Open Access Article
Open Access ArticleMultifunctional amphiphilic ionic liquid pathway to create water-based magnetic fluids and magnetically-driven mesoporous silica†
Jing Shen *a,
Wen Heb and
Tongwen Wangb
*a,
Wen Heb and
Tongwen Wangb
aDepartment of Applied Chemistry, College of Vocational Education, Yunnan Normal University, Kunming 650092, China. E-mail: shenjingbox0225@hotmail.com
bCollege of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650092, China
First published on 25th January 2019
Abstract
Amphiphilic ionic liquids, 1-alkyl-3-methylimidazolium chloride (CnmimCl with n = 10, 12, 14, 16) were firstly used as modifiers to construct a self-assembly bilayer on the surface of iron oxide nanoparticles for generation of highly stable, water-based magnetic fluids. Subsequently, a magnet-driven mesoporous silica was synthesized by in situ self-assembly in the bilayer CnmimCl-stabilized magnetic fluid using the C16mimCl as template and tetraethylorthosilicate (TEOS) as silicon source via a hydrothermal synthesis and following calcination procedure. A systematic study was carried out addressing the influence of the alkyl chain length of CnmimCl in the primary and secondary layers on the stability of magnetic fluids. The characterization of TEM, XRD, VSM, electrophoresis experiments, TGA and DTA showed that stable water-based magnetic fluids can be synthesized based on the assembly of the well-defined bilayer-CnmimCl structure with long-chain C16mimCl as secondary layer on the magnetite (Fe3O4) nanoparticles. The results of small and wide-angle XRD, TEM, VSM, and N2 absorption experiments indicated that the nano-scale magnetic Fe3O4 particles were inlayed into hexagonal p6mm mesoporous silica (MCM-41 type) framework. Importantly, it was found that the obtained Fe3O4/MCM-41 was an appropriate adsorbent for the adsorption of rhodamine B and methylene blue from their aqueous solution. In addition, the adsorbent could be separated and reclaimed fleetly from the solution under external magnetic field.
Introduction
The use of ionic liquids has opened new avenues in the synthesis and fabrication of various new materials with advanced properties.1–3 Particularly, amphiphilic ionic liquids, composed of a charged hydrophilic imidazolium head group and a hydrophobic ‘long tail’ domain, can provide not only the amphiphilic properties of conventional surfactants, but also the specificity of typical short-chain room temperature ionic liquids such as low melting point, strong polarity and high resolvability in aqueous solution etc. Much work has been made to explore self-organized behaviour of amphiphilic ionic liquids in aqueous and other solvents.4–6 For example, Zheng and co-workers measured the micelle formation and the micelle aggregation numbers of amphiphilic ionic liquids in aqueous solution by surface tension, electrical conductivity and fluorescence measurement etc.7–9 They found that the surface activity of amphiphilic ionic liquids in aqueous solution is slightly superior to that of the typical cationic surfactants. These self-organized advantages of amphiphilic ionic liquids have been applied to construct ordered mesoporous materials. For instance, ordered mesoporous silica with 2D hexagonal structure was synthesized by using amphiphilic ionic liquids, 1-alkyl-3-methylimidazolium chloride (CnmimCl, n is number of carbon atoms in alkyl chains), as templates.10 Zhou et al. reported a preparation of supermicroporous lamellar silica through 1-hexadecyl-3-methylimidazolium chloride (C16mimCl) as template.11 Our group has also contributed much effort to successfully synthesize mesoporous silica with high-quality cubic gyroid and 2D hexagonal mesoporous structures by using the C16mimCl as template.12 More recently, we have employed a C16mimCl-assisted synthesis method to prepare polymer/Pd microspheres and a bimodal porous Pd-decorated silica.13 These research results confirmed that CnmimCl possess a specific templating performance in the synthesis of ordered porous structural materials. However, their potential as modifying agent, especially for the formation of stable CnmimCl-functionalized magnetic Fe3O4 nanoparticles (NPs) or their multifunctional combination for the fabrication of hybrid porous materials is less commonly known.The aim of this work is twofold: firstly, it is intended to demonstrate that 1-alkyl-3-methylimidazolium chloride (CnmimCl) can serve as a surface functional molecule to construct a bilayer of CnmimCl in the surface of magnetic Fe3O4 NPs, which can effectively produce a stable water-based magnetic fluid. Secondly, based on the CnmimCl-based magnetic fluid, a more facile access to prepare magnetically-driven mesoporous silica with magnetic Fe3O4 NPs inlayed randomly into ordered hexagonal mesoporous silica framework by using C16mimCl as template and their adsorption properties is presented.
The CnmimCl-bilayer structure onto Fe3O4 NPs is similar to the surfactant-bilayer modified on the surface of iron oxide particles, which has been confirmed to form stable water-based magnetic fluid by effectively overcoming the agglomeration of Fe3O4 NPs. The combinations of various surfactants, such as fatty acid/fatty acid,14,15 oleate sodium/PEG-4000,16 oleic acid/succinimide,17 and oleic acid/sodium dodecyl sulfate18 etc., have been used as modified bilayer to stabilize Fe3O4 NPs in aqueous solution. In our design, the first research goal is to build a CnmimCl-bilayer on the surface of Fe3O4 NPs. We expect that the CnmimCl should exhibit the strong self-assembly ability on the surface of Fe3O4 NPs to form a high charge density and strong hydrophilic surface, which can form stable water-based magnetic fluid.
Recently, significant advances have been achieved in the synthesis of magnetic mesoporous silica materials because of their versatile application such as adsorption and separation,19 catalysis20 and biological technology.21 In the synthesis of these magnetic mesoporous silica materials, the commonly used approach is based on the construction of a core–shell structure, in which magnetite particle services as inner core and a layer mesoporous silica acts as outer shell.21 This synthesis procedure is complicated, and it is difficult to accurately control the experimental conditions. It is also impossible to exclude the formation of some irregular pore structures. Thus, the exploitation of a convenient way of homogeneous incorporation of magnetic particles into the framework of mesoporous silica by optimized various methods is still a valuable research issue. Another objective of our research is to demonstrate the supramolecular templating function of C16mimCl for the preparation of highly ordered magnetic mesoporous silica in the prepared Fe3O4/C16mimCl/C16mimCl magnetic fluid. We expect that CnmimCl can be manipulated in tandem as bilayer modifying agent and templating agent in one system, should provide important guidelines for the multifunctional applications of amphiphilic ionic liquids in the synthesis of various nanostructured materials.
