Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Goethite–titania composite: disinfection mechanism under UV and visible light†
Rosalina Lara-Ricoa,
Elia M. Múzquiz-Ramos a,
Claudia M. López-Badilloa,
Ulises M. García-Pérez
a,
Claudia M. López-Badilloa,
Ulises M. García-Pérez b and
Brenda R. Cruz-Ortiz
b and
Brenda R. Cruz-Ortiz *a
*a
aUniversidad Autónoma de Coahuila, Facultad de Ciencias Químicas, Blvd. V. Carranza s/n Col. República Ote., CP 25280, Saltillo, Coahuila, Mexico. E-mail: b.cruz@uadec.edu.mx
bUniversidad Autónoma de Nuevo León, Facultad de Ingeniería Mecánica y Eléctrica, Centro de Investigación e Innovación en Ingeniería Aeronáutica, Carretera a Salinas Victoria Km 2.3, Apodaca, Mexico
First published on 21st January 2019
Abstract
Goethite–titania (α-FeOOH–TiO2) composites were prepared by co-precipitation and mechanical milling. The structural, morphological and optical properties of as-synthesized composites were characterized by X-ray powder diffraction, scanning electron microscopy and UV-Vis diffuse reflectance spectroscopy, respectively. α-FeOOH–TiO2 composites and TiO2-P25, as reference, were evaluated as photocatalysts for the disinfection of Escherichia coli under UV or visible light in a stirred tank reactor. α-FeOOH–TiO2 exhibited better photocatalytic activity in the visible region than TiO2-P25. The mechanical activation increased the absorption in the visible range of TiO2-P25 and the photocatalytic activity of α-FeOOH–TiO2. In the experiments with UV light and α-FeOOH–TiO2, mechanically activated, a 5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction of bacteria was achieved after 240 min of treatment. Using visible light the α-FeOOH–TiO2 and the TiO2-P25 showed a 3.1 and a 0.7
log-reduction of bacteria was achieved after 240 min of treatment. Using visible light the α-FeOOH–TiO2 and the TiO2-P25 showed a 3.1 and a 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reductions at 240 min, respectively. The disinfection mechanism was studied by ROS detection and scavenger experiments, demonstrating that the main ROS produced in the disinfection process were superoxide radical anion, singlet oxygen and hydroxyl radical.
log-reductions at 240 min, respectively. The disinfection mechanism was studied by ROS detection and scavenger experiments, demonstrating that the main ROS produced in the disinfection process were superoxide radical anion, singlet oxygen and hydroxyl radical.
1. Introduction
In 2017 the WHO/UNICEF reported that 844 million people did not have a drinking water facility. Microbial contamination (E. coli or thermotolerant coliforms) of water is a worldwide concern.1 It was estimated that 477![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 children from 0 to 4 years died in 2016 due to diarrhea.2 In developing regions, the use of solar disinfection is one of the most reliable treatments for water disinfection. However, this technique can be improved using non-toxic and earth-abundant oxides with photocatalytic activity. TiO2 is a semiconductor employed in disinfection processes.3–9 The disinfection mechanism involves the production of reactive oxygen species (ROS), under UV light, through oxidation and reduction reactions by holes (h+) and electrons (e−) in the valence and conduction bands of TiO2, respectively. The main ROS produced are superoxide radical anion (O2˙−), singlet oxygen (1O2), hydroxyl radical (·OH) and hydrogen peroxide (H2O2). Investigations related to TiO2 have been carried out to decrease the recombination rate of e−–h+ pairs and increase its absorption to the visible range. Goethite (α-FeOOH) is an abundant iron oxyhydroxide,10 with a band gap value of 2.1 eV.11 Few works have been reported using FeOOH–TiO2 composites for E. coli disinfection.12,13 In these works, the route used to obtain the composites was the hydrothermal method. Chowdhury and Mpongwana12 studied the FeOOH–TiO2 composite, FeOOH was akaganeite (β-FeOOH), for E. coli disinfection in presence of H2O2 as electron acceptor; Mangayayam et al. studied the disinfection efficiency of E. coli using Ag–TiO2–FeOx (mainly goethite 37.3%) nanotubes.13 Goethite–titania composites can show enhanced photocatalytic activity due to the related studies reporting the increase of TiO2 light absorption attributed to Fe doping or iron oxides addition, and the photo-Fenton and photocatalytic activity of goethite.11,14–16 Additionally, the mechanical activation, through ball milling, has shown to create defects in materials, which contributes to improving its catalytic properties.17,18
291 children from 0 to 4 years died in 2016 due to diarrhea.2 In developing regions, the use of solar disinfection is one of the most reliable treatments for water disinfection. However, this technique can be improved using non-toxic and earth-abundant oxides with photocatalytic activity. TiO2 is a semiconductor employed in disinfection processes.3–9 The disinfection mechanism involves the production of reactive oxygen species (ROS), under UV light, through oxidation and reduction reactions by holes (h+) and electrons (e−) in the valence and conduction bands of TiO2, respectively. The main ROS produced are superoxide radical anion (O2˙−), singlet oxygen (1O2), hydroxyl radical (·OH) and hydrogen peroxide (H2O2). Investigations related to TiO2 have been carried out to decrease the recombination rate of e−–h+ pairs and increase its absorption to the visible range. Goethite (α-FeOOH) is an abundant iron oxyhydroxide,10 