Hollow multi-shelled structures for energy conversion and storage applications
Hao
Ren
ab and
Ranbo
Yu
 *a
*a
aSchool of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: ranboyu@ustb.edu.cn
bSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore
First published on 19th July 2019
Abstract
The shortage of fossil fuels and the issue of environmental pollution have drawn more attention towards sustainable and clean energy. In order to efficiently convert or store clean energy, suitable materials with excellent performance are highly required. Materials with hollow multi-shelled structures (HoMSs) have drawn extensive attention recently due to their advantages benefiting from their unique structures, making them promising candidates for energy conversion and storage applications. Besides structure, the composition is another important factor that affects the properties of materials, and relies on more precise control of the synthesis. More attention on the composition control of HoMSs should be paid for different energy conversion and storage applications, to better improve their performance. In this review, we have summarized recent research studies on HoMSs for energy conversion and storage fields, including synthesis and applications. General methods including the hard template method, soft template method and template-free method to synthesize HoMSs are reviewed initially. The following section covers various compositional types of HoMSs including AxBy, AxBy–CxDy, AxBy@CxDy, and AxByOz types, and other types. Afterwards, the promising energy conversion and storage applications of HoMSs have been reviewed, such as dye-sensitized solar cells, rechargeable batteries, supercapacitors, catalysis, and so on. Finally, a summary and outlook section is provided. We hope that this review will provide useful information for precise control of both structures and compositions of HoMSs to achieve enhanced properties for energy conversion and storage applications.
1. Introduction
Considering the scarcity of fossil fuels and environmental pollution, sustainable and clean energy sources like solar energy and wind energy are highly needed.1 One of the most effective devices to convert the solar energy to power is dye-sensitized solar cells (DSSCs).2 However, the intermittency nature of solar energy is another issue. In order to overcome this issue to guarantee smooth power supply, rechargeable batteries are one of the most promising and effective candidates for energy conversion and storage.3–6 Meanwhile, either the solar energy or the converted electricity can drive catalytic water splitting by using some suitable catalysts to generate hydrogen as clean gas energy.7,8 Besides, the solar energy can also be used for degradation of pollutants and catalytic CO2 conversion and in other areas.9–11 Suitable materials are the most important parts for the above energy conversion and storage areas to enhance the performance.Hollow multi-shelled structures (HoMSs) have been attracting extensive research interest on account of their advantages including low density, large specific surface area and high porosity.12,13 As is known, the material compositions and structures can greatly affect their performance when used for devices. As a consequence of their unique structures, materials with HoMSs can effectively enhance the performance in different ways based on their specific applications. For example, for solar energy conversion applications such as photodegradation, DSSCs and photocatalytic water splitting fields, efficient capture and utilization of light is one of the most important factors for high performance.7,9,14 Compared to single-shelled hollow structures, each shell of the HoMSs can reflect/scatter and absorb light efficiently, which can be called the multiple reflection/scattering effect.15,16 Consequently, the multiple shells can utilize the light more effectively to improve the performance. For lithium-ion batteries (LIBs), the cathodes or anodes using HoMSs as active materials benefit from the following aspects.3,17 (1) The large specific surface area and high porosity contribute to better contact with electrolytes, which can decrease the impedance effectively. (2) The hierarchical micro/nano-structures not only guarantee shortened lithium-ion diffusion pathways with nano-sized particles improving their rate capability, but also prevent structure destruction during the lithium-ion insertion/extraction processes by the unique buffer function of the HoMSs for volumetric expansion/contraction sustaining the cycling performance. (3) The volumetric capacity of HoMSs is higher as compared to that of hollow single-shelled structures since the mass loading is higher with the same volume. These advantages also work for other similar rechargeable batteries and supercapacitors.18–20 Due to their large specific surface area, porosity and cavity, the unique HoMSs composed of nanoparticles can also be used in other areas such as electrocatalysis, drug delivery and sensors with outstanding performance.8,21–23
Besides the effect of material structures, the composition can greatly affect material performance as well. There is no doubt that the compositional control relies on the synthesis procedure. To date, the promising applications of HoMSs have already pushed the design and synthesis forward greatly. Several general and reliable synthetic strategies have been developed to generate HoMSs, which are the hard template method, soft template method and template-free method.12 The conventional hard template method usually involves multiple layer-by-layer steps to coat either the precursors or template layers, which is quite complex.16 However, positively, some hard template methods can produce HoMSs whose inner shell and outer shell compositions are different. As a comparison, the procedures of soft template and template-free methods can be simplified.24,25 However, it is a challenge to acquire a uniform size or to tune the shell composition. The Wang group has proposed a sequential templating approach (STA) to synthesize metal oxide HoMSs.26 Using this method it is quite easy to obtain very uniform HoMSs with various controllable parameters including the shell thickness, shell number and the space between the outer shells.15,17,27 Multiple metal precursors can even be used at the same time to control the shell composition.18,28,29 Although the shell composition of HoMSs has been controlled to some extent in some studies, more precise control of the shell composition is still highly needed. The composition control will definitely be a necessity for achieving precise and sophisticated functional materials.
