Open Access Article
Open Access ArticleA computational study to determine whether substituents make E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) nitrogen (E13 = B, Al, Ga, In, and Tl) triple bonds synthetically accessible†
nitrogen (E13 = B, Al, Ga, In, and Tl) triple bonds synthetically accessible†
Shi-Lin Zhanga,
Ming-Chung Yanga and
Ming-Der Su *ab
*ab
aDepartment of Applied Chemistry, National Chiayi University, Chiayi 60004, Taiwan. E-mail: midesu@mail.ncyu.edu.tw
bDepartment of Medicinal and Applied Chemistry, Kaohsiung Medical University, Kaohsiung 80708, Taiwan
First published on 17th April 2019
Abstract
This study theoretically determines the effect of substituents on the stability of the triple-bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (E13 = B, Al, Ga, In, and Tl) compound using the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. Five small substituents (F, OH, H, CH3 and SiH3) and four large substituents (SiMe(SitBu3)2, SiiPrDis2, Tbt (
N–L (E13 = B, Al, Ga, In, and Tl) compound using the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. Five small substituents (F, OH, H, CH3 and SiH3) and four large substituents (SiMe(SitBu3)2, SiiPrDis2, Tbt (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H2-2,4,6-{CH(SiMe3)2}3) and Ar* (
C6H2-2,4,6-{CH(SiMe3)2}3) and Ar* (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H3-2,6-(C6H2-2,4,6-i-Pr3)2)) are used. Unlike other triply bonded L–E13
C6H3-2,6-(C6H2-2,4,6-i-Pr3)2)) are used. Unlike other triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P–L, L–E13
P–L, L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) As–L, L–E13
As–L, L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Sb–L and L–E13
Sb–L and L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Bi–L molecules that have been studied, the theoretical findings for this study show that both small (but electropositive) ligands and bulky substituents can effectively stabilize the central E13
Bi–L molecules that have been studied, the theoretical findings for this study show that both small (but electropositive) ligands and bulky substituents can effectively stabilize the central E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond. Nevertheless, these theoretical observations using the natural bond orbital and the natural resonance theory show that the central E13
N triple bond. Nevertheless, these theoretical observations using the natural bond orbital and the natural resonance theory show that the central E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond in these acetylene analogues must be weak, since these E13
N triple bond in these acetylene analogues must be weak, since these E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N compounds with various ligands do not have a real triple bond.
N compounds with various ligands do not have a real triple bond.
1. Introduction
Molecules that feature multiple bonds have been the subject of many studies because of their economic and academic importance.1–49 Recently, molecules containing a L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E15–L (E13 = B, Al, Ga, In, and Tl; E15 = P, As, Sb, and Bi) triple bond, which are isoelectronic to the alkyne analogues R–E14
E15–L (E13 = B, Al, Ga, In, and Tl; E15 = P, As, Sb, and Bi) triple bond, which are isoelectronic to the alkyne analogues R–E14![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E14–R (E14 = C, Si, Ge, Sn and Pb), have been the subject of theoretical study.52–65 This study focuses on the other acetylene analogues; i.e., the triply bonded L–E13
E14–R (E14 = C, Si, Ge, Sn and Pb), have been the subject of theoretical study.52–65 This study focuses on the other acetylene analogues; i.e., the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L compounds that contain group 13 (E13) and nitrogen atoms. As far as the authors are aware, only very few triply bonded compounds that contain a nitrogen element (i.e., L–B
N–L compounds that contain group 13 (E13) and nitrogen atoms. As far as the authors are aware, only very few triply bonded compounds that contain a nitrogen element (i.e., L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L,66–69 L–Ga
N–L,66–69 L–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L,70,71 and L–In
N–L,70,71 and L–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L70) have been successfully synthesized and isolated. No other triple bond molecules containing aluminum (L–Al
N–L70) have been successfully synthesized and isolated. No other triple bond molecules containing aluminum (L–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L) and thallium (L–Tl
N–L) and thallium (L–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L) have been both experimentally and theoretically reported.
N–L) have been both experimentally and theoretically reported.
Although the authors have already published 14 papers concerning group 13 group 15 triple bond molecules,52–65 the present computational evidence demonstrates that the results about the stability of the triply bonded RE13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR compounds are quite different from our previous theoretical examinations. For instance, the theoretical conclusions based on our previous papers show that only the bulky ligands can stabilize the triply bonded L–E13
NR compounds are quite different from our previous theoretical examinations. For instance, the theoretical conclusions based on our previous papers show that only the bulky ligands can stabilize the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E15–L (E15 = P, As, Sb, and Bi) molecules.52–65 Nevertheless, in this work, the authors' computations in this work reveal that both small (but electropositive) ligands and bulky substituents can effectively stabilize the triply bonded L–E13
E15–L (E15 = P, As, Sb, and Bi) molecules.52–65 Nevertheless, in this work, the authors' computations in this work reveal that both small (but electropositive) ligands and bulky substituents can effectively stabilize the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L compounds. In other words, the present theoretical evidences emphasize that both small (but electropositive) substituents and sterically bulky groups can successfully protect the central triple bond, which, in turn, can increase the bond order of this triple bond. Because of the difficulties in experimentally synthesizing these rare triply bonded molecules, this study theoretically determines the effect of substituents on the formation of L–E13
N–L compounds. In other words, the present theoretical evidences emphasize that both small (but electropositive) substituents and sterically bulky groups can successfully protect the central triple bond, which, in turn, can increase the bond order of this triple bond. Because of the difficulties in experimentally synthesizing these rare triply bonded molecules, this study theoretically determines the effect of substituents on the formation of L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L featuring a triple bond. The geometrical structures and associated properties of stable L–E13
N–L featuring a triple bond. The geometrical structures and associated properties of stable L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecules are theoretically predicted. Accordingly, the present work can conduct the experimental chemists how to design and synthesize the triply bonded RE13
N–L molecules are theoretically predicted. Accordingly, the present work can conduct the experimental chemists how to design and synthesize the triply bonded RE13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR compounds using the effective way.
NR compounds using the effective way.
2. General considerations
In order to determine the valence electronic structures of L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L, similarly to our previous studies,50–64 the L–E13
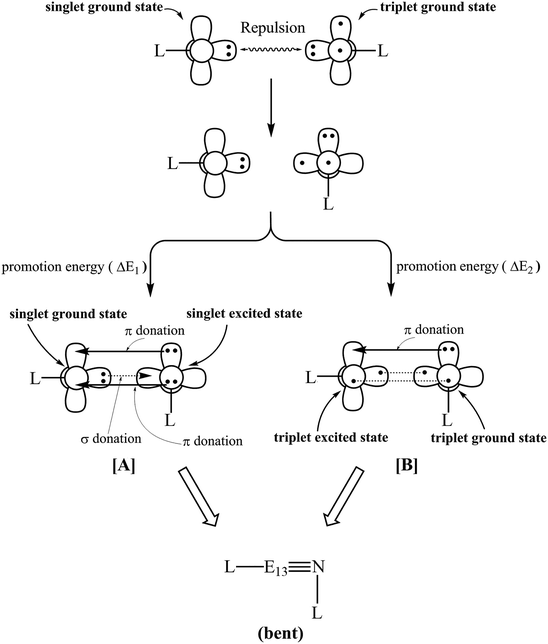
N–L, similarly to our previous studies,50–64 the L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L species is divided into two fragments: L–E13 and L–N. These are shown in Fig. 1.
N–L species is divided into two fragments: L–E13 and L–N. These are shown in Fig. 1.
As seen in Fig. 1, there are two mechanisms for the formation of the L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L triple bond species at the singlet ground state. The choice of mechanism [A] or mechanism [B] respectively depends on the promotion energy of L–N and L–E13 moieties. For mechanism [A], a singlet L–E13 and a singlet L–N combine to yield a singlet L–E13
N–L triple bond species at the singlet ground state. The choice of mechanism [A] or mechanism [B] respectively depends on the promotion energy of L–N and L–E13 moieties. For mechanism [A], a singlet L–E13 and a singlet L–N combine to yield a singlet L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecule, which is named a singlet–singlet bonding ([L–E13]1 + [L–N]1 → [L–E13
N–L molecule, which is named a singlet–singlet bonding ([L–E13]1 + [L–N]1 → [L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L]1). For mechanism [B], a triplet L–E13 and a triplet L–N couple to yield a singlet L–E13
N–L]1). For mechanism [B], a triplet L–E13 and a triplet L–N couple to yield a singlet L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L compound, which is called a triplet–triplet bonding ([L–E13]3 + [L–N]3 → [L–E13
N–L compound, which is called a triplet–triplet bonding ([L–E13]3 + [L–N]3 → [L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L]1).
N–L]1).
The chemical bonding nature of mechanism [A] in Fig. 1 contains three types of chemical bonds: a valence lone pair orbital of E13 → a valence p orbital of N, a valence p orbital of E13 ← a valence lone pair orbital of N, and a valence p orbital of E13 ← a valence p orbital of N. In other words, the E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond features one E13 → N σ donation bond and two E13 ← N π donation bonds. Therefore, the central E13
N triple bond features one E13 → N σ donation bond and two E13 ← N π donation bonds. Therefore, the central E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond in mechanism [A] can be regarded as L–E13
N triple bond in mechanism [A] can be regarded as L–E13 N–L.
N–L.