with a band gap value of 2.1 eV.11 Few works have been reported using FeOOH–TiO2 composites for E. coli disinfection.12,13 In these works, the route used to obtain the composites was the hydrothermal method. Chowdhury and Mpongwana12 studied the FeOOH–TiO2 composite, FeOOH was akaganeite (β-FeOOH), for E. coli disinfection in presence of H2O2 as electron acceptor; Mangayayam et al. studied the disinfection efficiency of E. coli using Ag–TiO2–FeOx (mainly goethite 37.3%) nanotubes.13 Goethite–titania composites can show enhanced photocatalytic activity due to the related studies reporting the increase of TiO2 light absorption attributed to Fe doping or iron oxides addition, and the photo-Fenton and photocatalytic activity of goethite.11,14–16 Additionally, the mechanical activation, through ball milling, has shown to create defects in materials, which contributes to improving its catalytic properties.17,18
The aim of this work was to investigate the photocatalytic mechanism of α-FeOOH–TiO2 composites in the disinfection of E. coli under UV and visible light irradiation. The composites were synthesized by co-precipitation and mechanical milling. The photocatalytic mechanism of the composite was studied by means of ROS detection and scavengers addition.
2. Materials and methods
2.1 Synthesis of goethite (α-FeOOH)
Goethite was synthesized by co-precipitation method (Fig. S1†). A solution of NaOH (Fermont, Mexico) 10 M was dripped in 200 mL of FeSO4·7H2O (Jalmek, Mexico) 0.04 M until pH 13 and kept under constant stirring with air sparging, after 4 h a yellow dark precipitate was obtained. The precipitate was washed with deionized water several times and centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min (Thermo Scientific, Sorvall ST-16). Finally, it was dried at 90 °C for 24 h.
000 rpm for 10 min (Thermo Scientific, Sorvall ST-16). Finally, it was dried at 90 °C for 24 h.
2.2 Synthesis of FeOOH–TiO2 composites
The syntheses of the composites were carried out by two techniques, the first labeled in situ (Fig. S2†), where TiO2-P25 (Aeroxide, Evonik, Degussa Corporation) was added to the solution after 4 h of goethite synthesis and kept for 1 h under constant stirring with air sparging. The second technique was by mechanical activation of TiO2-P25 and goethite previously synthesized (Fig. S3†), using a planetary mill (Retsch PM 100) with a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (balls
1 (balls![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) load) at 450 rpm for 1 h, with ethanol as dispersing agent, and zirconia container and balls. The powders were dried at 80 °C for 12 h. For both syntheses, the stoichiometric ratios used were 1
load) at 450 rpm for 1 h, with ethanol as dispersing agent, and zirconia container and balls. The powders were dried at 80 °C for 12 h. For both syntheses, the stoichiometric ratios used were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 3
3 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for FeOOH and TiO2-P25, respectively.
1 for FeOOH and TiO2-P25, respectively.
2.3 Characterization
The materials were characterized by X-ray powder diffraction (XRD, Panalytical, Empyrean) with Cu Kα radiation at 40 kV and 30 mA. The morphology was investigated by scanning electron microscopy (FE-SEM JEOL JSM-7041F and FEI Nova Nanosem 200). The UV-visible absorption was analyzed by diffuse reflectance in a UV-visible spectrometer (AvaSpec-2048L, Avantes).2.4 Photocatalytic disinfection of E. coli
| Identification | Description |
|---|---|
| P25 | TiO2-P25 without treatment |
| P25-M | TiO2-P25 milled at 400 rpm for 1 h |
| G | Goethite without treatment |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I 3-I |
Molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 goethite 3 goethite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2-P25, in situ TiO2-P25, in situ |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M 3-M |
Molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 goethite 3 goethite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2-P25, milled at 450 rpm for 1 h TiO2-P25, milled at 450 rpm for 1 h |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I 1-I |
Molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 goethite 1 goethite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2-P25, in situ TiO2-P25, in situ |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M 1-M |
Molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 goethite 1 goethite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2-P25, milled at 450 rpm for 1 h TiO2-P25, milled at 450 rpm for 1 h |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I 1-I |
Molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 goethite 1 goethite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2-P25, in situ TiO2-P25, in situ |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M 1-M |
Molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 goethite 1 goethite![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2-P25, milled at 450 rpm for 1 h TiO2-P25, milled at 450 rpm for 1 h |
Aliquots were collected at different times for 300 min and bacteria concentration was determined using the standard plated counting method on LB agar by triplicate. The detection limit was 2 CFU mL−1 and was achieved inoculating 500 μL of sample. The plates were incubated for 24 h at 37 °C. Control experiments were made without material to evaluate the photolysis and with material in the dark.