With more research studies on hollow structures, several review articles have reported the progress of this topic mainly focusing on single-shelled hollow structures.30,31 Recently, several reviews have focused on HoMSs.12,32 However, there is still no specific summary focusing on the composition of HoMS shells. Therefore, we aim to comprehensively summarize recent studies and to focus more attention on the composition of HoMS shells and the applications in energy conversion and storage fields. In this review, we have summarized general synthetic methodologies to control the composition of HoMSs including AxBy which contains only one substance composed of two elements, AxBy–CxDy which is a composite mixture containing two substances, AxBy@CxDy of which the inner shell composition is different from the outer shell composition, AxByOz which contains one substance consisting of three elements, and other types. The following section reviews the applications of HoMSs in energy conversion and storage fields. Finally, the summary and outlook of HoMSs are provided. This review will provide insight into precise control of the shell composition of HoMSs and their promising applications.
2. Synthesis of various compositional HoMSs
The composition of materials plays a key role in their properties; hence it is of great importance to pay more attention to the composition control for HoMSs. Based on their composition, materials with HoMSs can be divided into several types including AxBy type, AxBy–CxDy type, AxBy@CxDy type, AxByCz type and other types including carbon, polymers, MOFs, and so on. Table 1 lists typical materials with various types of HoMSs. With the extensive study on hollow structured materials, several general methods have been developed to synthesize the above types of HoMSs, including the hard template method, soft template method and template-free method. The synthesis of various types of materials with HoMSs will be covered in the following subsections in detail.| Material | Type | Method | Shell number | Application | Ref. |
|---|---|---|---|---|---|
| SiO2 | AxBy | Layer-by-layer | 1–3 | Optical imaging/drug delivery | 36 |
| TiO2 | AxBy | Layer-by-layer | 1–3 | DSSCs | 16 |
| SnO2 | AxBy | Layer-by-layer | 1–2 | — | 37 |
| ZnO | AxBy | STA | 1–4 | DSSCs | 15 |
| SnO2 | AxBy | STA | 1–5 | DSSCs | 38 |
| TiO2 | AxBy | STA | 1–4 | LIBs | 27 |
| Co3O4 | AxBy | STA | 1–4 | LIBs | 3 |
| α-Fe2O3 | AxBy | STA | 1–3 | LIBs | 17 |
| Mn2O3 | AxBy | STA | 1–4 | Supercapacitors | 20 |
| V2O5 | AxBy | STA | 1–3 | LIBs | 4 |
| MnO2 | AxBy | STA | 1–4 | Supercapacitors | 39 |
| Co3O4 | AxBy | MOF-based STA | 1–4 | Photocatalytic CO2 reduction | 11 |
| Co3O4 | AxBy | Soft template | 1–3 | LIBs | 40 |
| Cu2O | AxBy | Soft template | 1–4 | — | 24 |
| Cu2O | AxBy | Ostwald ripening | 1–4 | Sensors | 41 |
| Cu2S | AxBy | Ion exchange | 1–4 | — | 42 |
| SiO2–TiO2 | AxBy–CxDy | Layer-by-layer | 2 | DSSCs | 43 |
| TiO2/Fe2TiO5 | AxBy–CxDy | STA | 1–3 | Photocatalytic water oxidation | 28 |
| CeO2–MnOx | AxBy–CxDy | STA | 1–3 | Catalytic reduction of NO | 44 |
| Co3O4–CeO2−x | AxBy–CxDy | STA-derivative | 4 | Catalytic CO oxidation | 45 |
| ZnO–ZnFe2O4 | AxBy–CxDy | STA-derivative | 3 | Sensors | 46 |
| SrTiO3–TiO2 | AxBy–CxDy | STA-based | 1–3 | Water splitting | 7 |
| NiS–NiO | AxBy–CxDy | Sulphidation | 3 | Supercapacitors | 47 |
| CoO–Co9S8 | AxBy–CxDy | Sulphidation | 3 | Supercapacitors | 48 |
| CoP–Cu3P | AxBy–CxDy | Phosphidation | 6 | Supercapacitors | 49 |
| Anatase@rutile TiO2 | AxBy@CxDy | Layer-by-layer | 2 | Photocatalytic degradation | 10 |
| SiO2@TiO2@SiO2 | AxBy@CxDy | Layer-by-layer | 3 | Photocatalytic degradation | 9 |
| ZrO2/TiO2 | AxBy@CxDy | Layer-by-layer | 2 | — | 50 |
| TiO2/SiO2 | AxBy@CxDy | Layer-by-layer | 2 | Photocatalytic degradation | 51 |
| Co3O4@Co3V2O8 | AxBy@CxDy | Ion exchange | 1–3 | LIBs | 52 |
| LiMn2O4 | AxByCz | STA | 3–4 | LIBs | 53 |
| (Co2/3Mn1/3)(Co5/6Mn1/6)2O4 | AxByCz | STA | 3–7 | Alkaline rechargeable batteries | 29 |
| YVO4 | AxByCz | STA | 1–3 | Upconversion photoluminescence | 54 |