For mechanism [B], the chemical bonding character of the E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond in Fig. 1 involves three types of chemical bonds: a valence lone pair orbital of E13 — a valence p orbital of N, a valence p orbital of E13 — a valence p orbital of N, and a valence p orbital of E13 ← a valence lone pair orbital of N. The E13
N triple bond in Fig. 1 involves three types of chemical bonds: a valence lone pair orbital of E13 — a valence p orbital of N, a valence p orbital of E13 — a valence p orbital of N, and a valence p orbital of E13 ← a valence lone pair orbital of N. The E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond features one traditional E13–N σ bond, one traditional E13–N π bond and one E13 ← N π donation bond. Therefore, the principal E13
N triple bond features one traditional E13–N σ bond, one traditional E13–N π bond and one E13 ← N π donation bond. Therefore, the principal E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond in mechanism [B] can be described as L–E13
N triple bond in mechanism [B] can be described as L–E13 N–L. The two non-degenerate π bonding orbitals (π⊥ and π‖) for H–B
N–L. The two non-degenerate π bonding orbitals (π⊥ and π‖) for H–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–H are schematically represented in Fig. 2.
N–H are schematically represented in Fig. 2.
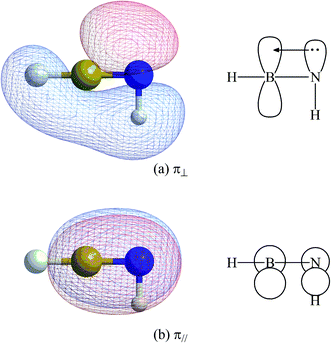 | ||
Fig. 2 The natural B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N π bonding orbitals (π⊥ and π‖ for (a) and (b), respectively) for H–B N π bonding orbitals (π⊥ and π‖ for (a) and (b), respectively) for H–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–H, based on Fig. 1. N–H, based on Fig. 1. | ||
It is noteworthy that, as demonstrated in Fig. 1, the vital bonding in the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L species contributes greatly to the lone pair of the N–L moiety, whose electron pair is donated to the empty p–π orbital of the L–E13 component. In particular, the lone pair orbital of the N–L unit includes the s valence orbital of nitrogen. The atomic size of E13 is also apparently different from that of nitrogen, especially for the E13 elements with a higher atomic number. Therefore, the overlap in the orbital populations between E13 and nitrogen is expected to be small. That is to say, from the overlap population viewpoint, this theoretical analysis anticipates that the triple bond between E13 and N must be weak. This prediction is verified in the following discussion.
N–L species contributes greatly to the lone pair of the N–L moiety, whose electron pair is donated to the empty p–π orbital of the L–E13 component. In particular, the lone pair orbital of the N–L unit includes the s valence orbital of nitrogen. The atomic size of E13 is also apparently different from that of nitrogen, especially for the E13 elements with a higher atomic number. Therefore, the overlap in the orbital populations between E13 and nitrogen is expected to be small. That is to say, from the overlap population viewpoint, this theoretical analysis anticipates that the triple bond between E13 and N must be weak. This prediction is verified in the following discussion.
3. Results and discussion
3.1 Small ligands on substituted L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) N–L
N–L
The effect of small ligands L (=F, OH, H, CH3 and SiH3) on the stability of the triply bonded L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecules is determined. For comparison, three computational methods (M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp), based on density functional theory (DFT), are used to determine the relative stability of the doubly bonded L2B
N–L molecules is determined. For comparison, three computational methods (M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp), based on density functional theory (DFT), are used to determine the relative stability of the doubly bonded L2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: and :B
N: and :B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NL2 and the triply bonded L–B
NL2 and the triply bonded L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L. The calculated potential energy surfaces are schematically shown in Fig. 3.
N–L. The calculated potential energy surfaces are schematically shown in Fig. 3.
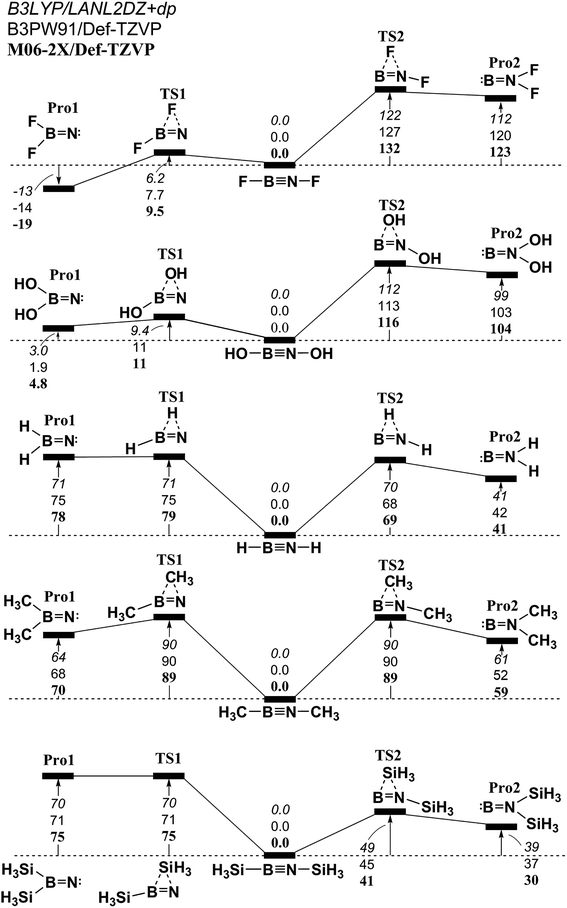 | ||
Fig. 3 The relative Gibbs free energy for L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table 1. N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table 1. | ||
Interestingly, unlike the other L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E15–L molecular systems that have been previously studied,50–64 the theoretical data for this study using three DFT methods suggest that when ligands are small and electropositive, the triply bonded L–B
E15–L molecular systems that have been previously studied,50–64 the theoretical data for this study using three DFT methods suggest that when ligands are small and electropositive, the triply bonded L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecule could be experimentally produced and detected, since these triple bonded species are more thermodynamically stable than their corresponding doubly bonded L2B
N–L molecule could be experimentally produced and detected, since these triple bonded species are more thermodynamically stable than their corresponding doubly bonded L2B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: and :B
N: and :B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR2 isomers. Actually, these triply bonded L–B
NR2 isomers. Actually, these triply bonded L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L species, which is isoelectronic to the alkynes L–C
N–L species, which is isoelectronic to the alkynes L–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–L and which contain small and electropositive substituents were experimentally isolated and structurally characterized about three decades ago.65–68
C–L and which contain small and electropositive substituents were experimentally isolated and structurally characterized about three decades ago.65–68
Several important geometrical parameters and the associated physical properties of L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (Table 1), L–Al
N–L (Table 1), L–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (Table S1†), L–Ga
N–L (Table S1†), L–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (Table S2†), L–In
N–L (Table S2†), L–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (Table S3†) and L–Tl
N–L (Table S3†) and L–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (Table S4†) are listed in ESI.†
N–L (Table S4†) are listed in ESI.†
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L using the B3PW91/Def2-TZVP, M06-2X/Def2-TZVP (in round bracket), and B3LYP/LANL2DZ+dp (in square bracket) levels of theory
N–L using the B3PW91/Def2-TZVP, M06-2X/Def2-TZVP (in round bracket), and B3LYP/LANL2DZ+dp (in square bracket) levels of theory
| L | F | OH | H | CH3 | SiH3 |
|---|---|---|---|---|---|
a The natural charge density on B.b The natural charge density on N.c ΔEST = E(triplet state for L–B) − E(singlet state for L–B).d ΔEST = E(triplet state for L–N) − E(singlet state for L–N).e BE = E(singlet state for L–B) + E(singlet state for L–B) – E(singlet state for L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L).f The Wiberg bond index (WBI) for the B N–L).f The Wiberg bond index (WBI) for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond: see ref. 71 and 72. N bond: see ref. 71 and 72. |
|||||
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (Å) N (Å) |
1.275 | 1.276 | 1.246 | 1.249 | 1.262 |
| (1.245) | (1.254) | (1.233) | (1.239) | (1.252) | |
| [1.220] | [1.238] | [1.231] | [1.236] | [1.248] | |
| ∠L–B–N (°) | 165.4 | 169.1 | 180.0 | 180.0 | 180.0 |
| (169.1) | (170.7) | (180.0) | (180.0) | (180.0) | |
| [180.0] | [174.1] | [179.9] | [179.9] | [178.6] | |
| ∠B–N–L (°) | 137.3 | 137.0 | 180.0 | 180.0 | 180.0 |
| (151.4) | (146.5) | (180.0) | (180.0) | (179.9) | |
| [160.0] | [158.2] | [179.9] | [179.9] | [178.9] | |
| ∠L–B–N–L (°) | 180.0 | 163.5 | 163.0 | 180.0 | 169.1 |
| (179.9) | (161.9) | (169.3) | (178.7) | (176.9) | |
| [180.0] | [159.7] | [178.8] | [178.4] | [172.1] | |
| QBa | 0.2569 | 0.0441 | 0.1196 | −0.1350 | −0.2670 |
| (0.1543) | (−0.0515) | (−0.1194) | (−0.2172) | (−0.1986) | |
| [0.1335] | [−0.0465] | [−0.0817] | [−0.2570] | [−0.2213] | |
| QNb | 0.1573 | 0.1411 | −0.2376 | −0.1295 | −0.0263 |
| (0.2340) | (0.1185) | (−0.2197) | (−0.1512) | (−0.0584) | |
| [0.2253] | [0.1226] | [−0.2575] | [−0.1150] | [−0.0537] | |
| ΔEST for L–Bc (kcal mol−1) | 73.97 | 64.90 | 25.39 | 36.79 | 22.28 |
| (73.60) | (62.12) | (27.65) | (32.69) | (21.77) | |
| [81.01] | [68.97] | [28.74] | [38.47] | [22.33] | |
| ΔEST for L–Nd (kcal mol−1) | −46.00 | −21.39 | −50.89 | 46.76 | 44.87 |
| (−48.48) | (−21.68) | (−55.08) | (48.23) | (46.86) | |
| [−45.47] | [−19.91] | [−49.44] | [50.99] | [48.02] | |
| HOMO–LUMO (kcal mol−1) | 147.8 | 128.3 | 197.6 | 162.2 | 165.6 |
| (173.2) | (145.9) | (206.1) | (182.3) | (165.4) | |
| [242.5] | [203.2] | [265.7] | [228.3] | [224.4] | |
| BEe (kcal mol−1) | 149.7 | 168.2 | 200.1 | 188.8 | 206.2 |
| (147.5) | (166.1) | (202.4) | (190.3) | (208.4) | |
| [157.4] | [171.4] | [210.5] | [199.4] | [217.4] | |
| WBIf | 1.880 | 1.843 | 2.114 | 1.962 | 1.908 |
| (1.951) | (1.911) | (2.149) | (2.000) | (1.963) | |
| [1.988] | [1.938] | [2.128] | [2.000] | [1.960] | |
As shown in Table 1, these computations predict that the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond distance (Å) lies in the range, 1.246–1.276 (B3PW91/Def2-TZVP), 1.233–1.254 (M06-2X/Def2-TZVP) and 1.220–1.248 (B3LYP/LANL2DZ+dp). The reported experimental values for the B
N triple bond distance (Å) lies in the range, 1.246–1.276 (B3PW91/Def2-TZVP), 1.233–1.254 (M06-2X/Def2-TZVP) and 1.220–1.248 (B3LYP/LANL2DZ+dp). The reported experimental values for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond length are 1.240 Å (ref. 65 and 66) and 1.258 Å,67,68 which agree well with the theoretical data for this study.