2.5 ROS detection
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, 1
3-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 3
1-M, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 1
1-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I, 1
3-I, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I or 3
1-I or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I at 300 mg L−1 were suspended in a solution containing RNO (Sigma Aldrich) 45 μM and imidazole (Sigma Aldrich) 8 mM. The experiments were performed under UV or visible light. Aliquots were taken during 24 min and centrifuged at 12
1-I at 300 mg L−1 were suspended in a solution containing RNO (Sigma Aldrich) 45 μM and imidazole (Sigma Aldrich) 8 mM. The experiments were performed under UV or visible light. Aliquots were taken during 24 min and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. The RNO concentration was determined at 440 nm in a Varian Cary 50 UV-Vis spectrometer. Negative controls were made without material.
000 rpm for 15 min. The RNO concentration was determined at 440 nm in a Varian Cary 50 UV-Vis spectrometer. Negative controls were made without material.2.6 Scavenger addition
Disinfection experiments were performed with different scavengers. tert-Butanol (TBA, 10 mM) was used as hydroxyl radical scavenger; KI (10 mM) quenched surface holes and surface bounded ·OH. The reduction pathway was inhibited by introducing nitrogen into the solution.2.7 Addition of H2O2 as electron acceptor
E. coli disinfection experiments in presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M (300 mg L−1) and H2O2 (10 mg L−1), as electron acceptor, under visible light were performed.
3-M (300 mg L−1) and H2O2 (10 mg L−1), as electron acceptor, under visible light were performed.
3. Results and discussion
Fig. 1 shows the XRD patterns of P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, 1
3-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 3
1-M, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 1
1-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I, 1
3-I, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I and 3
1-I and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I. The phases detected correspond to the indexed PDF crystallographic cards 01-075-2545 (anatase) and 01-080-2533 (rutile) for P25 and P25-M, and 98-007-1808 for goethite. The crystallite size of P25 and P25-M was calculated using the Scherrer equation (eqn (1)). The results showed a crystallite size of 19.4 nm for P25-M and 18.8 nm for P25.
1-I. The phases detected correspond to the indexed PDF crystallographic cards 01-075-2545 (anatase) and 01-080-2533 (rutile) for P25 and P25-M, and 98-007-1808 for goethite. The crystallite size of P25 and P25-M was calculated using the Scherrer equation (eqn (1)). The results showed a crystallite size of 19.4 nm for P25-M and 18.8 nm for P25.
D = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
 | ||
Fig. 1 XRD patterns in relative intensities of P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, 1 3-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 3 1-M, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 1 1-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I, 1 3-I, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I and 3 1-I and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I composites. 1-I composites. | ||
SEM characterization was carried out to investigate the shape and size of the photocatalysts. Fig. 2a and b shows SEM images of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I with densely packed FeOOH rods with TiO2-P25 spherical particles with a longitude of 126.5 ± 29 nm and 22.1 ± 2 nm, respectively. Fig. 2c and d corresponds to the composite 1
3-I with densely packed FeOOH rods with TiO2-P25 spherical particles with a longitude of 126.5 ± 29 nm and 22.1 ± 2 nm, respectively. Fig. 2c and d corresponds to the composite 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, the FeOOH and the TiO2-P25 show the same morphology that 1
3-M, the FeOOH and the TiO2-P25 show the same morphology that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I and longitudes of 86.1 ± 18 nm and 19.4 ± 6 nm, respectively. FE-SEM images of P25, P25-M and G are in Fig. S6.†
3-I and longitudes of 86.1 ± 18 nm and 19.4 ± 6 nm, respectively. FE-SEM images of P25, P25-M and G are in Fig. S6.†
Fig. 3 displays the UV-Vis absorption spectra of P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, 1
3-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 3
1-M, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 1
1-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I, 1
3-I, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I and 3
1-I and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I. For P25 and P25-M, a strong absorption in the UV until 410 and 415 nm, respectively, is observed. The diffuse reflectance spectra were used to estimate the bandgaps of all samples using the Kubelka–Munk function (eqn (2), Fig. S7†). The band gap values were 3.3, 3.2, 2.13, 2.86, 2.85, 2.83, 2.81, 2.76 and 2.73 eV for P25, P25-M, G, 1
1-I. For P25 and P25-M, a strong absorption in the UV until 410 and 415 nm, respectively, is observed. The diffuse reflectance spectra were used to estimate the bandgaps of all samples using the Kubelka–Munk function (eqn (2), Fig. S7†). The band gap values were 3.3, 3.2, 2.13, 2.86, 2.85, 2.83, 2.81, 2.76 and 2.73 eV for P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, 1
3-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 3
1-M, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 1
1-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I, 1
3-I, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I and 3
1-I and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I composites, respectively. The mechanical activation reduced the band gap value in P25 only.
