA h g−1).1,2 Hence, enormous efforts have been implemented to exploit anode materials with high capacity, such as metal oxide/sulfide,3–5 phosphide,6 Si-based compounds.7 In consideration of their nontoxicity, low cost and high energy density, transition metal oxides (TMOs) have attracted extraordinary attention, owing to their high theoretical reversible capacity, which is twice as high as that of graphite.8–10 Especially for ternary TMOs, they are considered as the most promising candidates for LIBs owing to their higher electrochemical activity and better ion/electron conductivity induced by their synergistic effects, compared with their corresponding single metal oxides.11,12
Among the numerous ternary TMOs, ZnMn2O4 are particularly attractive and extensively investigated, as it can offer richer redox reactions and display a relatively lower delithiation potential of 0.5 V than those of single-component oxides vs. Li/Li+ (ZnO, 1.2 V and MnO, 1.5 V).13,14 However, ZnMn2O4 will suffer from the unavoidable volume expansion, resulting in the detrimental structural collapse upon long-term lithiation/delithiation process.15 Generally, preparing the nano-size electrode is an effective approach to mitigate the intrinsical issue, which can enhance the electrochemical performance and stabilize the microstructure. While the achievement of nano-structured materials is heavily depended on the synthetic methods. For instance, flower-like ZnMn2O4 is synthesized by solvothermal process, and it displays an initial charge capacity of about 763 mA h g−1 and retains stable performance after 50 cycles.16 One-dimensional ZnMn2O4 nanowires have been fabricated by mixing α-MnO2 and Zn(CH3COO)2 under high temperature calcination at 480 °C in O2 atmosphere. However, the synthesis procedure introduces the template of precursor of α-MnO2 nanowires, which are complicated to obtain by hydrothermal approach.17 Besides, the ball-in-ball hollow microspheres of ZnMn2O4 electrode can only be manufactured by liquid phase method with reflux condensation and post-thermal treatment.18 Unfortunately, although the nanosized ZnMn2O4 prepared by the above studies deliver a good electrochemistry property, the operational process is fairly complex and the productivity is relatively low, hindering the further development of ZnMn2O4 anodes. Therefore, it is urgent to explore a novel and highly efficient synthetic method to fabricate nanosized ZnMn2O4 with improved electrochemical performance.
Herein, one-step room-temperature synthesis of ZnMn2O4 nano-particles based on facile KOH solution system and low-cost precursor materials has been designed and presented in this investigation. The synthesized ZnMn2O4 anode exhibits satisfied electrochemical properties in regard of high reversible capacity and excellent cycling stability. Compared with previously reported ZnMn2O4 materials synthesized at elevated temperature, it is facile, up-scalable and high-efficient for our work, which provide a new strategy to realize the large fabrication of other TMOs, such as ZnFe2O4, ZnCo2O4.
The ZnMn2O4 nano-particles were synthesized through a facile, low cost route in one-step process, which is depicted in Fig. 1a. In the basic medium of KOH aqueous solution, MnO could be slowly oxidized to Mn3O4 (Fig. S3†) by O2. Meanwhile, with the presence of the Zn2+, the ZnMn2O4 could be prepared at same time in oxidization.19 As displayed in the SEM image of ZnMn2O4 materials (Fig. 1b), the nano-sized particles with dimension of 20–30 nm get aggregation into bulk morphology, which is obviously different from the pristine samples (Fig. S1†) and is also confirmed by TEM (Fig. 1c). Predictably, after mixing the precursors in the solution process, the ZnMn2O4 materials are synthesized, which indicate that the approach is feasible and is confirmed by the following measurements of HRTEM and XRD. From the HRTEM image (inset of Fig. 1c), it can be clearly observed the interplanar spacing of 0.271 nm, well matching with the (103) planes of ZnMn2O4 phase, indicating the high crystallization of as-prepared ZnMn2O4. Moreover, the corresponding SAED pattern (Fig. 1d) is well indexed to the crystal planes of (211), (220), and (312) of ZnMn2O4, demonstrating the polycrystalline characters by the clear diffraction rings. The elemental mapping images (Fig. 1e) ascertains that the electrode is consisted of Zn, Mn and O with evenly distributed, and the molar ratio of Zn and Mn is approaching 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 1f), confirming the formation of ZnMn2O4.