N triple bond length are 1.240 Å (ref. 65 and 66) and 1.258 Å,67,68 which agree well with the theoretical data for this study.
In the case of the ΔEST (=E(triplet state) − E(singlet state)) for the L–B fragment (Table 1), its excited energy from the singlet ground state to the triplet excited state is theoretically estimated to be at least 22 kcal mol−1. However, for the L–N moiety, the modulus advancement energy between the ground state and the first excited state is calculated to be at least 20 kcal mol−1. On the basis of the theoretical analysis in Section 2, this theoretical data shows that mechanism [A] is feasible for the interpretation of the generation of the triply bonded L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L species that feature small ligands. Therefore, the bonding disposition of L–B
N–L species that feature small ligands. Therefore, the bonding disposition of L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L with small substituents must be viewed as L–B
N–L with small substituents must be viewed as L–B N–L, so one B → N σ donation bond and two B ← N π donation bonds constitute the B
N–L, so one B → N σ donation bond and two B ← N π donation bonds constitute the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond. All the values for the Wiberg bond index (WBI)71,72 in Table 1 show that B
N triple bond. All the values for the Wiberg bond index (WBI)71,72 in Table 1 show that B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds that are supported by small groups have values of less than 2.1, but the WBI for the C
N bonds that are supported by small groups have values of less than 2.1, but the WBI for the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond in ethyne is 2.99. These L–B
C bond in ethyne is 2.99. These L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L species that feature small substituents have a bond order of much less than 2.00 for the central B–N bond, as shown in Table 1. One explanation for this is that, as shown in Fig. 1, the lone pair orbitals of both the L–B and L–N components contain the valence s characters. This significantly decreases the bonding strength between boron and nitrogen. It is also possible that the covalent radii of boron and nitrogen, at 82 pm and 70 pm,73 result in a small overlapping population between B and N, which could result in small WBI values.
N–L species that feature small substituents have a bond order of much less than 2.00 for the central B–N bond, as shown in Table 1. One explanation for this is that, as shown in Fig. 1, the lone pair orbitals of both the L–B and L–N components contain the valence s characters. This significantly decreases the bonding strength between boron and nitrogen. It is also possible that the covalent radii of boron and nitrogen, at 82 pm and 70 pm,73 result in a small overlapping population between B and N, which could result in small WBI values.
Similar to the 1,2-migration reactions for L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L, the potential energy surfaces for the other triply bonded L–Al
N–L, the potential energy surfaces for the other triply bonded L–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L, L–Ga
N–L, L–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L, L–In
N–L, L–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L and L–Tl
N–L and L–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L compounds are schematically represented in Fig. 4–7, respectively. Being close to the L–B
N–L compounds are schematically represented in Fig. 4–7, respectively. Being close to the L–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (Fig. 3) compound, the computational results for the 1,2-ligand-shift reactions show that either a proton or the ligands containing the carbon atom (such as CH3) stabilize the triply bonded L–E13
N–L (Fig. 3) compound, the computational results for the 1,2-ligand-shift reactions show that either a proton or the ligands containing the carbon atom (such as CH3) stabilize the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (E13 = Al, Ga, In and Tl) species relative to their corresponding double-bond isomers. This theoretical finding is quite different from those for the other L–E13
N–L (E13 = Al, Ga, In and Tl) species relative to their corresponding double-bond isomers. This theoretical finding is quite different from those for the other L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E15–L systems that have been previously studied,52–64 in which regardless of whether the small ligands are electronegative or electropositive, the triple-bond L–E13
E15–L systems that have been previously studied,52–64 in which regardless of whether the small ligands are electronegative or electropositive, the triple-bond L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E15–L (except for E15 = N) compounds are not thermodynamically stable in the 1,2-migration reactions. To the authors' best knowledge, both monomeric imides Ar′–M
E15–L (except for E15 = N) compounds are not thermodynamically stable in the 1,2-migration reactions. To the authors' best knowledge, both monomeric imides Ar′–M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–Ar′′ (M = Ga or In; Ar′ or Ar′′ = terphenyl ligands) that were reported by Power and co-workers have been successfully synthesized and structurally characterized.69,70 The computed geometrical parameters and some physical properties of the L–E13
N–Ar′′ (M = Ga or In; Ar′ or Ar′′ = terphenyl ligands) that were reported by Power and co-workers have been successfully synthesized and structurally characterized.69,70 The computed geometrical parameters and some physical properties of the L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (E13 = Al, Ga, In and Tl) molecules featuring small groups are listed in Tables S1, S2, S3, and S4,† respectively. Several important conclusions can be drawn from Tables S1–S4.†
N–L (E13 = Al, Ga, In and Tl) molecules featuring small groups are listed in Tables S1, S2, S3, and S4,† respectively. Several important conclusions can be drawn from Tables S1–S4.†
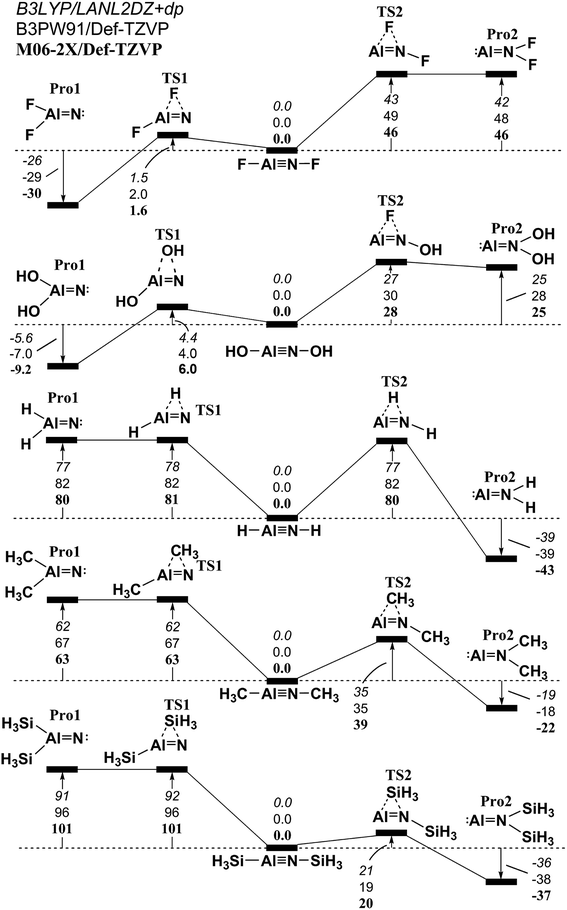 | ||
Fig. 4 The relative Gibbs free energy for L–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S1.† N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S1.† | ||
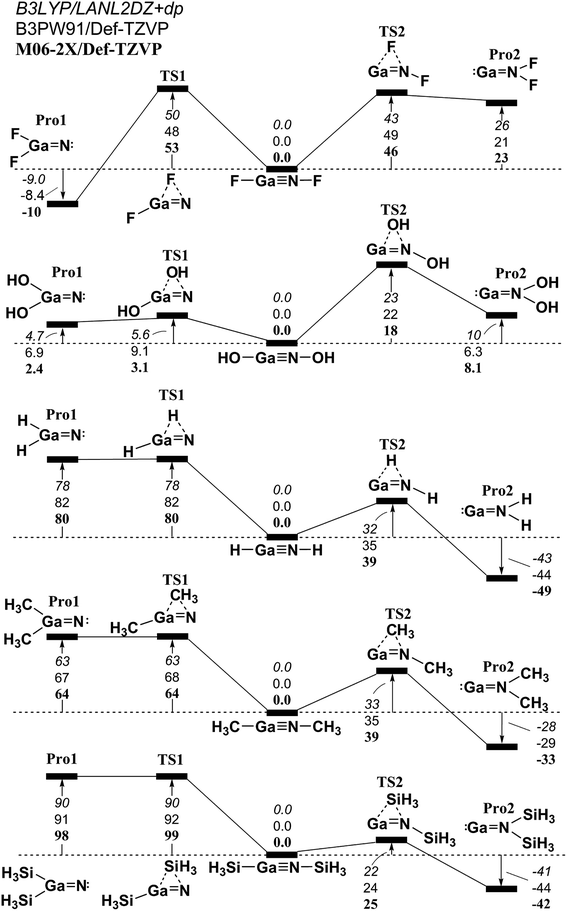 | ||
Fig. 5 The relative Gibbs free energy for L–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S2.† N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S2.† | ||
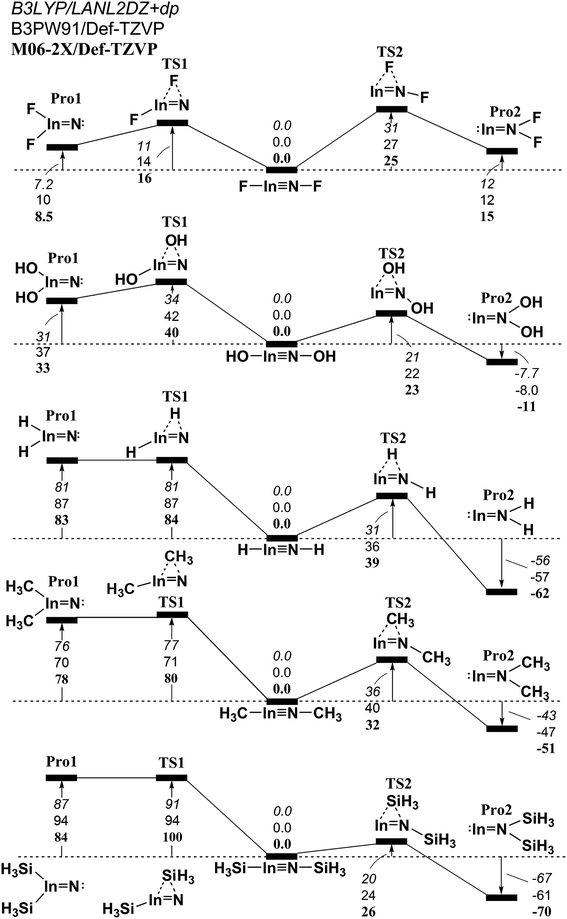 | ||
Fig. 6 The relative Gibbs free energy for L–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S3.† N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S3.† | ||
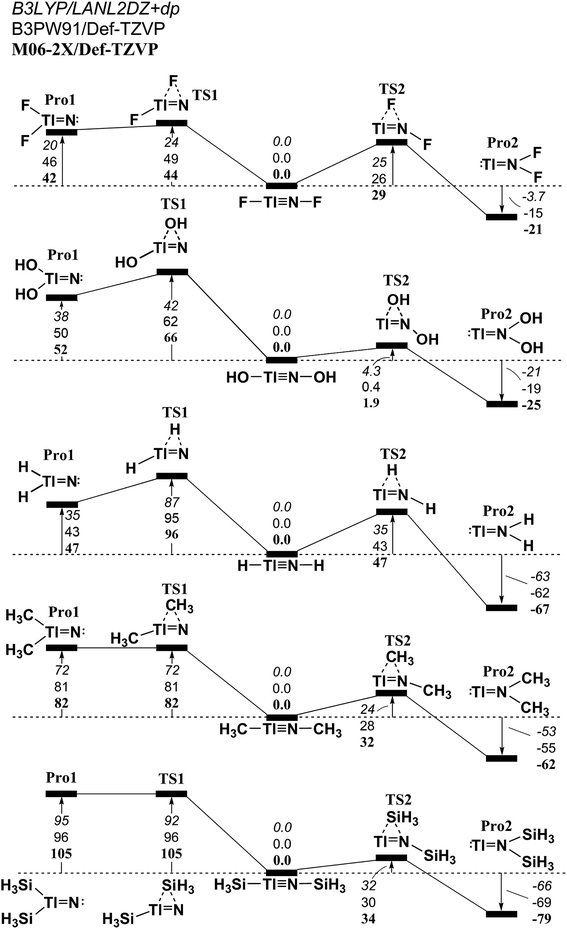 | ||
Fig. 7 The relative Gibbs free energy for L–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S4.† N–L (L = F, OH, H, CH3, and SiH3) calculated at the M06-2X/Def2-TZVP, B3PW91/Def2-TZVP, and B3LYP/LANL2DZ+dp levels of theory. For details see the text and Table S4.† | ||
(1) It is noteworthy that according to the available experimental detections, the lengths of the Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (1.701 Å)69,70 and In