1-I composites, respectively. The mechanical activation reduced the band gap value in P25 only.
| K/S = FKM(R) = (1 − R)2/2R | (2) |
 | ||
Fig. 3 UV-Vis absorption spectra of P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, 1 3-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 3 1-M, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M, 1 1-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I, 1 3-I, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I and 3 1-I and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I composites. 1-I composites. | ||
According to Dannangoda et al., the reduction in the band gap value after mechanical activation can be related with the change in bond angles and lengths in the crystal structure by the impact during the milling process.27
3.1 Photocatalytic disinfection with UV light
In Fig. 4 the disinfection plot using TiO2-P25 at 72, 150, 300 and 500 mg L−1 with UV light is shown. TiO2-P25 at 300 mg L−1 reached a 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction after 240 min of irradiation. With 72 and 150 mg L−1 of TiO2-P25 log-reductions of 3.7 and 3.8 at 240 min were achieved, respectively. TiO2-P25 at 500 mg L−1 gave the lower disinfection efficiency. The E. coli concentration remained constant during the 300 min in the dark. In the photolytic experiment, a 0.5
log-reduction after 240 min of irradiation. With 72 and 150 mg L−1 of TiO2-P25 log-reductions of 3.7 and 3.8 at 240 min were achieved, respectively. TiO2-P25 at 500 mg L−1 gave the lower disinfection efficiency. The E. coli concentration remained constant during the 300 min in the dark. In the photolytic experiment, a 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction at 240 min was observed. The following experiments with goethite and the composites under UV or visible irradiation were performed at 300 mg L−1.
log-reduction at 240 min was observed. The following experiments with goethite and the composites under UV or visible irradiation were performed at 300 mg L−1.
 | ||
| Fig. 4 Bacterial disinfection with TiO2-P25 at 72 mg L−1, 150 mg L−1, 300 mg L−1 and 500 mg L−1 under UV light. | ||
TiO2–FeOOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M) is more photocatalytic active under UV light than TiO2-P25. With 300 mg L−1 of TiO2–FeOOH (1
3-M) is more photocatalytic active under UV light than TiO2-P25. With 300 mg L−1 of TiO2–FeOOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M) a disinfection efficiency of 5.1
3-M) a disinfection efficiency of 5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction was reached at 240 min (Fig. 5). TiO2-P25-M showed a slightly better disinfection efficiency than TiO2-P25 with a 4.7
log-reduction was reached at 240 min (Fig. 5). TiO2-P25-M showed a slightly better disinfection efficiency than TiO2-P25 with a 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction at 240 min. In general, it is possible to observe that the composite with FeOOH at a low molar ratio and with mechanical activation increases the disinfection efficiency, the later can be due to the defects created during the mechanical activation.17,18 Ruales et al. reported that goethite acts as an efficient photocatalyst in absence of H2O2, in our case we only could appreciate a 1.34
log-reduction at 240 min. In general, it is possible to observe that the composite with FeOOH at a low molar ratio and with mechanical activation increases the disinfection efficiency, the later can be due to the defects created during the mechanical activation.17,18 Ruales et al. reported that goethite acts as an efficient photocatalyst in absence of H2O2, in our case we only could appreciate a 1.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction at 240 min.11 The dark controls of E. coli disinfection with the different photocatalysts are in Fig. S8.†
log-reduction at 240 min.11 The dark controls of E. coli disinfection with the different photocatalysts are in Fig. S8.†
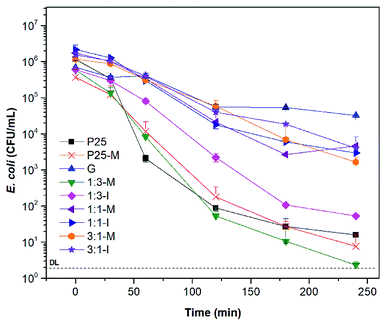 | ||
| Fig. 5 Bacterial disinfection with P25, P25-M, G and FeOOH–TiO2 composites under UV light at 300 mg L−1. | ||
3.2 Disinfection with visible light
Fig. 6 shows the disinfection kinetics of E. coli under visible light with P25, P25-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M and 1
3-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I. With 1
3-I. With 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M a 3.1
3-M a 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction after 240 min of treatment was achieved. In the case of P25, P25-M and 1
log-reduction after 240 min of treatment was achieved. In the case of P25, P25-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I showed a 0.7, 0.8 and 1.5
3-I showed a 0.7, 0.8 and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-reduction at the same time, respectively. The results confirm that 1
log-reduction at the same time, respectively. The results confirm that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M shows higher photoactivity and absorption range than TiO2-P25.