Experimental section
Synthesis of CnmimCl (n = 10, 12, 14, 16)
Amphiphilic ionic liquids, 1-alkyl-3-methylimidazolium chloride (abbreviated as CnmimCl, n is the number of carbon atoms in alkyl chains, n = 10, 12, 14, 16, respectively), were prepared according to a route reported in the literatures.13,22 All chemicals were purchased from Acrös and used as received. As a typical synthesis of C16mimCl, an excess of 1-hexadecyl chloride (33.41 g, 0.128 mol) was mixed with 1-methylimidazole (10.26 g, 0.125 mol). The mixture was put into a 250 mL flask, refluxed at 90 °C for 24 h, and then cooled to room temperature. The product was further purified by recrystallization in tetrahydrofuran (THF). After being washed several times with THF, the white crystalline C16mimCl powder was collected by filtration, and dried in air at room temperature. The preparation of the other CnmimCl was achieved by repeating the above procedure with substitution of the corresponding 1-alkyl chloride for 1-hexadecyl chloride, i.e., 1-decyl chloride for C10mimCl, 1-dodecyl chloride for C12mimCl and 1-tetradecyl chloride for C14mimCl. The structures of the obtained CnmimCl were identified by IR spectrum (see Fig. S1†).Synthesis of bilayer CnmimCl-stabilized magnetic fluids
The bilayer CnmimCl-stabilized magnetic fluids were synthesized by the chemical coprecipitation of Fe2+ and Fe3+ salts in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 M ratio from a basic aqueous solution containing a small number of CnmimCl, followed by the modifying process of the primary and secondary CnmimCl, respectively. In a typical preparation of primary C10mimCl-coated iron oxide particles, 0.86 g of FeCl2·4H2O, 2.35 g of FeCl3·6H2O, and 0.1 g of C10mimCl were dissolved in 40 mL of distilled water at 60 °C under mechanical paddle stirring and N2 purge. After 10 min, the temperature was raised to 80 °C, followed by 0.5 mL of 33% (w/w) NH4OH to adjust the pH value of the mixture to 9. After reaction for 10 min, the pH value of the mixture was adjusted again to 13 by adding tetramethylammonium hydroxide dropwise, and then, C10mimCl was further added to the suspension in five 0.2 g amounts over 5 min. The reaction was allowed to proceed for 30 min at 80 °C under constant stirring. Subsequently, the suspension was cooled slowly to room temperature and the precipitates were isolated from the solution by magnetic decantation. The precipitates were further purified with the magnetic decantation and redispersion in water and ethanol for three cycles. The resulted product was coded as Fe3O4/C10mimCl. The other primary CnmimCl-coated iron oxide particles were prepared by repeating above procedure with the corresponding CnmimCl as primary layer modifier and coded as Fe3O4/C12mimCl, Fe3O4/C14mimCl and Fe3O4/C16mimCl, respectively. For comparison, bare Fe3O4 nanoparticles were prepared by above similar procedure without addition of any CnmimCl.
2 M ratio from a basic aqueous solution containing a small number of CnmimCl, followed by the modifying process of the primary and secondary CnmimCl, respectively. In a typical preparation of primary C10mimCl-coated iron oxide particles, 0.86 g of FeCl2·4H2O, 2.35 g of FeCl3·6H2O, and 0.1 g of C10mimCl were dissolved in 40 mL of distilled water at 60 °C under mechanical paddle stirring and N2 purge. After 10 min, the temperature was raised to 80 °C, followed by 0.5 mL of 33% (w/w) NH4OH to adjust the pH value of the mixture to 9. After reaction for 10 min, the pH value of the mixture was adjusted again to 13 by adding tetramethylammonium hydroxide dropwise, and then, C10mimCl was further added to the suspension in five 0.2 g amounts over 5 min. The reaction was allowed to proceed for 30 min at 80 °C under constant stirring. Subsequently, the suspension was cooled slowly to room temperature and the precipitates were isolated from the solution by magnetic decantation. The precipitates were further purified with the magnetic decantation and redispersion in water and ethanol for three cycles. The resulted product was coded as Fe3O4/C10mimCl. The other primary CnmimCl-coated iron oxide particles were prepared by repeating above procedure with the corresponding CnmimCl as primary layer modifier and coded as Fe3O4/C12mimCl, Fe3O4/C14mimCl and Fe3O4/C16mimCl, respectively. For comparison, bare Fe3O4 nanoparticles were prepared by above similar procedure without addition of any CnmimCl.
To prepare stable water-based magnetic fluids, a series of secondary CnmimCl with similar chemical structures to the primary CnmimCl were coated on the primary CnmimCl-coated iron oxide particles. In a typical preparation of Fe3O4/C10mimCl/C16mimCl magnetic fluid, 1 mL of C16mimCl solution (40 g L−1) was firstly added to 20 mL of distilled water. 0.5 g of Fe3O4/C10mimCl obtained above was added to the solution under ultrasound at room temperature. After 10 min, C16mimCl solution (40 g L−1) was further added dropwise to the suspension in 1 mL amounts over 5 min under vigorous mechanical paddle stirring until no visible settling and phase separation was observed when the solution was placed over a permanent magnet for a period of 5 min. The sample was coded as Fe3O4/C10mimCl/C16mimCl. The preparations of the other bilayer structures were achieved by repeating the above procedure with corresponding bilayer combination of CnmimCl as primary and secondary layers. In order to make subsequent various analyses, a small portion of dried magnetic nanoparticles can be separated from the magnetic fluids by a decantation procedure using a permanent magnet placed next to the bottle to provide a magnetic field of about 0.3 T over 3–4 weeks.
Synthesis of magnetic mesoporous silica
The magnetic mesoporous silica was synthesized by a hydrothermal synthesis procedure in the Fe3O4/C16mimCl/C16mimCl fluid using tetraethylorthosilicate (TEOS) as silicon source and C16mimCl as template. In a typical synthesis procedure, C16mimCl and NaOH were dissolved in the Fe3O4/C16mimCl/C16mimCl fluid under mechanical paddle stirring. After homogenization of the mixture, TEOS was added dropwise at room temperature. The molar compositions of the starting mixtures were 1.0 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x Fe3O4/C16mimCl/C16mimCl
x Fe3O4/C16mimCl/C16mimCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1C16mimCl
0.1C16mimCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 NaOH
0.45 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140H2O (x = 0.025, 0.05, 0.10, respectively). The resulted mixtures were stirred at room temperature for 60 min, and then transferred into a PTFE-lined steel autoclave and heated at 100 °C for 3 days. After hydrothermal treatment, the mixtures were filtered, washed with deionized water, dried under atmosphere at room temperature, and finally calcined at 550 °C for 5 h with a temperature ramp of 2 °C min−1 under static air conditions to remove the template. The final product, coded as Fe3O4/MCM-41, was ground into powder for further characterization.