| Fe2(MoO4)3 | AxByCz | STA | 1–5 | Sodium-ion batteries | 18 |
| NiCo2O4 | AxByCz | STA-derivative | 3 | LIBs | 55 |
| NiCo2S4 | AxByCz | Ion exchange | 7 | Supercapacitors | 56 |
| Polypyrrole | Other | Layer-by-layer | 2 | — | 57 |
| Periodic mesoporous organosilica | Other | Multi-interface transformation | 1–3 | Drug loading/ultrasound imaging | 58 |
| Cr(III) terephthalate MOFs | Other | Layer-by-layer | 1–3 | Catalytic oxidation of styrene | 59 |
2.1 AxBy
This type of HoMS only contains one material composed of two elements; hence they can be synthesized by all three basic methods (hard template method, soft template method and template-free method) without considering other elements theoretically. A variety of this type of HoMS have been synthesized, especially metal oxides.The conventional layer-by-layer route is a typical hard template method to straightforwardly synthesize hollow structures or HoMSs.33,34 This method usually involves several layer-by-layer coating processes followed by removal of template layers. Hwang and co-workers have synthesized AxBy type multi-shelled porous TiO2 hollow nanoparticles (MS-TiO2-HNPs) via a layer-by-layer hard template method as shown in Fig. 1a.16 First, colloidal SiO2 spheres are synthesized by the Stöber method and used as the initial templates.35 Second, titanium(IV) isopropoxide (TTIP) is used as the TiO2 precursor to coat onto the SiO2 templates forming SiO2/TiO2 core/shell nanoparticles (ST-CSNPs). Third, polyvinylpyrrolidone (PVP) is used to modify the TiO2 surface, followed by coating a new SiO2 layer and a TiO2 layer forming SiO2/TiO2/SiO2/TiO2 (STST)-CSNPs. Further repeating the coating processes of SiO2 and TiO2 layers leads to SiO2/TiO2/SiO2/TiO2/SiO2/TiO2 (STSTST)-CSNPs. After the calcination process, the SiO2 templates can be removed by an etching process with a NaOH solution, which results in the formation of MS-TiO2-HNPs. The shell numbers can be controlled from 1 to 3 by controlling the layer-by-layer coating processes, as shown in Fig. 1b–d.
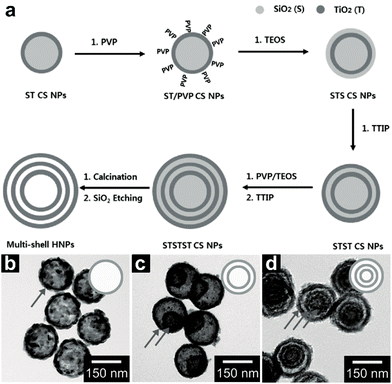 | ||
| Fig. 1 (a) Illustration of the MS-TiO2-HNP fabrication. Transmission electron microscopy (TEM) images of (b) single shell (SS), (c) double shell (DS), and (d) MS-TiO2-HNPs. Reproduced with permission from ref. 16. Copyright 2014, John Wiley & Sons. | ||
The conventional layer-by-layer hard template method is straightforward and effective to synthesize HoMSs, for instance, TiO2, SiO2, and SnO2.16,34,36,37,60 The size and thickness of shells of the HoMSs are usually tunable by this method. However, the procedure is tedious, complex and time-consuming. The Wang group has developed a hard template method called a sequential templating approach (STA) using carbonaceous microspheres (CMSs) as templates to synthesize HoMSs more efficiently.26,61,62 This method includes the template synthesis, metal ion adsorption and template removal processes. The formation of HoMSs accompanies the template removal process during calcination. As shown in Fig. 2a, CMS templates are firstly synthesized by a hydrothermal reaction.63 Then the CMSs penetrate into metal ion solution with high concentration. Metal ions can be adsorbed not only on the surface of the CMSs, but also deep inside. Afterwards, the one-step calcination process in air is performed to remove the CMS templates. During the calcination process, the adsorbed metal ions shrink together with the shrinking CMS until a solid shell is formed (Fig. 2b–d). The rest of the adsorbed metal ions inside the CMSs can repeat the shell formation process sequentially until the CMS is entirely removed or there is not enough metal source to form a stable shell (Fig. 2e and f). The amount of the adsorbed metal ions can be adjusted by controlling the penetration process to control the shell number. Besides the shell number, parameters including the shell thickness, shell space and shell composition are adjustable. Through the STA, a variety of metal oxide HoMSs can be synthesized, indicating its generality.3,15,17,27,38,64 The STA is much easier as compared to the conventional layer-by-layer method.