3-M shows higher photoactivity and absorption range than TiO2-P25.
 | ||
Fig. 6 Bacterial disinfection with P25, P25-M and FeOOH–TiO2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 composites under visible light at 300 mg L−1. 3 composites under visible light at 300 mg L−1. | ||
The fitting tool GInaFit, version 1.7 was employed to analyze the disinfection kinetic curves.28 The results are shown in Table 2. The shoulder indicates the time before the bacteria concentration begins to diminish, and the tail the moment in which an additional reduction is not achieved, probably due to bacteria resistance or a protective effect of the residual cell components to the still viable bacteria.
| Material (mg L−1) | Parameter | ||||
|---|---|---|---|---|---|
| Material (mg L−1) | SI (shoulder length, min) | Kmax (min−1) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nres (residual bacterial concentration) Nres (residual bacterial concentration) |
No (initial bacterial concentration) | |
| a No fits on tail model. | |||||
| UV light | P25 (72)a | 46.72 ± 22.08 | 0.05 ± 0.01 | 2.43 ± 0.31 | 6.15 ± 0.43 |
| P25 (150) | 14.00 ± 30.37 | 0.06 ± 0.02 | 2.36 ± 0.34 | 6.17 ± 0.43 | |
| P25 (300) | 25.00 ± 10.73 | 0.18 ± 0.05 | 1.53 ± 0.22 | 6.07 ± 0.38 | |
| P25 (500) | 10.93 ± 1.01 | 0.13 ± 0.01 | 2.77 ± 0.01 | 6.08 ± 0.02 | |
| P25-M (300) | 15.25 ± 4.05 | 0.07 ± 0.01 | 0.91 ± 0.06 | 5.58 ± 0.07 | |
| G (300) | 70.11 ± 23.03 | 0.07 ± 0.04 | 4.62 ± 0.12 | 5.74 ± 0.14 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M (300) 3-M (300) |
13.03 ± 14.79 | 0.09 ± 0.01 | 0.68 ± 0.24 | 5.78 ± 0.32 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I (300)a 3-I (300)a |
29.10 ± 1.11 | 0.06 ± 0.01 | 1.72 ± 0.02 | 5.77 ± 0.01 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M (300)a 1-M (300)a |
33.43 ± 8.11 | 0.05 ± 0.00 | 3.41 ± 0.07 | 6.24 ± 0.08 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I (300) 1-I (300) |
23.61 ± 12.73 | 0.05 ± 0.01 | 3.58 ± 0.11 | 6.37 ± 0.12 | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-M (300)a 1-M (300)a |
38.58 ± 17.18 | 0.04 ± 0.01 | 3.68 ± 0.14 | 6.08 ± 0.11 | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-I (300)a 1-I (300)a |
9.92 ± 38.87 | 0.03 ± 0.01 | 3.44 ± 0.51 | 6.25 ± 0.20 | |
| Visible light | P25 (300)a | 67.79 ± 5.24 | 0.03 ± 0.00 | 6.21 ± 0.02 | 7.00 ± 0.01 |
| P25-M (300)a | 46.02 ± 5.19 | 0.02 ± 0.01 | 5.77 ± 0.28 | 6.73 ± 0.05 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M (300)a 3-M (300)a |
63.96 ± 6.14 | 0.06 ± 0.01 | 3.07 ± 0.07 | 6.14 ± 0.05 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I (300) 3-I (300) |
68.98 ± 7.84 | 0.08 ± 0.01 | 4.44 ± 0.05 | 5.96 ± 0.06 | |
3.3 ROS production
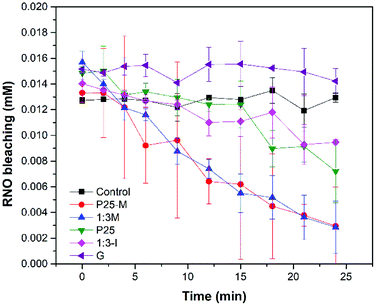
Eqn (3) shows the reaction between the RNO and the hydroxyl radical, which produces the bleaching of the RNO.| RNO + HO˙ → RNO·OH | (3) |
In Fig. 7 the materials P25, P25-M, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M and 1
3-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I produced ·OH under UV irradiation. However, it was not possible to detect ·OH with goethite. P25-M and 1
3-I produced ·OH under UV irradiation. However, it was not possible to detect ·OH with goethite. P25-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M produced almost the same quantity of ·OH. The reaction rate coefficients (k) for the control, P25, P25-M, G, 1
3-M produced almost the same quantity of ·OH. The reaction rate coefficients (k) for the control, P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M and 1
3-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I are 5 × 10−6, 0.0119, 0.0281, 0.0005, 0.0306 and 0.007, respectively. The data were analyzed by a factorial design and the interaction graphs for each ROS test are in the ESI (Fig. S9–S11†).
3-I are 5 × 10−6, 0.0119, 0.0281, 0.0005, 0.0306 and 0.007, respectively. The data were analyzed by a factorial design and the interaction graphs for each ROS test are in the ESI (Fig. S9–S11†).