N (1.701 Å)69,70 and In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (1.928 Å)69 triple bonds are consistent with the computational results (1.662–1.804 Å and 1.828–2.073 Å) in Tables S2 and S3,† respectively. This theoretical evidence strongly suggests that the computational methods that are used in this study provide reliable information for further theoretical observations.
N (1.928 Å)69 triple bonds are consistent with the computational results (1.662–1.804 Å and 1.828–2.073 Å) in Tables S2 and S3,† respectively. This theoretical evidence strongly suggests that the computational methods that are used in this study provide reliable information for further theoretical observations.
(2) The results using DFT that are shown in Table S1† (L–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L) and Table S4† (L–Tl
N–L) and Table S4† (L–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L) predict that the central Al
N–L) predict that the central Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and Tl
N and Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond distances are in the range, 1.608–1.753 Å and 1.849–2.300 Å, respectively. The calculated WBI for the central Al–N, Ga–N, In–N, and Tl–N bonds are all estimated to be less than 1.50. This theoretical evidence strongly suggests that all of these central triple bonds in L–E13
N bond distances are in the range, 1.608–1.753 Å and 1.849–2.300 Å, respectively. The calculated WBI for the central Al–N, Ga–N, In–N, and Tl–N bonds are all estimated to be less than 1.50. This theoretical evidence strongly suggests that all of these central triple bonds in L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecules that feature small substituents must be quite weak, possibly because of the hybridized lone pair orbitals for both L–E13 and L–N fragments and the different atomic radius for E13 and N elements, both of which do not produce good overlap populations between nitrogen and the group 13 elements.
N–L molecules that feature small substituents must be quite weak, possibly because of the hybridized lone pair orbitals for both L–E13 and L–N fragments and the different atomic radius for E13 and N elements, both of which do not produce good overlap populations between nitrogen and the group 13 elements.
(3) The DFT data in Tables S1–S4† shows that the singlet–triplet energy splitting (ΔEST) for the L–E13 fragment is much higher than that for the L–N moiety. Therefore, the electron for the latter jumps from the triplet ground state to the singlet excited state more easily than the electron from the singlet ground state for the former. As a result, it is better to use mechanism [A] to describe the bonding characteristic of the L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecule bearing the small substituents. For mechanism [A] in Fig. 1, the bonding constitution for the E13
N–L molecule bearing the small substituents. For mechanism [A] in Fig. 1, the bonding constitution for the E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond in L–E13
N triple bond in L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L that feature small ligands must be L–E13
N–L that feature small ligands must be L–E13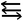 N–L.
N–L.
3.2 Large ligands on substituted L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) N–L′
N–L′
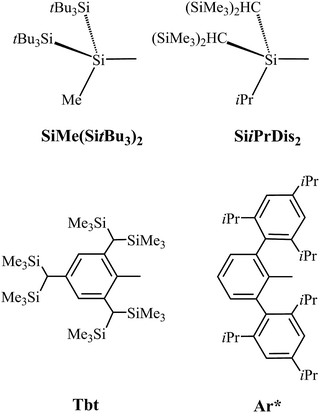
The possibility of bulky substituents (L′) stabilizing the central E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond is determined. Similarly to previous studies,52–64 as shown in Scheme 1, SiMe(SitBu3)2, SiiPrDis2, Tbt and Ar* are used for this study. London dispersion forces, which are the non-valent interactions between large groups, can greatly affect the structure and stability of sterically congested molecules.74 Therefore, the dispersion-corrected M06-2X/Def2-TZVP method75 is used to gain more information about producing stable, triple-bonded L′–E13
N triple bond is determined. Similarly to previous studies,52–64 as shown in Scheme 1, SiMe(SitBu3)2, SiiPrDis2, Tbt and Ar* are used for this study. London dispersion forces, which are the non-valent interactions between large groups, can greatly affect the structure and stability of sterically congested molecules.74 Therefore, the dispersion-corrected M06-2X/Def2-TZVP method75 is used to gain more information about producing stable, triple-bonded L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ species. The key geometrical parameters and the associated physical properties of L′–B
N–L′ species. The key geometrical parameters and the associated physical properties of L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ are listed in Table 2. This information for other triply bonded molecules that feature bulky ligands, i.e., L′–Al
N–L′ are listed in Table 2. This information for other triply bonded molecules that feature bulky ligands, i.e., L′–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S5†), L′–Ga
N–L′ (Table S5†), L′–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S6†), L′–In
N–L′ (Table S6†), L′–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S7†), and L′–Tl
N–L′ (Table S7†), and L′–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S8†), is collected in ESI.†
N–L′ (Table S8†), is collected in ESI.†
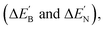 natural charge densities
natural charge densities 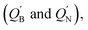 binding energies (BE), the Wiberg bond index (WBI), HOMO–LUMO energy gaps, and some reaction enthalpies for L′–B
binding energies (BE), the Wiberg bond index (WBI), HOMO–LUMO energy gaps, and some reaction enthalpies for L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ at the M06-2X/Def2-TZVP level of theory
N–L′ at the M06-2X/Def2-TZVP level of theory
| L′ | SiMe(SitBu3)2 | SiiPrDis2 | Tbt | Ar* |
|---|---|---|---|---|
a The natural charge density on boron.b The natural charge density on nitrogen.c 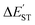 (kcal mol−1) = E(triplet state for L′–B) − E(singlet state for L′–B).d (kcal mol−1) = E(triplet state for L′–B) − E(singlet state for L′–B).d 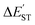 (kcal mol−1) = E(triplet state for L′–N) − E(singlet state for L′–N).e BE (kcal mol−1) = E(triplet state for L′–B) + E(triplet state for L′–N) − E(singlet for L′–B (kcal mol−1) = E(triplet state for L′–N) − E(singlet state for L′–N).e BE (kcal mol−1) = E(triplet state for L′–B) + E(triplet state for L′–N) − E(singlet for L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′).f See Scheme 2.g The Wiberg bond index (WBI) for the B N–L′).f See Scheme 2.g The Wiberg bond index (WBI) for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond: see ref. 71 and 72. N bond: see ref. 71 and 72. |
||||
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (Å) N (Å) |
1.257 | 1.242 | 1.273 | 1.267 |
| ∠L′–B–N (°) | 175.2 | 165.2 | 171.9 | 171.2 |
| ∠B–N–L′ (°) | 163.1 | 166.6 | 157.7 | 166.2 |
| ∠L′–B–N–L′ (°) | 180.0 | 180.0 | 178.7 | 179.5 |
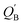 a a |