140H2O (x = 0.025, 0.05, 0.10, respectively). The resulted mixtures were stirred at room temperature for 60 min, and then transferred into a PTFE-lined steel autoclave and heated at 100 °C for 3 days. After hydrothermal treatment, the mixtures were filtered, washed with deionized water, dried under atmosphere at room temperature, and finally calcined at 550 °C for 5 h with a temperature ramp of 2 °C min−1 under static air conditions to remove the template. The final product, coded as Fe3O4/MCM-41, was ground into powder for further characterization.
Adsorption experiment
The adsorption experiments were carried out by the adsorption of two dyes, rhodamine B (RhB) and methylene blue (MB), using the prepared Fe3O4/MCM-41 as adsorbent at room temperature. For comparison, the adsorption experiments of two dyes on the pure mesoporous silica MCM-41 (without Fe3O4 NPs) prepared using C16mimCl template method which we reported earlier,12 and on the bare Fe3O4 particles synthesized using the same chemical coprecipitation method without adding ionic liquid were also studied at room temperature. In a typical adsorption procedure, Fe3O4/MCM-41 (0.1 g) was added to the aqueous suspensions (50 mL) of the dye solutions with concentration of x mg L−1 (where x = 10, 20, 50, 100, 200, and 400 mg L−1, respectively) under mechanical stirring. At fixed intervals, the precipitates were isolated from the suspensions by magnetic decantation, and the absorbance of the solutions was measured by UV-vis spectrophotometer (SHIMADZU UV-1780) at 554 and 664 nm for RhB and MB solutions, respectively. The decolorization rates of the solutions were calculated according to eqn (1)| D% = (A0 − At) × 100%/A0 | (1) |
| qt = (C0 − Ct)V/m | (2) |
| qe = KFCe1/n | (3) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + (1/n)log KF + (1/n)log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (4) |
Moreover, after the adsorption process, the precipitates were calcined at 550 °C for 5 h to remove the adsorbed dyes. The reusability of the recovered Fe3O4/MCM-41 adsorbents was also studied by tracking the changes in UV-vis absorption spectrograph of the adsorption system under different cycles.
Characterization
Small- and wide-angle X-ray diffraction (XRD) patterns were measured on a TTR III powder X-ray diffractometer using Cu Kα radiation (wavelength 0.154 nm) at a rate of 0.05° 2θ s−1 and operated at 40 kV and 30 mA. Transmission electron microscopy (TEM) images were taken on a JEM-2100 electron microscope at an acceleration voltage of 200 kV. The specimens for TEM were prepared by dropping a small drop of the solutions onto a carbon-coated copper grid. Electrophoresis experiments were performed on a Nanjing Sangli DYL-3 electrophoresis apparatus with 15 V of external voltage. The magnetization of the samples was measured by a vibrating sample magnetometer (VSM 7407) at room temperature. Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were performed with a ZRY-1P thermal analysis system. Nitrogen sorption experiments were conducted using a Micromeritics Tristar 3000 automated gas adsorption analyzer. A TENSOR27 Fourier transform infrared (FT-IR) spectrometer was employed for recording IR spectra.Results and discussion
Synthesis and characterization of CnmimCl bilayer assembled onto Fe3O4 particles
As schematically depicted in Scheme 1, the strategy to prepare the hydrophilic magnetic Fe3O4 NPs with ionic liquid CnmimCl bilayer as shell involves the first synthesis of Fe3O4 NPs, followed by coating magnetite NPs with CnmimCl (n = 10, 12, 14, 16) as inner layer and outer layers, respectively. The Fe3O4 NPs were synthesized based on the well-established coprecipitation method using Fe2+ and Fe3+ salts from a basic aqueous solution.23 We found that during the precipitation process, the size of Fe3O4 NPs can be easily controlled with the initial presence of a small quantity of CnmimCl (2.5 g L−1). Before coating primary CnmimCl, the pH value of the suspension was adjusted to 13 by adding TMAOH, which led to negatively charged iron oxide particles.24 The precipitated Fe3O4 NPs were first coated with a primary layer of CnmimCl adsorbed to the iron oxide surfaces through an electrostatic attraction between the negatively charged Fe3O4 NPs and the positively charged imidazole heads of CnmimCl. For example, the particles coated with monolayer C10mimCl were unstable and settled from the aqueous solution in a few minutes due to its hydrocarbon chains extending from the particle surface (Fig. S2a†). These particles were attracted by placing a magnet near the glass bottle, demonstrating that the particles possess magnetic properties (Fig. S2b†). Compared with the frequently-used surfactants such as fatty acids that are sparingly soluble in water and their solubility was enhanced by the presence of acetone,15 CnmimCl is highly soluble in water because of its special hydrophilic imidazole head. The excellent solubility of CnmimCl in water provide the possibility of direct interactions between individual molecules of CnmimCl and Fe3O4 particle, which is especially important for limiting the growth of Fe3O4 particle and preventing the agglomeration of the particles. Moreover, it was found that a drop by drop and slow adding way for the CnmimCl aqueous solution is especially important for preventing the micelle formation of CnmimCl in the dispersion. For example, the cmc of C16mimCl at 298 K was reported to be 1.21 mM,25 and the concentration of one drop of the C16mimCl aqueous solution in the suspension is about 0.043 mM, which is well below the cmc of C16mimCl. Hence, this adding way is favourable to coat the individual C16mimCl molecule onto Fe3O4 particles efficiently.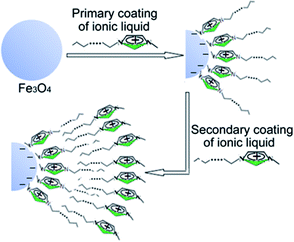 | ||
| Scheme 1 Schematic diagram of the synthesis procedure of Fe3O4/CnmimCl/CnmimCl particle using CnmimCl as bilayer modifier. | ||
To prepare stable water-based magnetic fluids, CnmimCl was secondly coated on the Fe3O4/CnmimCl particles to form Fe3O4/CnmimCl/CnmimCl particles. As shown in Fig. S2c,† Fe3O4/C10mimCl/C16mimCl colloidal suspension exhibited a remarkable stability as evidenced by a lack of observable precipitation over periods of more than 12 months. The zeta potential (ζ) of the Fe3O4/C10mimCl/C16mimCl colloidal suspension was found to be approximately +40 mV measured by electrophoresis experiments. These results imply a hypothetical structure of the exposure of imidazole head groups of the secondary layer C16mimCl molecules toward the surrounding solution, and their hydrophobic long alkyl chain extending or inserting into the fence of alkyl chain of Fe3O4/C10mimCl by means of a hydrophobic interaction. The exposure of imidazole head groups in the surface of Fe3O4/C10mimCl/C16mimCl provided high charge density and strong hydrophilic surfaces, resulting in the formation of stable and water-based magnetic fluid. We observed the formation of similar stable water-based magnetic fluids for Fe3O4/C12mimCl/C16mimCl, Fe3O4/C14mimCl/C16mimCl and Fe3O4/C16mimCl/C16mimCl.