2 (Fig. 1f), confirming the formation of ZnMn2O4.
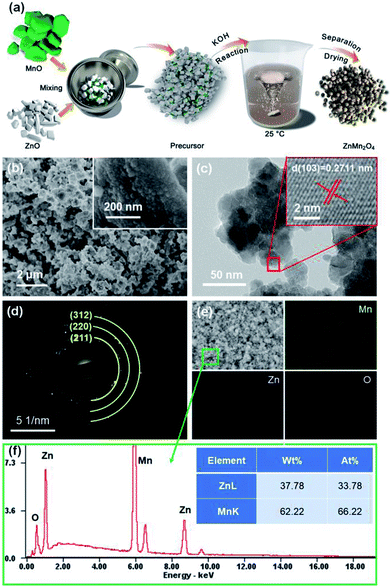 | ||
| Fig. 1 (a) The schematic illustration of the fabrication procedure of ZnMn2O4; the SEM (b), TEM (c), SAED pattern (d), mapping profile (e) and the EDS spectroscopy pattern (f). | ||
The XRD pattern of the ZnMn2O4 nanoparticles is shown in Fig. 2a, which can be well indexed into the tetragonal structure of ZnMn2O4 with a space group of I41/amd (141) (JCPDS no. 24–1133). Meanwhile, as presented in the Raman spectra (Fig. 2b), it exhibits three obvious peaks located at 326, 369 and 671 cm−1, which is consistent with the typical vibration modes of ZnMn2O4.20 The peak at 671 cm−1 is corresponding to the oxygen motion in the tetrahedral AO4 group with A1g symmetry, and the other two modes are characteristic of the octahedral site (BO6).21 The valence state and chemical composition are analysed by XPS (Fig. 2c and d). The full spectrum displays four elements, Zn, Mn, O, and C in Fig. 2c and S3,† and the C 1s spectrum (282.8 eV) is assigned to carbonate materials existed in the XPS instrument. The Mn 2p peak can be split into two peaks at 653.4 and 641.7 eV with an energy margin of 11.7 eV, associated to the Mn 2p1/2 and 2p3/2, respectively, which confirms the oxidation state of Mn3+ in the ZnMn2O4 nanoparticles.22,23
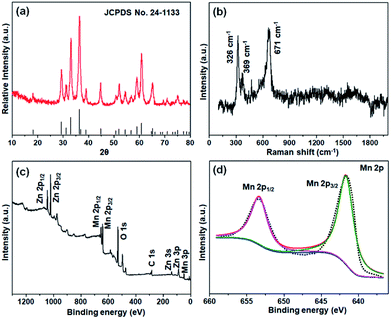 | ||
| Fig. 2 The results of XRD (a), Raman spectra (b), XPS full spectrum (c) and the high-resolution spectrum of Mn 2p (d). | ||
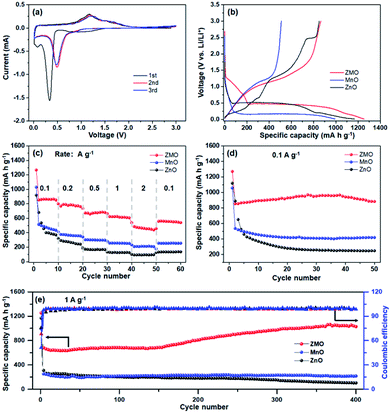
To evaluate the lithium storage performance of ZnMn2O4, various electrochemical measurements were conducted as anodes in the coin cells. Cyclic voltammetry (CV) test at a rate of 0.1 mV s−1 in potential range of 0.01–3.0 V is employed to explore the lithium-ion reaction mechanism of ZnMn2O4, as shown in Fig. 3a. During the initial cathodic sweep, the first occurred peak at 1.08 V is consistent to the reduction from Mn3+ to Mn2+, and the following peak located at 0.75 V is ascribed to the electrolyte decomposition accompanied with the formation of the solid electrolyte interphase (SEI), respectively, which disappears in the subsequent scans. Meanwhile, the peak at 0.41 V is assigned to the irreversible reduction reaction of Mn2+ and Zn2+ into Zn0 and Mn0 embedded in amorphous Li2O matrix. While the peak at low potential (about 0.08 V) is corresponding to the formation of Li–Zn alloy (Fig. S6†), which is consistent with the earlier CV profiles.16 In the corresponding anodic scan, two oxidation peaks at approximately 1.2 and 1.5 V are associated to the reversible oxidation of Zn0 and Mn0, and formation of ZnO and MnO, respectively.24,25 In the following scans, the reduction peak for conversion reaction shifts from 0.41 V to 0.49 V, ascribing to the small overpotentials induced by smaller particles.22 Furthermore, the following CV profiles almost completely superimposed for each other, suggesting the excellent reversibility and stability of ZnMn2O4 anode.