0.2413 | 0.0818 | −0.2133 | −0.1600 |
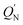 b b |
−0.3076 | −0.4369 | −0.1566 | −0.1471 |
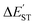 for L′–Bc (kcal mol−1) for L′–Bc (kcal mol−1) |
13.59 | 11.24 | 21.47 | 20.75 |
| ΔEST for L′–Nd (kcal mol−1) | −22.30 | −25.05 | −25.52 | −28.63 |
| HOMO–LUMO (kcal mol−1) | 103.3 | 114.2 | 66.97 | 68.28 |
| BEe (kcal mol−1) | 380.0 | 383.8 | 375.9 | 426.3 |
| ΔH1f (kcal mol−1) | 98.78 | 80.03 | 91.69 | 89.84 |
| ΔH2f (kcal mol−1) | 94.05 | 71.21 | 92.96 | 75.25 |
| WBIg | 2.188 | 2.161 | 2.078 | 2.135 |
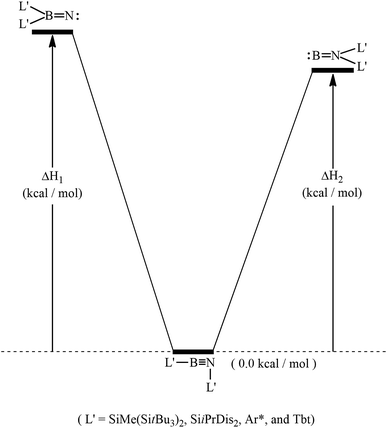
The same computational method is used to determine the 1,2-ligand-shift reactions for L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ molecules that are substituted with bulky groups; i.e., L′–E13
N–L′ molecules that are substituted with bulky groups; i.e., L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ → L2′E13
N–L′ → L2′E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: and L′–E13
N: and L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ →:E13
N–L′ →:E13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NL2′, as shown in Scheme 2. The results in Table 2 show that because of steric crowding, the potential energies of both double-bond molecules (:B
NL2′, as shown in Scheme 2. The results in Table 2 show that because of steric crowding, the potential energies of both double-bond molecules (:B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NL2′ and L2′B
NL2′ and L2′B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) are respectively higher than that of the corresponding triple-bond L′–B
N) are respectively higher than that of the corresponding triple-bond L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ isomer by at least 80 and 71 kcal mol−1. These theoretical findings strongly suggest that sterically hindered ligands shield the central weak B
N–L′ isomer by at least 80 and 71 kcal mol−1. These theoretical findings strongly suggest that sterically hindered ligands shield the central weak B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond, since the Wiberg bond index (WBI) for the C
N triple bond, since the Wiberg bond index (WBI) for the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond in acetylene was computed to be 2.99.
C bond in acetylene was computed to be 2.99.
The computational data in Tables 2 and S5–S8† shows that the central triple bond distances are predicted to be in the range of 1.242–1.273 Å (L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′), 1.681–1.719 Å (L′–Al
N–L′), 1.681–1.719 Å (L′–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′), 1.698–1.722 Å (L′–Ga
N–L′), 1.698–1.722 Å (L′–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′), 1.866–1.902 Å (L′–In
N–L′), 1.866–1.902 Å (L′–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′), and 1.877–1.930 Å (L′–Tl
N–L′), and 1.877–1.930 Å (L′–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′). These predicted bond lengths are consistent with other reported experimental data, such as, 1.240 Å (ref. 65 and 66) and 1.258 Å (ref. 67 and 68) for the B
N–L′). These predicted bond lengths are consistent with other reported experimental data, such as, 1.240 Å (ref. 65 and 66) and 1.258 Å (ref. 67 and 68) for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond, 1.701 Å (ref. 69 and 70) for the Ga
N bond, 1.701 Å (ref. 69 and 70) for the Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and 1.928 Å (ref. 69) for the In
N bond and 1.928 Å (ref. 69) for the In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond. Since there is good agreement between the available experimental values and the dispersion-corrected M06-2X data for the central triple bond lengths, the computational method that is used in this study must be reliable.
N bond. Since there is good agreement between the available experimental values and the dispersion-corrected M06-2X data for the central triple bond lengths, the computational method that is used in this study must be reliable.
The M06-2X results in Table 2 show that the 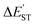 for the L′–B fragment is calculated to be at least 11 kcal mol−1, but the modulus of
for the L′–B fragment is calculated to be at least 11 kcal mol−1, but the modulus of 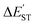 for the L′–N component is computed to be at least 22 kcal mol−1. In other words, L′–B jumps easily from the singlet ground state to the triplet state because the
for the L′–N component is computed to be at least 22 kcal mol−1. In other words, L′–B jumps easily from the singlet ground state to the triplet state because the 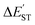 value for L′–B is smaller than that for L′–N. Therefore, the L′–B and L′–N fragments must follow a triplet–triplet bonding mechanism; i.e., mechanism [B]: [L′–B]3 + [L′–N]3 → [L′–B
value for L′–B is smaller than that for L′–N. Therefore, the L′–B and L′–N fragments must follow a triplet–triplet bonding mechanism; i.e., mechanism [B]: [L′–B]3 + [L′–N]3 → [L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′]1. As schematically shown in Fig. 1, the bonding nature of the bulkily substituted L′–B
N–L′]1. As schematically shown in Fig. 1, the bonding nature of the bulkily substituted L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ can be viewed as L′–B
N–L′ can be viewed as L′–B N–L′. That is to say, this B
N–L′. That is to say, this B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond consists of a usual σ bond, a conventional π bond and a donor–acceptor π bond.
N triple bond consists of a usual σ bond, a conventional π bond and a donor–acceptor π bond.
However, each lone-pair orbital of L′–B and L′–N respectively contains s and p valence orbitals of boron and nitrogen. As shown in Fig. 1, this phenomenon means that the overlap population between L′–B and L′–N is small. Therefore, the bond order for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond must be small. This study's M06-2X computations are shown in Table 2 and confirm this prediction. Similarly, the values for
N triple bond must be small. This study's M06-2X computations are shown in Table 2 and confirm this prediction. Similarly, the values for 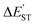 for L′–N and the other L′–B fragments in Tables S5–S8† show that the modulus of
for L′–N and the other L′–B fragments in Tables S5–S8† show that the modulus of 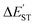 (>22 kcal mol−1) for the former is always larger than those for the latter, e.g., L′–Al (>18 kcal mol−1), L′–Ga (>15 kcal mol−1), L′–In (>17 kcal mol−1) and L′–Tl (>19 kcal mol−1). These computational values show that all of the bonding in these triply bonded L′–E13
(>22 kcal mol−1) for the former is always larger than those for the latter, e.g., L′–Al (>18 kcal mol−1), L′–Ga (>15 kcal mol−1), L′–In (>17 kcal mol−1) and L′–Tl (>19 kcal mol−1). These computational values show that all of the bonding in these triply bonded L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ species can be represented as L′–E13
N–L′ species can be represented as L′–E13 N–L′.
N–L′.