Fig. 1A and B show the typical TEM images of ionic liquid bilayer-stabilized Fe3O4/C10mimCl/C16mimCl and Fe3O4/C16mimCl/C16mimCl particles, respectively. It is clear that both magnetic oxides formed a well-dispersed quasi spherical morphology with clear boundary and mean diameter of approximately 10.5 and 10.1 nm. Electron diffractions (the insets in Fig. 1A and B) measured from a large zone present a series of rings that can be indexed to the magnetite structure, which is consistent with the results obtained from wide-angle XRD patterns described in Fig. S3.†
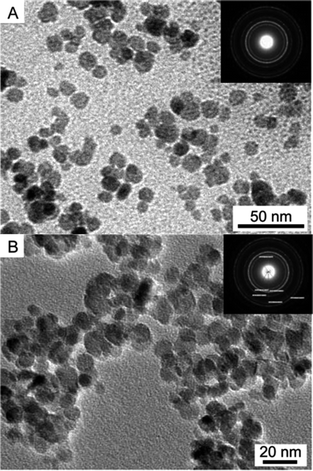 | ||
| Fig. 1 TEM images of (A) ionic liquid bilayer-stabilized Fe3O4/C10mimCl/C16mimCl and (B) Fe3O4/C16mimCl/C16mimCl particles. The insets show the corresponding electron diffraction images. | ||
The magnetization curves of bare Fe3O4, Fe3O4/C10mimCl, Fe3O4/C10mimCl/C16mimCl (Fig. 2A) and bare Fe3O4, Fe3O4/C16mimCl, Fe3O4/C16mimCl/C16mimCl (Fig. 2B) displayed a typical magnetization “S” curve, where no reduced remanence and coercivity were observed, revealing the superparamagnetic nature of these magnetic particles. The saturation magnetization (Ms) of 74.2, 65.1 and 55.1 emu g−1 was determined for bare Fe3O4, Fe3O4/C10mimCl and Fe3O4/C10mimCl/C16mimCl, respectively. The similar results of the gradual reducing Ms from 74.2 to 61.8 and 55.0 emu g−1 can be observed for bare Fe3O4, Fe3O4/C16mimCl and Fe3O4/C16mimCl/C16mimCl (see Fig. 2B). Obviously, the coating of primary C10mimCl (or C16mimCl) layer onto Fe3O4 NPs led to a significant decrease in the values of Ms, and the coating of secondary C16mimCl layer onto Fe3O4/C10mimCl (or Fe3O4/C16mimCl) particles showed lower value of Ms, i.e. Ms(Fe3O4) > Ms(Fe3O4/C10mimCl) > Ms(Fe3O4/C10mimCl/C16mimCl). These phenomena can be attributed to the detachment from dipole coupling due to the introduction of nonmagnetic species (CnmimCl) on the surface of Fe3O4 particles.26,27
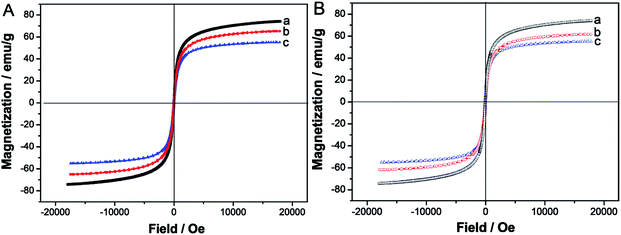 | ||
| Fig. 2 (A) Magnetization curves of (a) bare Fe3O4, (b) Fe3O4/C10mimCl, (c) Fe3O4/C10mimCl/C16mimCl, and (B) magnetization curves of (a) bare Fe3O4, (b) Fe3O4/C16mimCl, (c) Fe3O4/C16mimCl/C16mimCl. | ||
To demonstrate the existence of CnmimCl bilayer on the surface of iron oxide NPs, thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were performed for the samples dried at room temperature. Fig. 3A and B present the typical TGA and DTA curves of Fe3O4/C10mimCl and Fe3O4/C10mimCl/C16mimCl, respectively. As can be seen in Fig. 3A (TGA curves), at the temperature below 150 °C, both samples exhibited a small decrease step with a percentage weight loss of about 4.0% and 3.6% owing to the removal of adsorbed water in the samples. For the monolayer C10mimCl-coated particles (Fe3O4/C10mimCl), a well-defined percentage weight loss of about 4.5% was detected over a temperature range of 170–400 °C. The weight loss should be assigned to desorption and subsequent combustion of the monolayer C10mimCl on the surface of particles. For the bilayer C10mimCl/C16mimCl-coated particles (Fe3O4/C10mimCl/C16mimCl), a more significant percentage weight loss of about 12.7% was observed between 170 and 450 °C. The difference of weight loss between Fe3O4/C10mimCl and Fe3O4/C10mimCl/C16mimCl can be attributed to the removal of the secondary C16mimCl shell layer. The DTA curve (Fig. 3B) of Fe3O4/C10mimCl exhibited one well-defined exothermic peak in the temperature range of 200–350 °C, corresponding to decomposition of the monolayer C10mimCl on the surface of particles, while The DTA curve of Fe3O4/C10mimCl/C16mimCl (Fig. 3B) displayed two distinguishable exothermic peaks between 250 and 450 °C, which might be due to the decomposition of the outer layer (the secondary layer) C16mimCl and the inner layer (the primary layer) C10mimCl, respectively. A small exothermic peak at around 500–600 °C is possibly due to the structural transformation from magnetite to hematite when the temperature of magnetite NPs was exceeded 400 °C.28 These observations can be easily explained in terms of the different molecular weights and boiling points of C10mimCl and C16mimCl molecules. Moreover, the two exothermic peaks taken place at