The first discharge–charge curves of ZnMn2O4 nano-particles (abb. ZMO) and precursor materials (ZnO and MnO) are shown in Fig. 3b, recorded at 0.1 A g−1 in the potential range of 0.01–3.0 V. The as-prepared ZnMn2O4 shows a voltage plateau at 0.43 V, which is in accordance with the CV profiles. While it displays an initial charge capacity of 865.6 mA h g−1 with an acceptable coulombic efficiency (70.1%), higher than those of the ZnO (851.6 mA h g−1) and MnO (509.8 mA h g−1). The rate capability of ZnMn2O4 is demonstrated in Fig. 3c. The ZnMn2O4 anode delivers 863.4, 781.9, 676, 622.7 and 470 mA h g−1 at rate increasing from low current to ultrahigh (0.1, 0.2, 0.5, 1.0 and 2.0 A g−1). When returned back to 0.1 A g−1, it immediately recovered to 555.6 mA h g−1, much higher than that of pristine ZnO and MnO samples, indicating their advanced kinetic properties. The cycling performance of ZnMn2O4 is also superior compared with pristine ZnO and MnO. It is noteworthy that ZnMn2O4 shows the reversible capacity of 884.5 mA h g−1 after 50 cycles at rate of 0.1 A g−1 (Fig. 3d). Furthermore, it maintains a reversible capacity of 1028.9 mA h g−1 after 400 cycles at rate of 1 A g−1, equivalent to capacity retention of 82.08%. Interestingly, it suffers from capacity loss during the initial dozens of cycles, but thereafter, it begins to increase significantly, which is normally found in other TMO-material anodes. This phenomenon is generally attributed to the polymeric gel-like film with continuous and reversible formation during the kinetic activation process of anode.18,26
The EIS profiles of the three samples are Nyquist plots, fitted by the corresponding equivalent circuit as displayed inset of Fig. 4a. The charge-transfer resistance is noted as Rct and Li+ diffusion coefficient is calculated as followed:27,28
| D = (R2T2)/(2A2n4F4C2σ2) | (1) |
| Z′= Rs + Rct + σω−1/2 | (2) |
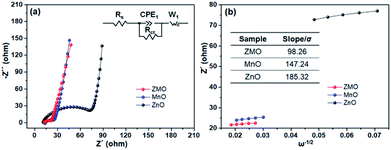 | ||
| Fig. 4 (a) The EIS spectra of all samples and the equivalent electrical circuit (inset), (b) the liner fitting of Z′ vs. ω −1/2 in the low-frequency and the value of σ (inset). | ||
In summary, nano-particles ZnMn2O4 with high yields were synthesized by a facile solution reaction route without template at room temperature. Unlike other materials for amorphous state, the as-prepared ZnMn2O4 exhibits strong crystalline with a nanostructure morphology. Electrochemical measurements demonstrate that the as-prepared ZnMn2O4 exhibits remarkable reversible capacity and cycling stability, superior to those of pristine materials. The facile strategy opens up a new approach to fabricate ternary or multiphase transition metal oxides at room temperature, which can promote the development and keep highly promising to scale-up of transition metal oxides as anodes for LIBs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 51772334, 51778627 and 51822812).Notes and references
- C. Zhu, R. E. Usiskin, Y. Yu and J. Maier, Science, 2017, 358, easo2808 CrossRef PubMed.
- S. Wei, S. Choudhury, Z. Tu, K. Zhang and L. A. Archer, Acc. Chem. Res., 2018, 51, 80–88 CrossRef CAS PubMed.
- C. J. Heard, J. Čejka, M. Opanasenko, P. Nachtigall, G. Centi and S. Perathoner, Adv. Mater., 2018, 1801712 Search PubMed.