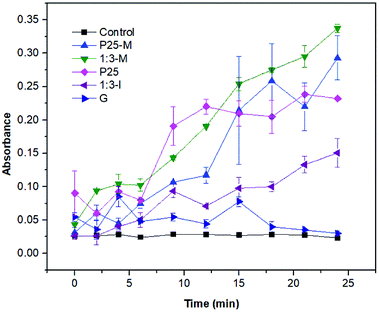
For superoxide detection (Fig. 8) the XTT reduction by superoxide to XTT-formazan was monitored. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M composite exhibited more superoxide production than P25-M. Thus, the interaction between FeOOH and TiO2-P25-M increased the disinfection photocatalytic efficiency compared to α-FeOOH or TiO2-P25. The reaction rate coefficients (k) for the control, P25, P25-M, G, 1
3-M composite exhibited more superoxide production than P25-M. Thus, the interaction between FeOOH and TiO2-P25-M increased the disinfection photocatalytic efficiency compared to α-FeOOH or TiO2-P25. The reaction rate coefficients (k) for the control, P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M and 1
3-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I are 0.0006, 0.0241, 0.0394, 0.0084, 0.0327 and 0.0318, respectively.
3-I are 0.0006, 0.0241, 0.0394, 0.0084, 0.0327 and 0.0318, respectively.
According to Kralji and El Mohsni, the reaction of imidazole with 1O2 generates an intermediate that reacts with RNO.20 The singlet oxygen detection (Fig. 9) showed that only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M, P25-M, P25 and 1
3-M, P25-M, P25 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I produced 1O2 under UV irradiation. The reaction rate coefficients (k) for the control, P25, P25-M, G, 1
3-I produced 1O2 under UV irradiation. The reaction rate coefficients (k) for the control, P25, P25-M, G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M and 1
3-M and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-I are 0.0003, 0.0411, 0.0561, 0.0006, 0.101 and 0.0197, respectively.
3-I are 0.0003, 0.0411, 0.0561, 0.0006, 0.101 and 0.0197, respectively.
The tests between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M and Ti(IV) ions showed no significant H2O2 formation during 24 min under UV or visible light. The same result was observed using different material loadings (data not shown).
3-M and Ti(IV) ions showed no significant H2O2 formation during 24 min under UV or visible light. The same result was observed using different material loadings (data not shown).
ROS detection under visible irradiation was negative, probably due to the low detection limit of spectroscopic probes. The same behavior was observed in previous work,29 where hydroxyl radical was detected only under UV irradiation.
3.4 Scavenger study under visible irradiation
The addition of tert-butanol (TBA) showed a reduction in the disinfection efficiency compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M without TBA (Fig. S12†), suggesting that the disinfection is mediated by hydroxyl radicals in the bulk. When KI was used, as a surface hole and hydroxyl radical scavenger, the disinfection efficiency increased considerably. Probably due to the disinfectant action of iodine formed after oxidation of iodide, such behavior was also observed in previous work.29 Under anoxic conditions the disinfection also decreased, pointing out that the oxygen reduction pathway plays an important role in ROS production. It is necessary to emphasize that the study with scavengers must be interpreted with caution since they can be involved in side reactions, mainly in disinfection processes.
3-M without TBA (Fig. S12†), suggesting that the disinfection is mediated by hydroxyl radicals in the bulk. When KI was used, as a surface hole and hydroxyl radical scavenger, the disinfection efficiency increased considerably. Probably due to the disinfectant action of iodine formed after oxidation of iodide, such behavior was also observed in previous work.29 Under anoxic conditions the disinfection also decreased, pointing out that the oxygen reduction pathway plays an important role in ROS production. It is necessary to emphasize that the study with scavengers must be interpreted with caution since they can be involved in side reactions, mainly in disinfection processes.
In the experiment of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M with visible light and H2O2 (Fig. S4†) significant E. coli disinfection was observed compared to 1
3-M with visible light and H2O2 (Fig. S4†) significant E. coli disinfection was observed compared to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M under UV or visible irradiation without H2O2 (Fig. 5 and 6). Hydrogen peroxide also showed good disinfection efficiency at the concentration tested. These results follow those observed by Ruales et al. with goethite and peroxide.11
3-M under UV or visible irradiation without H2O2 (Fig. 5 and 6). Hydrogen peroxide also showed good disinfection efficiency at the concentration tested. These results follow those observed by Ruales et al. with goethite and peroxide.11
The eqn (4) and (5) were used to calculate the conduction band (CB) and the valence band (VB) potentials of P25-M and FeOOH.