different temperature can be assigned to the difference of interactions, such as electrostatic attraction between C10mimCl and magnetite NPs, and hydrophobic interaction between the alkyl chain of C10mimCl and C16mimCl. The similar results can be observed in Fig. 3C for Fe3O4/C16mimCl (weight loss of about 9.2%) and Fe3O4/C16mimCl/C16mimCl (weight loss of about 19.2%), and Fig. 3D for Fe3O4/C16mimCl with one exothermic peak and for Fe3O4/C16mimCl/C16mimCl with two exothermic peaks. These results revealed the existence of two different molecular layers coated on the iron oxide surface. Analogous to our results, Shen et al. observed the significant difference in the weight loss for monolayer and bilayer fatty acid surfactant-coated magnetite particles.15
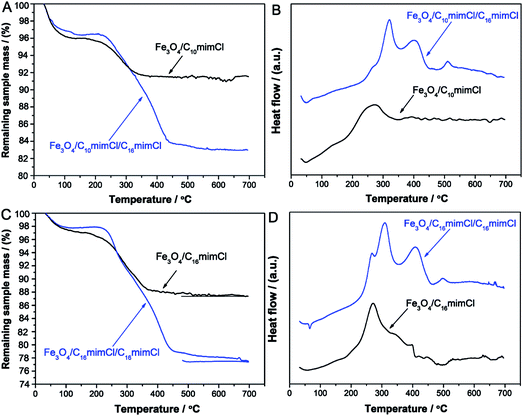 | ||
| Fig. 3 (A) TGA and (B) DTA curves of Fe3O4/C10mimCl and Fe3O4/C10mimCl/C16mimCl. (C) TGA and (D) DTA curves of Fe3O4/C16mimCl and Fe3O4/C16mimCl/C16mimCl. | ||
We calculated further the coverage parameters of the monolayer CnmimCl and the secondary layer CnmimCl on the Fe3O4 particle surface, respectively, in terms of the above TGA results. In these calculations, Fe3O4 particle was regard as spherical shape with mean diameter D of 10 nm on the basis of the results of TEM (Fig. 1). The surface areas of the spheres were determined with diameters D and D + 2l for the monolayer and secondary coating of surfactants, respectively,15 with l being the chain length of the primary surfactant. For example, the surfactant with chain length of C10 was estimated to be ca. 1.415 nm based on the method proposed by Shaw.29 Table 1 lists the coverage parameters of the monolayer and secondary layer CnmimCl assembled with C10mimCl and C16mimCl on Fe3O4 particle surface. It is clear that the increase in the number of carbon atoms of the monolayer layer ionic liquid molecule from 10 to 16 increased the weight percent of the ionic liquid from 4.5 to 9.2, and the number of the ionic liquid molecules on each particle increased from 316 to 501. Meanwhile, the number of the C16mimCl molecules in secondary layer on each particle increased from 469 to 608. These results demonstrated that the long-chain C16mimCl has stronger anchoring capacity on the iron oxide surface. The molecule number of 608 on each particle is close to that of the saturated fatty acids (e.g. myristic acid) adhered to iron oxide particle by a densely packed pattern.14 We observed that the long-chain C16mimCl exhibited a stronger assembly capability than that of the short-chain C10mimCl for the secondary layer construction. The phenomenon can be explained on the basis of a competition between affinity of the imidazole head groups of CnmimCl for water and hydrophobic interaction between hydrocarbon tails of the primary and secondary CnmimCl. It was known that the positively charged imidazole head groups possess intense hydrophilic properties.2 The shorter the hydrocarbon tail of CnmimCl is, the stronger the hydrophilic ability of CnmimCl has. Hence, as the short-chain C10mimCl was used as the secondary layer stabilizer, a highly affinity of water might make its molecules to have a tendency to remain in aqueous solution, resulting in a loose array of molecules on the particle surface. We found that the construction of Fe3O4/C10mimCl/C10mimCl, Fe3O4/C12mimCl/C10mimCl, Fe3O4/C14mimCl/C10mimCl and Fe3O4/C16mimCl/C10mimCl cannot produce stable magnetic fluids, and their particles settled from the aqueous solution in a few minutes. When the carbon atom number of the secondary layer CnmimCl increases to 16, the hydrophobic interaction is evidently enhanced, leading to a significant increase in the assembly capability on the particle coated with the primary layer CnmimCl. The bilayer CnmimCl-coated Fe3O4 NPs, such as Fe3O4/C16mimCl/C16mimCl, having close-packed imidazole headgroups exposing to the surrounding solution would provide a highly charge-density and strongly hydrophilic surface. The special structure surface can prevent the particles not only from aggregating but also from oxidizing through the electrostatic and steric repulsions between the particles, leading to the highly stable, water-based magnetic fluids.