The theoretically calculated values for the 1,2-shifted energy barriers and the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond orders (WBI) in Table 2 strongly indicate that large substituents protect the central fragile B
N bond orders (WBI) in Table 2 strongly indicate that large substituents protect the central fragile B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond and increase its bond order. The same conclusions can also be drawn from the computational results for the other triply bonded L′–E13
N triple bond and increase its bond order. The same conclusions can also be drawn from the computational results for the other triply bonded L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ molecules, which are listed in Tables S5–S8.†
N–L′ molecules, which are listed in Tables S5–S8.†
Both natural bond orbital (NBO)71,72 and natural resonance theory (NRT)76–78 are used to determine the electronic densities of the triply bonded L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ molecules that feature large substituents. The M06-2X results are listed in Table 3. The same theoretical analysis for the other triply bonded L′–E13
N–L′ molecules that feature large substituents. The M06-2X results are listed in Table 3. The same theoretical analysis for the other triply bonded L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ species is listed in ESI:† L′–Al
N–L′ species is listed in ESI:† L′–Al![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S9†), L′–Ga
N–L′ (Table S9†), L′–Ga![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S10†), L′–In
N–L′ (Table S10†), L′–In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S11†), and L′–Tl
N–L′ (Table S11†), and L′–Tl![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ (Table S12†). The NRT values in Table 3 show that the bond order for the B
N–L′ (Table S12†). The NRT values in Table 3 show that the bond order for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond is 2.18 (L′ = SiMe(SitBu3)2), 2.17 (L′ = SiiPrDis2), 2.24 (L′ = Tbt), and 2.22 (L′ = Ar*). This NRT data is similar to the WBI values (2.19, 2.16, 2.08, and 2.14, respectively) in Table 3. The NBO and NRT data in Table 3 also shows that the triply bonded L′–B
N bond is 2.18 (L′ = SiMe(SitBu3)2), 2.17 (L′ = SiiPrDis2), 2.24 (L′ = Tbt), and 2.22 (L′ = Ar*). This NRT data is similar to the WBI values (2.19, 2.16, 2.08, and 2.14, respectively) in Table 3. The NBO and NRT data in Table 3 also shows that the triply bonded L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ molecules for this study all have an analogous electronic structure. As seen in Table 3, (SiMe(SitBu3)2)–B
N–L′ molecules for this study all have an analogous electronic structure. As seen in Table 3, (SiMe(SitBu3)2)–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–(SiMe(SitBu3)2) is predicted to have one σ bond and two π (π⊥ and π‖) bonds, which are occupied by two electrons: that is, 1.99 (σ), 1.96 (π⊥) and 1.96 (π‖). The M06-2X results also show that the σ bond is heavily polarized towards nitrogen (78%) and that there are two non-degenerate π bonds that are also heavily polarized towards nitrogen (π⊥, 80% and π‖, 80%). This is consistent with the fact that nitrogen (3.066) is more electronegative than boron (2.051).79 The two non-degenerate π bonding orbitals (π⊥ and π‖) are schematically given in ESI.†
N–(SiMe(SitBu3)2) is predicted to have one σ bond and two π (π⊥ and π‖) bonds, which are occupied by two electrons: that is, 1.99 (σ), 1.96 (π⊥) and 1.96 (π‖). The M06-2X results also show that the σ bond is heavily polarized towards nitrogen (78%) and that there are two non-degenerate π bonds that are also heavily polarized towards nitrogen (π⊥, 80% and π‖, 80%). This is consistent with the fact that nitrogen (3.066) is more electronegative than boron (2.051).79 The two non-degenerate π bonding orbitals (π⊥ and π‖) are schematically given in ESI.†
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–R′ molecules that feature bulky ligands (L′ = SiMe(SitBu3)2, Tbt, SiiPrDis2, and Ar*) at the M06-2X/Def2-TZVP level of theorya,b
N–R′ molecules that feature bulky ligands (L′ = SiMe(SitBu3)2, Tbt, SiiPrDis2, and Ar*) at the M06-2X/Def2-TZVP level of theorya,b
L′–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–R′ N–R′ |
WBI | NBO analysis | NRT analysis | |||
|---|---|---|---|---|---|---|
| Occupancy | Hybridization | Polarization | Total/covalent/ionic | Resonance weight | ||
a The value of the Wiberg bond index (WBI) for the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and the occupancy of the corresponding σ and π bonding NBO (see ref. 71 and 72).b NRT; see ref. 76–78. N bond and the occupancy of the corresponding σ and π bonding NBO (see ref. 71 and 72).b NRT; see ref. 76–78. |
||||||
| L′ = SiMe(SitBu3)2 | 2.19 | σ: 1.99 | σ: 0.4743 B (sp1.43) + 0.8803 N (sp0.80) | 22.50% (B) | 2.18/0.88/1.30 | B–N: 6.14% |
| 77.50% (N) | ||||||
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: 69.80% N: 69.80% |
||||||
| π⊥: 1.96 | π⊥: 0.4506 B (sp99.99) + 0.8927 N (sp99.99) | 20.30% (B) | ||||
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: 24.06% N: 24.06% |
||||||
| 79.70% (N) | ||||||
| π‖: 1.96 | π‖: 0.4483 B (sp99.99) + 0.8939 N (sp1.00) | 20.10% (B) | ||||
| 79.90% (N) | ||||||
| L′ = SiiPrDis2 | 2.16 | σ: 1.99 | σ: 0.4747 B (sp1.44) + 0.8801 N (sp0.83) | 22.54% (B) | 2.17/0.91/1.26 | B–N: 72.26% |
| 77.46% (N) | B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: 27.74% N: 27.74% |
|||||
| π⊥: 1.96 | π⊥: 0.4530 B (sp99.99) + 0.8915 N (sp99.99) | 20.52% (B) | ||||
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: 0.00% N: 0.00% |
||||||
| 79.48% (N) | ||||||
| π‖: 1.96 | π‖: 0.4430 B (sp21.83) + 0.8965 N (sp79.29) | 19.63% (B) | ||||
| 80.37% (N) | ||||||
| L′ = Tbt | 2.08 | σ: 1.99 | σ: 0.4855 B (sp1.34) + 0.8742 N (sp0.81) | 23.57% (B) | 2.24/0.49/1.75 | B–N: 81.96 |
| 76.43% (N) | ||||||
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: 18.04 N: 18.04 |
||||||
| π⊥: 1.94 | π⊥: 0.4515 B (sp99.99) + 0.8923 N (sp1) | 20.38% (B) | ||||
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: 0.00% N: 0.00% |
||||||
| 79.62% (N) | ||||||
| π‖: 1.88 | π‖: 0.4433 B (sp99.99) + 0.8964 N (sp99.99) | 19.65% (B) | ||||
| 80.35% (N) | ||||||
| L′ = Ar* | 2.14 | σ: 1.99 | σ: 0.4918 B (sp1.30) + 0.8707 N (sp0.84) | 24.18% (B) | 2.22/0.49/1.09 | B–N: 42.68% |
| 75.82% (N) | ||||||
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N: 56.9% N: 56.9% |
||||||
| π⊥: 1.95 | σ: 0.4580 B (sp99.99) + 0.8889 N (sp99.99) | 20.98% (B) | ||||
B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: 0.42% N: 0.42% |
||||||
| 79.02% (N) | ||||||
| π‖: 1.85 | σ: 0.4433 B (sp99.99) + 0.8964 N (sp99.99) | 19.65% (B) | ||||
| 80.35% (N) | ||||||
4. Conclusions
This study uses DFT computational methods to determine the effect of both small and bulky substituents on the triple-bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (E13 = B, Al, Ga, In, and Tl) compounds, in order to determine how to successfully design and synthesize a molecule featuring an E13
N–L (E13 = B, Al, Ga, In, and Tl) compounds, in order to determine how to successfully design and synthesize a molecule featuring an E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond. This study represents the first theoretical investigation of the stability of the triply bonded L–E13
N triple bond. This study represents the first theoretical investigation of the stability of the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecules. Four important conclusions are drawn, based on the results of this theoretical study:
N–L molecules. Four important conclusions are drawn, based on the results of this theoretical study:
(1) Previous theoretical conclusions52–64 showed that only sterically bulky ligands, and not small groups, thermodynamically stabilize the triple bond of the L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) E15–L (E13 = B, Al, Ga, In and Tl; E15 = P, As, Sb and Bi) molecules. However,52–64 this theoretical study finds that both small (but electropositive) ligands and bulky substituents stabilize the triply bonded L–E13
E15–L (E13 = B, Al, Ga, In and Tl; E15 = P, As, Sb and Bi) molecules. However,52–64 this theoretical study finds that both small (but electropositive) ligands and bulky substituents stabilize the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L compounds.
N–L compounds.
(2) The theoretical analysis shows that the bonding nature of a triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L molecule that features small substituents can be represented as L–E13
N–L molecule that features small substituents can be represented as L–E13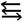 N–L.
N–L.
(3) The bonding character of the central triple bond in an L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ compound that features bulkier substituents can be regarded as L′–E13
N–L′ compound that features bulkier substituents can be regarded as L′–E13 N–L′.
N–L′.
(4) Since two central heteroatoms (E13 and N) are involved in the triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L (and L′–E13
N–L (and L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′) species, they belong to different rows of the periodic table so they have different quantum numbers. Therefore, E13 and N have different electronegativity values and different atomic sizes. Due to the poor overlap populations between E13 and N in both triply bonded L–E13
N–L′) species, they belong to different rows of the periodic table so they have different quantum numbers. Therefore, E13 and N have different electronegativity values and different atomic sizes. Due to the poor overlap populations between E13 and N in both triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L and L′–E13
N–L and L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ molecules, it is expected that the bond order of the E13
N–L′ molecules, it is expected that the bond order of the E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond must be small, so their E13
N triple bond must be small, so their E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bonds must be weak.
N triple bonds must be weak.
The results of this theoretical study should allow the production and synthesis of stable triply bonded L–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L and L′–E13
N–L and L′–E13![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–L′ molecules.
N–L′ molecules.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the National Center for High-Performance Computing in Taiwan for the donation of generous amounts of computing time. The authors are also grateful for financial support from the Ministry of Science and Technology of Taiwan. Special thanks are also due to reviewers 1 and 2 for very help suggestions and comments.References
- P. P. Power, Boron-Phosphorus Compounds and Multiple Bonding, Angew. Chem., Int. Ed. Engl., 1990, 29, 449–460 CrossRef.
- P. P. Power, A. Moezzi, D. C. Pestana, M. A. Petrie, S. C. Shoner and K. M. Waggoner, Multiple Bonding, π-bonding Contributions and Aromatic Character in Isoelectronic Boron-phosphorus, Boron-arsenic, Aluminum-nitrogen and Zinc-sulfur Compounds, Pure Appl. Chem., 1991, 63, 859–866 CAS.
- R. T. Paine and H. Nöth, Recent Advances in Phosphinoborane Chemistry, Chem. Rev., 1995, 95, 343–379 CrossRef.
- R. Okazaki and R. West, Chemistry of Stable Disilenes, Adv. Organomet. Chem., 1996, 39, 231–273 CrossRef CAS.
- P. P. Power, π-Bonding and the Lone Pair Effect in Multiple Bonds between Heavier Main Group Elements, Chem. Rev., 1999, 99, 3463–3504 CrossRef CAS PubMed.
- G. H. Robison, Gallanes, Gallenes, Cyclogallenes, and Gallynes:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Organometallic Chemistry about the Gallium-Gallium Bond, Acc. Chem. Res., 1999, 32, 773–782 CrossRef.