| EVB = X − Ee + 0.5Eg | (4) |
| ECB = EVB − Eg | (5) |
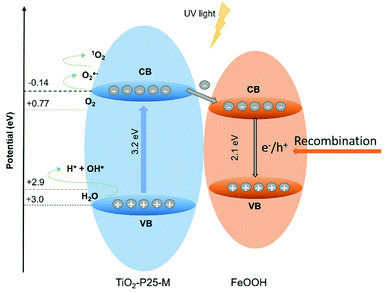
Goethite did not show appreciable ROS production to determine its photoactivity; however, this does not mean that it does not contribute to the photocatalytic activity observed in the FeOOH–TiO2 composites. The band positions of TiO2-P25-M enable the generation of the ROS detected in this study. The heterojunction between these oxides improved the electron and hole mobility with a slight increase in the photoactivity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-M composite compared to TiO2-P25-M. In Fig. 10 the proposed photocatalytic mechanism, under UV light, between TiO2-P25-M and goethite shows the availability of holes and electrons in the VB and CB of TiO2-P25-M for oxidation and reduction reactions (eqn (6)–(8)). According to the ROS tests results, the goethite did not show production of ROS, this is attributed to rapid recombination of electron–hole pairs. The electrons in the CB of TiO2-P25-M that did not participate in the oxygen-reduction reactions migrate to the CB of goethite that acts as an electron capture site, which contributes to decrease the recombination in TiO2.7 Under visible light the composites showed photoactivity; however, a mechanism cannot be proposed because the ROS detection, under visible light, was negative, probably due to the low detection limit of the spectroscopic probes employed. Cruz-Ortiz et al. also studied the photoactivity of TiO2-P25, and according to the ROS study, the photocatalyst showed singlet oxygen production using the singlet oxygen sensor green (Invitrogen), a molecular probe that shows more sensitivity than the RNO-imidazole used in the present work.29 Also, the UV-visible absorption spectra of TiO2-P25 and TiO2-P25-M (Fig. 3) shows that these materials absorb in the visible region until 410 and 415 nm.
3-M composite compared to TiO2-P25-M. In Fig. 10 the proposed photocatalytic mechanism, under UV light, between TiO2-P25-M and goethite shows the availability of holes and electrons in the VB and CB of TiO2-P25-M for oxidation and reduction reactions (eqn (6)–(8)). According to the ROS tests results, the goethite did not show production of ROS, this is attributed to rapid recombination of electron–hole pairs. The electrons in the CB of TiO2-P25-M that did not participate in the oxygen-reduction reactions migrate to the CB of goethite that acts as an electron capture site, which contributes to decrease the recombination in TiO2.7 Under visible light the composites showed photoactivity; however, a mechanism cannot be proposed because the ROS detection, under visible light, was negative, probably due to the low detection limit of the spectroscopic probes employed. Cruz-Ortiz et al. also studied the photoactivity of TiO2-P25, and according to the ROS study, the photocatalyst showed singlet oxygen production using the singlet oxygen sensor green (Invitrogen), a molecular probe that shows more sensitivity than the RNO-imidazole used in the present work.29 Also, the UV-visible absorption spectra of TiO2-P25 and TiO2-P25-M (Fig. 3) shows that these materials absorb in the visible region until 410 and 415 nm.
| O2˙− + h+ → 1O2 | (6) |
| H2O + h+ → HO˙ + H+ | (7) |
| O2 + e− → O2˙− | (8) |
4. Conclusions
FeOOH–TiO2 composite is a photocatalyst with increased disinfection activity than TiO2-P25. The better disinfection efficiency using UV or visible light was observed with FeOOH–TiO2 treated with mechanical milling. The ROS detected employing UV light were hydroxyl radical, superoxide radical anion and singlet oxygen. A reduction in the disinfection efficiency was detected when hole and hydroxyl radical scavengers, KI and tert-butanol, were added. Under N2 sparging the same effect was observed. A photocatalytic mechanism is proposed based on the band edge positions of TiO2 and FeOOH, and the ROS detected. Where the hydroxyl radical and singlet oxygen are generated after oxidation of H2O and superoxide radical in the valence band, respectively, and the superoxide is produced after oxygen reduction in the conduction band of the TiO2-P25.Conflicts of interest
There are no conflicts to declare.Acknowledgements
RLR acknowledge to the National Council of Science and Technology (CONACyT-México) for the scholarship No. 446683, to Dr Silvia Solís from the Research Center in Applied Chemistry (CIQA, México) for the facilities during the summer research of RLR. Also, the authors thank Química Anher S. A. de C. V. for supplying the TiO2-P25 (Aeroxide®) and to the Department of Biotechnology and Dr Marco García (UAdeC) for the facilities. Finally, the authors acknowledge to CGEPI-UAdeC for the economic support for this publication.References
- WHO/UNICEF, progress on drinking water, sanitation and hygiene 2017, https://www.unicef.org/publications/index_96611.html, accessed July 2018.
- WHO, Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000-2016. Geneva 2018, http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html, accessed July 2018.
- A. García, Y. Quintero, N. Vicencio, B. Rodríguez, D. Ozturk, E. Mosquera, T. P. Corrales and U. G. Volmann, RSC Adv., 2016, 6, 82941–82948 RSC.
- C. Adán, J. Marugán, S. Mesones, C. Casado and R. van Grieken, Chem. Eng. J., 2016, 318, 29–38 CrossRef.
- S. Yu, T. Lin and W. Chen, RSC Adv., 2014, 4, 31370–31377 RSC.
- S. G. Ullattil, S. B. Narendranath, S. C. Pillai and P. Periyat, Chem. Eng. J., 2018, 343, 708–736 CrossRef CAS.