| Sample | wta% (adsorbed water) | wta% (CnmimCl onto Fe3O4 NPs) | wt% (out layer CnmimCl) | wtoutb (weight of out layer CnmimCl on per g Fe3O4) (g) | Noutc (number of out layer CnmimCl molecules on each particle) |
|---|---|---|---|---|---|
| a The mass percent content of component was determined on the basis of the results of TGA.b wtout = wt% (out)/wt% Fe3O4, where the mass percent content of Fe3O4 was calculated by a formula that subtracts the mass percent of CnmimCl and corresponding adsorbed water.c Nout = N1/N2, where N1 = wtout × NA/Mout is the number of CnmimCl molecules per g Fe3O4, with NA being the Avogadro constant and Mout being the molar mass of out layer CnmimCl, and N2 = V1/V2 is the number of Fe3O4 particles per g Fe3O4, with V1 = 1/ρ and V2 = 4πR13/3 (or V2 = 4πR23/3) being the volume per g Fe3O4 and the volume per particle, respectively, where ρ is the density of Fe3O4 (5.18 g cm−3) and R1 is the radius of Fe3O4 particle, and R2 = R1 + l, with l being the length of primary layer CnmimCl. | |||||
| Fe3O4/C10mimCl | 4.0 | 4.5 | 4.5 | 0.05 | 316 |
| Fe3O4/C10mimCl/C16mimCl | 3.6 | 12.7 | 8.2 | 0.098 | 469 |
| Fe3O4/C16mimCl | 2.8 | 9.2 | 9.2 | 0.105 | 501 |
| Fe3O4/C16mimCl/C16mimCl | 2.2 | 19.2 | 10 | 0.127 | 608 |
Characterization of C16mimCl-based magnetic mesoporous silica
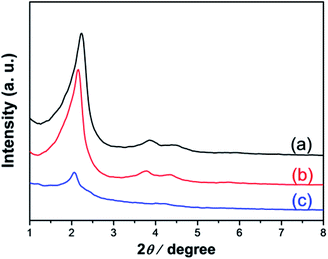
As shown in the Scheme 2, in the above synthesized Fe3O4/C16mim/C16mim magnetic fluid, a magnetic mesoporous silica was synthesized by a hydrothermal synthesis using C16mimCl as template and TEOS as silicon source. Fig. 4 shows small-angle XRD patterns of the calcined samples prepared from reaction mixtures with an initial molar ratio n(Fe3O4/C16mimCl/C16mimCl)/n(TEOS) of 0.025, 0.05 and 0.10, respectively. It is seen that very similar diffraction patterns with three well-resolved characteristic peaks attributed to (100), (110) and (200) planes of p6mm hexagonal structure (with reciprocal spacing, 1/dhkl, ratios of 31/2, 2, 71/2) were detected for two samples (Fig. 4a and b). These well-defined reflections peaks are in good agreement with the peaks of patterns from the siliceous MCM-41 prepared using quaternary ammonium ion surfactants,30 which suggests that the presence of magnetic particles with n(Fe3O4/C16mimCl/C16mimCl)/n(TEOS) of 0.025 and 0.05 did not destroy the ordered array of mesoporous cannels. However, when the molar ratio was increased to 0.10 (Fig. 4c), only one well-resolved diffraction peak (100) in the 2θ range between 2 and 8 was detected, indicating that the ordered degree of mesopores is reduced. This is probably owing to the overfull addition of Fe3O4/C16mimCl/C16mimCl particles, disrupting the charge density matching between the silicon species and C16mimCl liquid crystal phase, finally, leading to the partial breakdown of long-range ordered mesoporous silica structure. Generally, when guest species are loaded in the mesopores, the intensity of their XRD lines decreases.31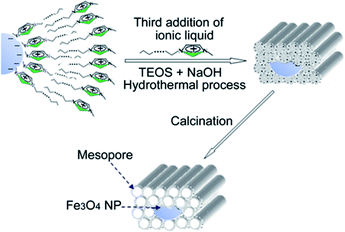 | ||
| Scheme 2 Schematic diagram of prepared route of magnetic mesoporous silica using C16mimCl as template and TEOS as silicon source in the Fe3O4/C16mimCl/C16mimCl magnetic fluid. | ||
TEM images recorded along [100] (Fig. 5A) and [001] (Fig. 5B) directions for the calcined sample with n(Fe3O4/C16mimCl/C16mimCl)/n(TEOS) of 0.05 showed ordered hexagonal arrangement of mesopore channels as well as dark quasi spherical Fe3O4 NPs. These Fe3O4 NPs were enwrapped by the ordered mesoporous silica channels and distributed randomly throughout the mesoporous silica matrix. The TEM images provided a direct and conclusive evidence of the coexistence of Fe3O4 NPs and well-defined ordered mesoporous silica. Wide-angle XRD pattern (Fig. S4A†) of this sample measured at 2θ of 20–80° presented some distinguishable weak Bragg peaks that can be assigned to magnetite crystalline phase (JCPDS card no. 28-0491), which further confirms the existence of magnetite Fe3O4 NPs in the sample. A gradual protuberance at low angle can be attributed to amorphous silica of the pore walls.32 Magnetization curve of this sample shown in Fig. S4B† exhibited also a typical magnetization “S” curve without obvious remanence and coercivity, revealing the superparamagnetic feature of the magnetic mesoporous silica.
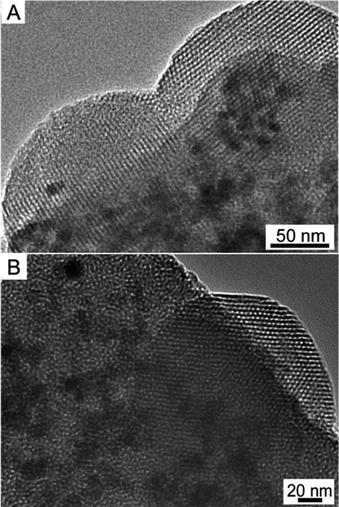 | ||
| Fig. 5 TEM images recorded along the (A) [100] and (B) [001] directions of calcined sample prepared using C16mimCl as template in the Fe3O4/C16mimCl/C16mimCl magnetic fluid. | ||
Nitrogen physisorption isotherm (Fig. 6A) of this sample displayed a type IV adsorption isotherm with an obvious hysteresis loop at a relatively high p/p0 values according to IUPAC,33 indicating the presence of open pores. A steep increasing occurs at a relative pressure 0.30 < p/p0 < 0.43, which is due to the filling of mesoporous walls by capillary condensation. In the previous publication,12 the hysteresis phenomenon was observed and attributable to the filling of a secondary pore structure, which resulted from grain boundaries, creating small cavities between adjacent ordered regions. In this sample, the Fe3O4 NPs can be regarded as grain, and its boundaries might create small cavities, therefore, resulting in the visible hysteresis loop. Pore size distribution calculated by BJH model (Fig. 6B) showed a narrow pore size distribution with the mean size of about 2.5 nm, which is in agreement with the value from TEM images. The BET surface area and total pore volume are 915 m2 g−1 and 0.78 cm3 g−1, respectively.