Organometallic Chemistry about the Gallium-Gallium Bond, Acc. Chem. Res., 1999, 32, 773–782 CrossRef. - M. Haaf, T. A. Schmedake and R. West, Stable Silylenes, Acc. Chem. Res., 2000, 33, 704–714 CrossRef CAS PubMed.
- B. Gehrhus and M. F. Lappert, Chemistry of Thermally Stable Bis(amino)silylenes, J. Organomet. Chem., 2001, 617, 209–223 CrossRef.
- M. Weidenbruch, Triple Bonds of the Heavy Main-Group Elements: Acetylene and Alkylidyne Analogues of Group 14, Angew. Chem., Int. Ed., 2003, 42, 2222–2224 CrossRef CAS PubMed.
- L. E. Gusel’nikov, Hetero-π-systems from 2+2 Cycloreversions. Part 1. Gusel'nikov-Flowers Route to Silenes and Origination of the Chemistry of Doubly Bonded Silicon, Coord. Chem. Rev., 2003, 244, 149–204 CrossRef.
- M. Weidenbruch, From a Cyclotrisilane to a Cyclotriplumbane:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Low Coordination and Multiple Bonding in Group 14 Chemistry, Organometallics, 2003, 22, 4348–4360 CrossRef CAS.
Low Coordination and Multiple Bonding in Group 14 Chemistry, Organometallics, 2003, 22, 4348–4360 CrossRef CAS. - P. P. Power, Silicon, Germanium, Tin and Lead Analogues of Acetylenes, Chem. Commun., 2003, 2091–2101 RSC.
- M. Lein, A. Krapp and G. Frenking, Why Do the Heavy-Atom Analogues of Acetylene E2H2 (E = Si−Pb) Exhibit Unusual Structures?, J. Am. Chem. Soc., 2005, 127, 6290–6299 CrossRef CAS PubMed.
- B. Gehrhus, P. B. Hitchcock, R. Pongtavornpinyo and L. Zhang, Insights Into the Making of a Stable Silylene, Dalton Trans., 2006, 15, 1847–1857 RSC.
- A. Sekiguchi, M. Ichinohe and R. Kinjo, The Chemistry of Disilyne with a Genuine Si–Si Triple Bond: Synthesis, Structure, and Reactivity, Bull. Chem. Soc. Jpn., 2006, 79, 825–832 CrossRef CAS.
- M. Kira, T. Iwamoto and S. Ishida, A Helmeted Dialkylsilylene, Bull. Chem. Soc. Jpn., 2007, 80, 258–275 CrossRef CAS.
- Y. Wang and G. H. Robinson, Organometallics of the Group 13 M-M Bond (M = Al, Ga, In) and the Concept of Metalloaromaticity, Organometallics, 2007, 26, 2–11 CrossRef CAS.
- P. P. Power, Bonding and Reactivity of Heavier Group 14 Element Alkyne Analogues, Organometallics, 2007, 26, 4362–4372 CrossRef CAS.
- A. Sekiguchi, Disilyne With a Silicon-Silicon Triple Bond: A New Entry to Multiple Bond Chemistry, Pure Appl. Chem., 2008, 80, 447–457 CAS.
- A. Sekiguchi, R. Kinjo and M. Ichinohe, Interaction of π-bonds of the Silicon-Silicon Triple Bond with Alkali Metals: An Isolable Anion Radical Upon Reduction of a Disilyne, Synth. Met., 2009, 159, 773–775 CrossRef CAS.
- D. Scheschkewitz, Anionic Reagents with Silicon-Containing Double Bonds, Chem. - Eur. J., 2009, 15, 2476–2485 CrossRef CAS PubMed.
- Y. Wang and G. H. Robinson, Unique Homonuclear Multiple Bonding in Main Group Compounds, Chem. Commun., 2009, 5201–5213 RSC.
- R. C. Fischer and P. P. Power, π-Bonding and the Lone Pair Effect in Multiple Bonds Involving Heavier Main Group Elements: Developments in the New Millennium, Chem. Rev., 2010, 110, 3877–3923 CrossRef CAS PubMed.
- M. Kira, An isolable dialkylsilylene and its derivatives. A step toward comprehension of heavy unsaturated bonds, Chem. Commun., 2010, 46, 2893–2903 RSC.
- T. Sasamori, J. S. Han, K. Hironaka, N. Takagi, S. Nagase and N. Tokitoh, Synthesis and Structure of Stable 1,2-Diaryldisilyne, Pure Appl. Chem., 2010, 82, 603 CAS.
- Y. Peng, R. C. Fischer, W. A. Merrill, J. Fischer, L. Pu, B. D. Ellis, J. C. Fettinger, R. H. Herber and P. P. Power, Substituent Effects in Ditetrel Alkyne Analogues: Multiple vs. Single Bonded Isomers, Chem. Sci., 2010, 1, 461–468 RSC.
- A. Sekiguchi, R. Kinjo and M. Ichinohe, A Stable Compound Containing A Silicon-Silicon Triple Bond, Science, 2004, 305, 1755–1757 CrossRef CAS PubMed.
- N. Wiberg, S. K. Vasisht, G. Fischer and P. Mayer, Disilynes. III [1] a Relatively Stable Disilyne RSi≡SiR (R = SiMe(SitBu3)2), Z. Anorg. Allg. Chem., 2004, 630, 1823–1828 CrossRef CAS.
- T. Sasamori, K. Hironaka, T. Sugiyama, N. Takagi, S. Nagase, Y. Hosoi, Y. Furukawa and N. Tokitoh, Synthesis and Reactions of a Stable 1,2-diaryl-1,2-dibromodisilene: A Precursor for Substituted Disilenes and 1,2-diaryldisilyne, J. Am. Chem. Soc., 2008, 130, 13856–13857 CrossRef CAS PubMed.
- M. Stender, A. D. Phillips, R. J. Wright and P. P. Power, Synthesis and Characterization of a Digermanium Analogue of an Alkyne, Angew. Chem., Int. Ed., 2002, 41, 1785–1787 CrossRef CAS PubMed.
- M. Stender, A. D. Phillips and P. P. Power, Formation of [Ar*Ge{CH2C(Me)C(Me)CH2}CH2C(Me)N]2 (Ar* = C6H3-2,6-Trip2; Trip = C6H2-2,4,6-i-Pr3) Via Reaction of Ar*GeGeAr* with 2,3-dimethyl-1,3-butadiene: Evidence for the Existence of a Germanium Analogue of an Alkyne, Chem. Commun., 2002, 1312–1313 RSC.
- L. Pu, A. D. Phillips, A. F. Richards, M. Stender, R. S. Simons, M. M. Olmstead and P. P. Power, Germanium and Tin Analogues of Alkynes and Their Reduction Products, J. Am. Chem. Soc., 2003, 125, 11626–11636 CrossRef CAS PubMed.
- Y. Sugiyama, T. Sasamori, Y. Hosoi, Y. Furukawa, N. Takagi, S. Nagase and N. Tokitoh, Synthesis and Properties of a New Kinetically Stabilized Digermyne: New Insights for a Germanium Analogue of an Alkyne, J. Am. Chem. Soc., 2006, 128, 1023–1031 CrossRef CAS PubMed.
- G. H. Spikes and P. P. Power, Lewis Base Induced Tuning of the Ge–Ge Bond Order in a ‘‘digermyne’’, Chem. Commun., 2007, 85–87 RSC.
- A. D. Phillips, R. J. Wright, M. M. Olmstead and P. P. Power, Synthesis and Characterization of 2,6-Dipp2-H3C6SnSnC6H3-2,6-Dipp2 (Dipp = C6H3-2,6-Pri2): A Tin Analogue of an Alkyne, J. Am. Chem. Soc., 2002, 124, 5930–5931 CrossRef CAS PubMed.
- L. Pu, B. Twamley and P. P. Power, Synthesis and Characterization of 2,6-Trip2H3C6PbPbC6H3-2,6-Trip2 (Trip = C6H2-2,4,6-i-Pr3): a Stable Heavier Group 14 Element Analogue of an Alkyne, J. Am. Chem. Soc., 2000, 122, 3524–3525 CrossRef CAS.
- A. Bino, M. Ardon and E. Shirman, Formation of a Carbon-Carbon Triple Bond by Coupling Reactions In Aqueous Solution, Science, 2005, 308, 234–235 CrossRef CAS PubMed.
- P. Su, J. Wu, J. Gu, W. Wu, S. Shaik and P. C. Hiberty, Bonding Conundrums in the C2 Molecule: A Valence Bond Study, J. Chem. Theory Comput., 2011, 7, 121–130 CrossRef CAS PubMed.
- E. Ploshnik, D. Danovich, P. C. Hiberty and S. Shaik, The Nature of the Idealized Triple Bonds Between Principal Elements and the σ Origins of Trans-Bent Geometries—A Valence Bond Study, J. Chem. Theory Comput., 2011, 7, 955–968 CrossRef CAS PubMed.
- I. Seidu, M. Seth and T. Ziegler, Role Played by Isopropyl Substituents in Stabilizing the Putative Triple Bond in Ar'EEAr′ [E = Si, Ge, Sn; Ar' = C6H3-2,6-(C6H3-2,6-Pri2)2] and Ar*PbPbAr* [Ar* = C6H3-2,6-(C6H2-2,4,6-Pri3)2], Inorg. Chem., 2013, 52, 8378–8388 CrossRef CAS PubMed.
- D. Danovich, A. Bino and S. Shaik, Formation of Carbon–Carbon Triply Bonded Molecules from Two Free Carbyne Radicals via a Conical Intersection, J. Phys. Chem. Lett., 2013, 4, 58–64 CrossRef CAS PubMed.