- X. Chia and M. Pumera, Chem. Soc. Rev., 2018, 47, 5602–5613 RSC.
- W. H. Ren, D. N. Liu, C. L. Sun, X. H. Yao, J. Tan, C. M. Wang, K. N. Zhao, X. P. Wang, Q. Li and L. Q. Mai, Small, 2018, 14, 1800659 CrossRef PubMed.
- M. Sun, H. Liu, J. Qu and J. Li, Adv. Energy Mater., 2016, 6, 1600087 CrossRef.
- H. Shang, Z. Zuo, L. Yu, F. Wang, F. He and Y. Li, Adv. Mater., 2018, 30, e1801459 CrossRef PubMed.
- M. V. Reddy, G. V. Subba Rao and B. V. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- Y. Zhao, L. P. Wang, M. T. Sougrati, Z. Feng, Y. Leconte, A. Fisher, M. Srinivasan and Z. Xu, Adv. Energy Mater., 2017, 7, 1601424 CrossRef.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2008, 53, 2380–2385 CrossRef CAS.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381–384 CrossRef PubMed.
- Y. Deng, S. Tang, Q. Zhang, Z. Shi, L. Zhang, S. Zhan and G. Chen, J. Mater. Chem., 2011, 21, 11987–11995 RSC.
- Q. Gao, Z. Yuan, L. Dong, G. Wang and X. Yu, Electrochim. Acta, 2018, 270, 417–425 CrossRef CAS.
- M. H. Alfaruqi, A. K. Rai, V. Mathew, J. Jo and J. Kim, Electrochim. Acta, 2015, 151, 558–564 CrossRef CAS.
- L. F. Xiao, Y. Y. Yang, J. Yin, Q. Li and L. Z. Zhang, J. Power Sources, 2009, 194, 1089–1093 CrossRef CAS.
- S.-W. Kim, H.-W. Lee, P. Muralidharan, D.-H. Seo, W.-S. Yoon, D. K. Kim and K. Kang, Nano Res., 2011, 4, 505–510 CrossRef CAS.
- G. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
- B. G. S. Raj, A. M. Asiri, J. J. Wu and S. Anandan, J. Alloy. Compd., 2015, 626, 234–240 CrossRef.
- J. G. Kim, S. H. Lee, Y. Kim and W. B. Kim, ACS Appl. Mater. Interfaces, 2013, 5, 11321–11328 CrossRef CAS PubMed.
- T. Zhang, H. Yue, H. Qiu, K. Zhu, L. Zhang, Y. Wei, F. Du, G. Chen and D. Zhang, RSC Adv., 2015, 5, 99107–99114 RSC.
- X.-F. Chen, L. Qie, L.-L. Zhang, W.-X. Zhang and Y.-H. Huang, J. Alloy. Compd., 2013, 559, 5–10 CrossRef CAS.
- L. Huang, Y. Huang, J. Liang, X. Wan and Y. Chen, Nano Res., 2011, 4, 675–684 CrossRef CAS.
- H. Rong, G. Xie, S. Cheng, Z. Zhen, Z. Jiang, J. Huang, Y. Jiang, B. Chen and Z.-J. Jiang, J. Alloy. Compd., 2016, 679, 231–238 CrossRef CAS.
- Z. Bai, N. Fan, C. Sun, Z. Ju, C. Guo, J. Yang and Y. Qian, Nanoscale, 2013, 5, 2442–2447 RSC.
- L. Hou, L. Lian, L. Zhang, G. Pang, C. Yuan and X. Zhang, Adv. Funct. Mater., 2015, 25, 238–246 CrossRef CAS.
- L. Cao, B. Zhang, X. Ou, C. Wang, C. Peng and J. Zhang, Small, 2019, 15, 1804861 CrossRef PubMed.
- C. Shen, H. Long, G. Wang, W. Lu, L. Shao and K. Xie, J. Mater. Chem. A, 2018, 6, 6007–6014 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, material characterizations, electrochemical characterizations, Fig. S1–S5. See DOI: 10.1039/c9ra00553f |
| ‡ These authors contributed equally and mainly to this study. |
| This journal is © The Royal Society of Chemistry 2019 |