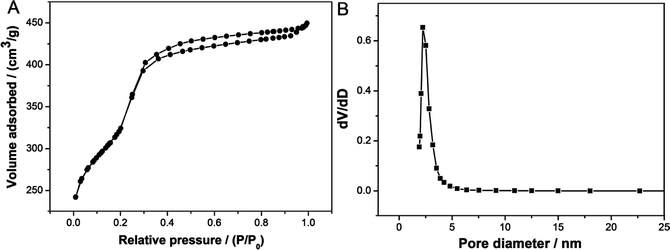 | ||
| Fig. 6 (A) N2 adsorption–desorption isotherm of calcined sample prepared using C16mimCl as template in the Fe3O4/C16mimCl/C16mimCl magnetic fluid, and (B) its BJH pore size distribution. | ||
Adsorption of magnetic mesoporous silica
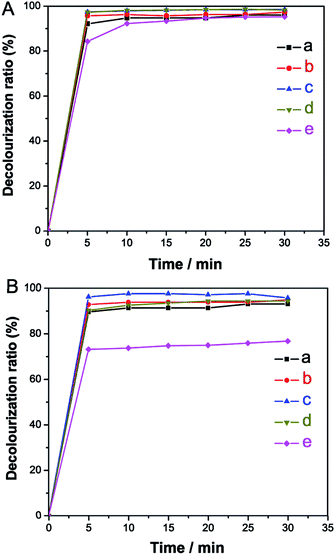
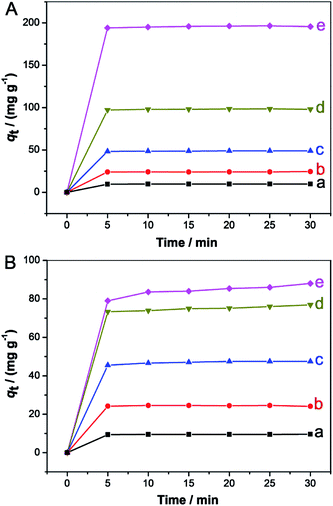
To evaluate the adsorption properties of the prepared Fe3O4/MCM-41, we employed rhodamine B (RhB) and methylene blue (MB) solutions as model systems at room temperature. Fig. 7 shows the time-dependent decolorization rates of two dye solutions measured at different initial concentrations after adsorption using the Fe3O4/MCM-41 as adsorbent. It is clear that the decolorization rates of both solutions increased fast and reached a nearly plateau slope (about 90%) within 10 min except for MB with initial concentration of 200 mg L−1. Fig. 8 shows the effect of contact time on the adsorption capacity of the Fe3O4/MCM-41 in RhB (Fig. 8A) and MB (Fig. 8B) solutions with different initial concentrations of 20, 50, 100, 200 and 400 mg L−1, respectively. The two dyes were adsorbed rapidly in the initial 5 min. After that, the adsorption capacity did not change with increasing of the contact time, indicating the adsorption equilibrium was approached. For adsorption of RhB, the adsorption capacity of the Fe3O4/MCM-41 in RhB solutions with different initial concentrations of 20, 50, 100, 200 and 400 mg L−1 at equilibrium (30 min) can reach 9.74, 24.4, 48.8, 98.0 and 196 mg g−1, respectively (Fig. 8A). Compared with the maximum adsorption capacity (qm = 196 mg g−1) of the Fe3O4/MCM-41, the pure mesoporous silica MCM-41 had higher adsorption capacity (qm = 393 mg g−1), which is exactly two times that of the Fe3O4/MCM-41. The reason is probably that the specific surface area (1200 m2 g−1)12 of the pure MCM-41 is higher than that of the Fe3O4/MCM-41 (915 m2 g−1). However, the pure mesoporous silica MCM-41 can only be recovered from the solution by centrifugation, while the Fe3O4/MCM-41 can be easily recovered by magnetic separation technology. The qm of the bare Fe3O4 particles prepared using the same chemical coprecipitation method without adding ionic liquid was found to be 7.5 mg g−1, which is lower than that of the Fe3O4/MCM-41. This may be attributed to the fact that the Fe3O4/MCM-41 has adequate mesoporous channels.Similarly, for adsorption of MB, the adsorption capacity of the Fe3O4/MCM-41 in MB solutions with different initial concentrations of 20, 50, 100, 200 and 400 mg L−1 at equilibrium (30 min) was found to be 9.62, 24.1, 47.5, 76.9 and 88.0 mg g−1, respectively (Fig. 8B). It is clear that when the initial concentration of dyes is less than 100 mg L−1, the adsorption capacity of the Fe3O4/MCM-41 for both dye solutions on is close. However, when the initial concentration of dyes was increased from 200 to 400 mg L−1, the adsorption capacity of the Fe3O4/MCM-41 increased from 98.0 to 196 mg g−1 for RhB solution, while for MB solution, the value increased slowly from 76.9 to 88.0 mg g−1. It can be seen that for both dye solutions with high concentration (≥200 mg g−1), the Fe3O4/MCM-41 is more beneficial for adsorption of RhB than MB. This phenomenon may be attributed to the fact the Fe3O4/MCM-41 is more likely to adsorb RhB with larger molecular structure because of the special mesoporous structure of the Fe3O4/MCM-41. It was also found that for the adsorption of MB, the qm of 97.5, 88.0 and 5.50 mg g−1 was determined for pure MCM-41, Fe3O4/MCM-41 and bare Fe3O4 particles, respectively, which showed a similar trend with that of the adsorption of RhB.
The well-defined linear relations (see Fig. S5†), fitted by Freundlich isotherm equation were observed for RhB solution in the range of 20–400 mg L−1 and for MB solution in the range of 10–100 mg L−1 adsorbed on the Fe3O4/MCM-41. The obtained values of KF and 1/n were found to be 20 mg g−1 and 1.074 for RhB, and 11 mg g−1 and 0.957 for MB, respectively. Obviously, the adsorption capacity of the Fe3O4/MCM-41 to RhB is higher than that of MB.
These results of adsorption analyses imply that the Fe3O4/MCM-41 has luxuriant mesoporous channels and high surface area, and can be potentially used as an adsorbent in liquid-phase processes. It is well-known that silicon-based MCM-41 mesopores have abundant and negatively charged surface silicon hydroxyl groups, which can adsorb the positively charged cationic dye, such as RhB or MB, by means of an electrostatic adsorption.
The magnetic separability of the dye-adsorbed Fe3O4/MCM-41 adsorbents was tested by placing a conventional laboratory magnet near the small beaker containing the mixtures of two dye solutions (100 mg L−1) and the adsorbent powder. As shown in Fig. S6,† after a certain time interval, the powder was attracted by the magnet (on the right), and the colour of the powder changed from grey to orange-red (Fig. S6A†) and grey to blue (Fig. S6B†) for RhB-adsorbed and MB-adsorbed Fe3O4/MCM-41, respectively. The clear solution could be decanted off or removed by pipette. These simple experiments confirmed that the Fe3O4/MCM-41 is magnetic and can be used as a magnetic adsorbent to remove dyes in liquid-phase.