- M. Karni, Y. Apeloig, D. Schröder, W. Zummack, R. Rabezzana and H. Schwarz, HCSiF and HCSiCl: The First Detection of Molecules with Formal C≡Si Triple Bonds, Angew. Chem., Int. Ed., 1999, 38, 331–335 CrossRef PubMed , and related references therein..
- D. Danovich, F. Ogliaro, M. Karni, Y. Apeloig, D. L. Cooper and S. Shaik, Silynes (RC≡SiR') and Disilynes (RSi≡SiR'): Why Are Less Bonds Worth Energetically More?, Angew. Chem., Int. Ed., 2001, 40, 4023–4026 CrossRef CAS PubMed.
- D. Gau, T. Kato, N. Saffon-Merceron, A. D. Cozar, F. P. Cossio and A. Baceiredo, Synthesis and Structure of a Base-Stabilized C-Phosphino-Si-AminoSilyne, Angew. Chem., Int. Ed., 2010, 49, 6585–6588 CrossRef CAS PubMed.
- N. Lühmann and T. Müller, A Compound with a Si–C Triple Bond, Angew. Chem., Int. Ed., 2010, 49, 10042–10044 CrossRef PubMed.
- H.-Y. Liao, M.-D. Su and S.-Y. Chu, A Stable Species with a Formal Ge≡C Triple Bond — A Theoretical Study, Chem. Phys. Lett., 2001, 341, 122–128 CrossRef CAS.
- P.-C. Wu and M.-D. Su, Theoretical Designs for Germaacetylene (RC≡GeR’): A New Target For Synthesis, Dalton Trans., 2011, 40, 4253–4259 RSC.
- P.-C. Wu and M.-D. Su, Effects of Substituents on the Thermodynamic and Kinetic Stabilities of HCGeX (X = H, CH3, F, and Cl) Isomers. A Theoretical Study, Inorg. Chem., 2011, 50, 6814–6822 CrossRef CAS PubMed.
- P.-C. Wu and M.-D. Su, A New Target for Synthesis of Triply Bonded Plumbacetylene (RC≡PbR): A Theoretical Design, Organometallics, 2011, 30, 3293–3301 CrossRef CAS.
- X.-T. Wen, Y.-C. Li and M.-D. Su, Substituent Effects on the Geometries and Energies of the Antimony-Silicon Multiple Bond, Bull. Chem. Soc. Jpn., 2014, 87, 816–818 CrossRef CAS.
- M.-D. Su, Doubly Bonded Molecules Containing Bismuth and Other Group 15 Elements in the Singlet and Triplet States, in Advances in Chemistry Research, ed. J. C. Taylor, Nova Science Publishers, Inc., New York, 2014, vol. 21, ch. 4, pp.149–184 Search PubMed.
- J.-S. Lu, S.-H. Su, M.-C. Yang, X.-T. Wen, J.-Z. Xie and M.-D. Su, Substituent Effects on Boron-Bismuth Triple Bond: A New Target for Synthesis, Organometallics, 2016, 35, 3924–3931 CrossRef CAS.
- J.-S. Lu, M.-C. Yang and M.-D. Su, The Effect of Substituents on the Stability of Triply Bonded Gallium≡Antimony Molecules: A New Target for Synthesis, Dalton Trans., 2017, 46, 1848–1856 RSC.
- J.-S. Lu, M.-C. Yang and M.-D. Su, The Effect of Substituents on the Triply Bonded Boron≡Antimony Molecules: A Theoretical Approach, Phys. Chem. Chem. Phys., 2017, 19, 8026–8033 RSC.
- J.-S. Lu, M.-C. Yang and M.-D. Su, Substituent Effects on the Stability of Thallium and Phosphorus Triple Bonds: A Density Functional Study, Molecules, 2017, 22, 1111–1124 CrossRef PubMed.
- J.-S. Lu, M.-C. Yang and M.-D. Su, Triply-bonded Indium≡Phosphorus Molecules: Theoretical Designs and Characterization, RSC Adv., 2017, 7, 20597–20603 RSC.
- J.-S. Lu, M.-C. Yang, S.-H. Su, X.-T. Wen, J.-Z. Xie and M.-D. Su Triple Bonds between Bismuth and Group 13 Elements: Theoretical Designs and Characterization, in Recent Progress in Organometallic Chemistry, ed. M. M. Rahman and A. M. Asiri, InTechOpen, London, 1st edn, 2017, ch. 4, pp. 71–99 Search PubMed.
- J.-S. Lu, M.-C. Yang and M.-D. Su, Triply Bonded Gallium≡Phosphorus Molecules: Theoretical Designs and Characterization, J. Phys. Chem. A, 2017, 121, 6630–6637 CrossRef CAS PubMed.
- J.-S. Lu, M.-C. Yang and M.-D. Su, Aluminum−Phosphorus Triple Bonds: Do Substituents Make Al≡P Synthetically Accessible?, Chem. Phys. Lett., 2017, 686, 60–67 CrossRef CAS.
- J.-S. Lu, M.-C. Yang and M.-D. Su, The Indium−Arsenic Molecules with an In≡As Triple Bond: A Theoretical Approach, ACS Omega, 2017, 2, 1172–1179 CrossRef CAS.
- J.-S. Lu, M.-C. Yang and M.-D. Su, Triple-Bonded Boron≡Phosphorus Molecule: Is That Possible?, ACS Omega, 2018, 3, 76–85 CrossRef CAS.
- J.-S. Lu, M.-C. Yang and M.-D. Su, A Possible Target: the Triply Bonded Indium≡Antimony Molecules With High Stability, New J. Chem., 2018, 42, 6932–6941 RSC.
- J.-S. Lu, M.-C. Yang, S.-H. Su and M.-D. Su, The Effect of Substituent on Molecules that Contain a Triple Bond Between Arsenic and Group 13 Elements: Theoretical Designs and Characterizations, in Chemical Reactions in Inorganic Chemistry, ed. C. Saravanan, InTechOpen, London, 1st edn, 2018, ch. 4, pp. 51–73 Search PubMed.
- J.-S. Lu, M.-C. Yang and M.-D. Su, Is It Possible To Prepare and Stabilize the Triply Bonded Thallium≡Antimony Molecules Using Substituents?, ACS Omega, 2018, 3, 10163–10171 CrossRef CAS.
- J.-S. Lu, M.-C. Yang, S.-H. Su and M.-D. Su, The Triply Bonded Al≡Sb Molecules: A Theoretical Prediction, in Basic Concepts Viewed from Frontier in Inorganic Coordination Chemistry, ed. T. Akitsu, InTechOpen, London, 1st edn, 2018, ch. 5, pp. 83–97 Search PubMed.
- P. Paetzold, Iminoboranes, Adv. Inorg. Chem., 1987, 31, 123–170 CrossRef CAS.
- P. Paetzold, Born Chemistry in Proceedings of the 6th International Meeting on Born Chemistry, Reactions at the Boron-Nitrogen Triple Bond, ed. S. Hermanek, World Scientific, Singapore, 1987, pp. 446–475 Search PubMed.
- P. Paetzold, New Perspectives in Boron Nitrogen Chemistry I, Pure Appl. Chem., 1991, 63, 345–350 CAS.
- P. Paetzold, Boron-Nitrogen Analogues of Cyclobutadiene, Benzene and Cyclooctatetraene: Interconversions, Phosphorus, Sulfur Silicon Relat. Elem., 1994, 93–94, 39–50 CrossRef CAS.
- R. J. Wright, A. D. Phillips, T. L. Allen, W. H. Fink and P. P. Power, Synthesis and Characterization of the Monomeric Imides Ar‘MNAr‘
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‘ (M = Ga or In; Ar‘ or Ar‘
‘ (M = Ga or In; Ar‘ or Ar‘![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‘ = Terphenyl Ligands) with Two-Coordinate Gallium and Indium, J. Am. Chem. Soc., 2003, 125, 1694–1695 CrossRef CAS PubMed.
‘ = Terphenyl Ligands) with Two-Coordinate Gallium and Indium, J. Am. Chem. Soc., 2003, 125, 1694–1695 CrossRef CAS PubMed. - R. J. Wright, M. Brynda, J. C. Fettinger, A. R. Betzer and P. P. Power, Quasi-Isomeric Gallium Amides and Imides GaNR2 and RGaNR (R = Organic Group): Reactions of the Digallene, Ar′GaGaAr′ (Ar′ = C6H3-2,6-(C6H3-2,6-Pri2)2) with Unsaturated Nitrogen Compounds, J. Am. Chem. Soc., 2006, 128, 12498–12509 CrossRef CAS PubMed.
- K. B. Wiberg, Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane, Tetrahedron, 1968, 24, 1083–1096 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint, Chem. Rev., 1998, 88, 899–926 CrossRef.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Pergamon, Oxford, England, 1984, pp. 452–513 Search PubMed.
- D. J. Liptrot and P. P. Power, London Dispersion Forces in Sterically Crowded Inorganic and Organometallic Molecules, Nat. Rev. Chem., 2017, 1, 1–12 CrossRef.
- Y. Zhao and D. G. Truhlar, Density Functionals with Broad Applicability in Chemistry, Acc. Chem. Res., 2008, 41, 157–166 CrossRef CAS PubMed.
- E. D. Glendening and F. Weinhold, Natural resonance theory: I. general formalism, J. Comput. Chem., 1998, 19, 593–609 CrossRef CAS.
- E. D. Glendening and F. Weinhold, Natural resonance theory: II. natural bond order and valency, J. Comput. Chem., 1998, 19, 610–627 CrossRef CAS.
- E. D. Glendening, J. K. Badenhoop and F. Weinhold, Natural resonance theory: iii. Chemical Applications, J. Comput. Chem., 1998, 19, 628–646 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00318e |
| This journal is © The Royal Society of Chemistry 2019 |