- S. Malato, M. I. Maldonado, P. Fernández-Ibáñez, I. Oller, I. Polo and R. Sánchez-Moreno, Mater. Sci. Semicond. Process., 2016, 42, 15–23 CrossRef CAS.
- C. S. Uyguner-Demirel, C. N. Birben and M. Bekbolet, Chemosphere, 2018, 211, 420–448 CrossRef CAS PubMed.
- P. V. Laxma Reddy, B. Kavitha, P. A. Kumar Reddy and K. H. Kim, Environ. Res., 2017, 154, 296–303 CrossRef CAS PubMed.
- R. M. Cornell and U. Schwertmann, The Iron Oxides: Structure, properties, reactions, occurrences and uses, Wiley-VCH, Weinheim, 2006 Search PubMed.
- C. Ruales-Lonfat, J. F. Barona, A. Sienkiewicz, M. Bensimon, J. Vélez-Colmenares, N. Benítez and C. Pulgarín, Appl. Catal., B, 2015, 166–167, 497–508 CrossRef CAS.
- M. Chowdhury and N. Mpongwana, β-FeOOH/TiO2 heterojunction for visible light-driven photocatalytic inactivation of E. coli, in Semiconductor Photocatalysis, ed. W. Cao, IntechOpen, Rijeka, 2016, pp. 367–378 Search PubMed.
- M. Mangayayam, J. Kiwi, S. Giannakis, C. Pulgarin, I. Zivkovic, A. Magrez and S. Rtimi, Appl. Catal., B, 2017, 202, 438–445 CrossRef CAS.
- X. I. E. Weimiao, C. Hui, Z. Xuanhui, H. U. Xianchao and L. I. Guohua, Chin. J. Catal., 2013, 34, 1076–1086 CrossRef.
- X. Zuo, M. Chen, D. Fu and H. Li, Chem. Eng. J., 2016, 294, 202–209 CrossRef CAS.
- A. R. Amani-Ghadim, S. Alizadeh, F. Khodam and Z. Rezvani, J. Mol. Catal. A: Chem., 2015, 408, 60–68 CrossRef CAS.
- C. Shifu, C. Lei, G. Shen and C. Gengyu, Mater. Chem. Phys., 2006, 98, 116–120 CrossRef.
- S. Petrović, L. Rožić, V. Jović, S. Stojadinović, B. Grbić, N. Radić, J. Lamovec and R. Vasilić, Adv. Powder Technol., 2018, 29, 2129–2139 CrossRef.
- A. M. Braun, M. T. Maurette and E. Oliveros, Photochemical Technology, Wiley, New York, 1991 Search PubMed.
- I. Kraljic and S. E. Mohsni, Photochem. Photobiol., 1978, 28, 577–581 CrossRef CAS.
- S. P. Zhang, J. Q. Zhao and L. J. Jiang, Free Radical Res., 2000, 33, 489–496 CrossRef CAS PubMed.
- M. Rajendran, Photodiagn. Photodyn. Ther., 2016, 13, 175–187 CrossRef CAS PubMed.
- L. Brunet, D. Y. Lyon, E. M. Hotze, P. J. J. Alvarez and M. R. Wiesner, Environ. Sci. Technol., 2009, 43, 4355–4360 CrossRef CAS PubMed.
- M. E. Simonsen, J. Muff, L. R. Bennedsen, K. P. Kowalski and E. G. Søgaard, J. Photochem. Photobiol., A, 2010, 216, 244–249 CrossRef CAS.
- C. Kim, H. Park, S. Cha and J. Yoon, Chemosphere, 2013, 93, 2011–2015 CrossRef CAS PubMed.
- B. J. Deadman, K. Hellgardt and K. K. Hii, React. Chem. Eng., 2017, 2, 462–466 RSC.
- G. C. Dannangoda, C. Key, M. Sumets and K. S. Martirosyan, J. Electron. Mater., 2018, 47, 5800–5809 CrossRef CAS.
- A. H. Geeraerd, V. P. Valdramidis and J. F. Van Impe, Int. J. Food Microbiol., 2005, 102, 95–105 CrossRef CAS PubMed.
- B. R. Cruz-Ortiz, J. W. J. Hamilton, C. Pablos, L. Díaz-Jiménez, D. A. Cortés-Hernández, P. K. Sharma, M. Castro-Alférez, P. Fernández-Ibañez, P. S. M. Dunlop and J. A. Byrne, Chem. Eng. J., 2017, 316, 179–186 CrossRef CAS.
- H. Dong, G. Chen, J. Sun, Y. Feng, C. Li, G. Xiong and C. Lv, Dalton Trans., 2014, 43, 7282–7289 RSC.
- N. Tian, H. Huang, Y. He, Y. Guo, T. Zhang and Y. Zhang, Dalton Trans., 2015, 44, 4297–4307 RSC.
- C. Yu, G. Li, S. Kumar, H. Kawasaki and R. Jin, J. Phys. Chem. Lett., 2013, 4, 2847–2852 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08412b |
| This journal is © The Royal Society of Chemistry 2019 |