The regeneration of the Fe3O4/MCM-41 was conducted by calcination of the dye-adsorbed Fe3O4/MCM-41 powder at 550 °C for 5 h to remove the dyes from the pores. The reclaimed powder can be used as an adsorbent again. Table 2 shows the decolorization rates of RhB and MB solutions (100 mg L−1) after four cycles. After the three cycles, the decolorization rates of both dye solutions can be maintained at more than 80%. However, after the fourth cycle, the decolorization rates were reduced to about 70%. The phenomenon may be caused by partial collapse of the mesoporous structure of the adsorbent after several cycles.
| Dye | Cycle | Decolorization rates (%) |
|---|---|---|
| Rhodamine B | 1 | 95 |
| 2 | 88.5 | |
| 3 | 82.1 | |
| 4 | 72.2 | |
| Methylene blue | 1 | 93 |
| 2 | 87.6 | |
| 3 | 80.5 | |
| 4 | 73.7 |
Conclusions
In summary, amphiphilic 1-alkyl-3-methylimidazolium chloride (CnmimCl) can serve as a surface functional molecule to construct bilayer CnmimCl structure in the surface of magnetic Fe3O4 nanoparticles. It was found that CnmimCl (n = 10, 12, 14, 16) can be used to assemble the primer layer, and long chain C16mimCl is favourable for the construction of the secondary layer. The bilayer CnmimCl-based Fe3O4 particles provide a highly charge-density and strongly hydrophilic surface, leading to the highly stable, water-based magnetic fluids. Moreover, in the CnmimCl-based magnetic fluid, a magnetically-driven mesoporous silica with magnetic Fe3O4 NPs inlayed randomly into ordered hexagonal mesoporous silica (MCM-41 type) framework can be prepared by using C16mimCl as template. Furthermore, the obtained Fe3O4/MCM-41 with high surface area of up to 915 m2 g−1 exhibited enhanced decolorization rates (about 95%) within 5 min for the rhodamine B and methylene blue from their aqueous solutions (100 mg L−1). This work may provide a new approach for the development of multifunctional amphiphilic ionic liquid in the practical nanomaterial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from National Natural Science Foundation of China (21564018, 21063017 and 21363029), and Yunnan Provincial Science and Technology Department (2015FD014).References
- K. R. Seddon, Nat. Mater., 2003, 22, 363–365 CrossRef PubMed.
- M. Antonietti, D. Kuang, B. Smarsly and Y. Zhou, Angew. Chem., Int. Ed., 2004, 43, 4988–4992 CrossRef CAS PubMed.
- B. Xin and J. Hao, Chem. Soc. Rev., 2014, 43, 7171–7187 RSC.
- M. Blesic, M. H. Marques, N. V. Plechkova, K. R. Seddon, L. P. N. Rebelo and A. Lopes, Green Chem., 2007, 9, 481–490 RSC.
- J. Łuczak, J. Hupka, J. Thöming and C. Jungnickel, Colloids Surf., A, 2008, 329, 125–133 CrossRef.
- Y. Pei, L. Hao, J. Ru, Y. Zhao, H. Wang, G. Bai and J. Wang, J. Mol. Liq., 2018, 254, 130–136 CrossRef CAS.
- B. Dong, N. Li, L. Zheng, L. Yu and T. Inoue, Langmuir, 2007, 23, 4178–4182 CrossRef CAS PubMed.
- T. Inoue, H. Ebina, B. Dong and L. Zheng, J. Colloid Interface Sci., 2007, 314, 236–241 CrossRef CAS PubMed.
- B. Dong, X. Zhao, L. Zheng, J. Zhang, N. Li and T. Inoue, Colloids Surf., A, 2008, 317, 666–672 CrossRef CAS.
- C. J. Adams, A. E. Bradley and K. R. Seddon, Aust. J. Chem., 2001, 54, 679–681 CrossRef CAS.
- Y. Zhou and M. Antonietti, Adv. Mater., 2003, 15, 1452–1455 CrossRef CAS.
- T. Wang, H. Kaper, M. Antonietti and B. Smarsly, Langmuir, 2007, 23, 1489–1495 CrossRef CAS PubMed.
- T. Wang, T. Fu, Y. Meng, J. Shen and T. Wang, RSC Adv., 2018, 8, 25141–25149 RSC.
- A. Wooding, M. Kilner and D. B. Lambrick, J. Colloid Interface Sci., 1991, 144, 236–242 CrossRef CAS.
- L. F. Shen, P. E. Laibinis and T. A. Hatton, Langmuir, 1999, 15, 447–453 CrossRef CAS.
- R. Y. Hong, S. Z. Zhang, Y. P. Han, H. Z. Li, J. Ding and Y. Zheng, Powder Technol., 2006, 170, 1–11 CrossRef CAS.
- B. Bateer, Y. Qu, X. Meng, C. Tian, S. Du, R. Wang, K. Pan and H. Fu, J. Magn. Magn. Mater., 2013, 332, 151–156 CrossRef CAS.
- M. Soleymani and M. Edrissi, J. Dispersion Sci. Technol., 2016, 37, 693–698 CrossRef CAS.
- S. Hou, X. Li, H. Wang, M. Wang, Y. Zhang, Y. Chi and Z. Zhao, RSC Adv., 2017, 7, 51993–52000 RSC.
- Q. Y. Li, K. R. Ma, Z. J. Ma, Q. Wei, J. G. Liu, S. P. Cui and Z. R. Nie, Microporous Mesoporous Mater., 2018, 265, 18–25 CrossRef CAS.
- N. Z. Knezevic, E. Ruiz-Hernandez, W. E. Hennink and M. Vallet-Regi, RSC Adv., 2013, 3, 9584–9593 RSC.
- Y. Zhou and M. Antonietti, Chem. Mater., 2004, 16, 544–550 CrossRef CAS.
- R. Massart and V. Cabuil, J. Chem. Phys., 1987, 84, 967–973 CAS.
- E. Dubois, J. Chevalet and R. Massart, J. Mol. Liq., 1999, 83, 243–254 CrossRef CAS.
- C. Jungnickel, J. Łuczak, J. Ranke, J. F. Fernández, A. Müller and J. Thfiming, Colloids Surf., A, 2008, 316, 278–284 CrossRef CAS.
- J. Wang, Q. Chen, C. Zeng and B. Hou, Adv. Mater., 2004, 16, 137–140 CrossRef CAS.
- D. Wang, C. Cao, S. Xue and H. Zhu, J. Cryst. Growth, 2005, 277, 238–245 CrossRef CAS.
- N. Pinna, S. Grancharov, P. Beato, P. Bonville, M. Antonietti and M. Niederberger, Chem. Mater., 2005, 17, 3044–3049 CrossRef CAS.
- D. J. Shaw, Introduction to Colloid and Surface Chemistry, Butterworths, London, 3rd edn, 1980, p. 90 Search PubMed.
- C. T. Kresge, M. E. Lconowiez, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- C. K. Krishnan, T. Hayashi and M. Ogura, Adv. Mater., 2008, 20, 2131–2136 CrossRef.
- M. Thommes, R. Köhn and M. Fröba, J. Phys. Chem. B, 2000, 104, 7932–7943 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra10065a |
| This journal is © The Royal Society of Chemistry 2019 |