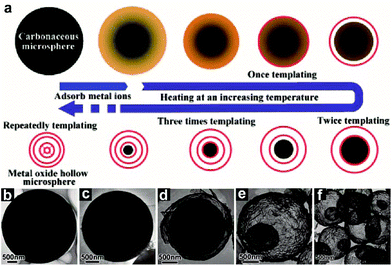 | ||
| Fig. 2 (a) Illustration of the STA to synthesize metal oxide HoMSs. TEM images of CMSs after soaking in a 3 mol L−1 solution of iron nitrate: (b) before heating, and after heating at (c) 270 °C, (d) 350 °C, (e) 430 °C, and (f) 500 °C. Reproduced with permission from ref. 26. Copyright 2011, John Wiley & Sons. | ||
The STA using CMSs as hard templates is one of the most powerful methods for the synthesis of AxBy type metal oxide HoMSs. By using the STA, Dong and co-workers have synthesized ZnO HoMSs.15 Besides the controllable shell number from 1 to 4, a programmable heating process plays a key role in adjusting the inter-shell spacing by a comprehensive investigation on the evolution of ZnO HoMSs (Fig. 3a). Both fast heating and medium heating processes lead to normal HoMSs with relatively large spacing between adjacent shells (Fig. 3b and c), while a slow heating process results in ZnO HoMSs with close spacing between the exterior two shells as shown in Fig. 3d. In fact, the calcination process controls the separation of the exterior shell from the inner CMS core originating from differential shrinking rates. However, based on other studies afterwards, it seems that the effect of heating conditions on the shell spacing for different materials is quite different. SnO2 HoMSs synthesized by the STA are not so sensitive to the heating conditions.38,65 A common annealing process of the as-penetrated CMSs in aqueous Sn4+ solution can achieve SnO2 HoMSs with close spacing between the exterior two shells, as shown in Fig. 3e. However, for TiO2, the annealing conditions are more important to the spacing between the exterior shells, as investigated by Ren and co-workers (Fig. 3f–i).27
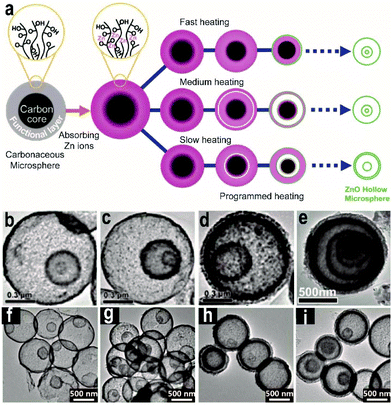 | ||
| Fig. 3 (a) Illustration of the synthesis of ZnO HoMSs by programming the heating processes. TEM images of triple-shelled ZnO HoMSs via different annealing processes: (b) fast heating, (c) medium heating and (d) slow heating. Reproduced with permission from ref. 15. Copyright 2012, John Wiley & Sons. (e) TEM image of SnO2 HoMSs with close spacing between the exterior two shells. Reproduced with permission from ref. 38. Copyright 2013, John Wiley & Sons. TEM images of TiO2 HoMSs: (f) double-shelled and (g) triple-shelled TiO2 HoMSs with relatively large spacing between the shells, (h) double-shelled and (i) triple-shelled TiO2 HoMSs with close exterior two shells. Adapted with permission from ref. 27. Copyright 2014 American Chemical Society. | ||
The number of shells for HoMSs synthesized by the STA is usually tuned by the amount of the adsorbed metal ions and the annealing conditions. However, for some metal precursors, the large radii of hydrated metal ions are obstacles to realize a high enough adsorption amount for the production of HoMSs. To remedy this issue, Wang and co-workers have controlled the size of hydrated metal cations and the adsorption capability of CMSs, thus having precisely achieved accurate control of the synthesis of Co3O4 HoMSs, as shown in Fig. 4a–d.3 Thereafter, Xu and co-workers have controlled the ethanol content of the aqueous infusion solution to tune the Fe3+ concentration in the CMS templates and have synthesized α-Fe2O3 HoMSs with a controllable shell thickness, porosity and number of shells, as shown in Fig. 4e–j.17 The key is that ethanol can lower surface tension and enhance wettability by disrupting hydrogen bonding and cohesive forces between water molecules, which affects the adsorption of Fe3+ into the CMSs. Except for the effect of ethanol, the pH value also plays an important part in the adsorption of metal ions by CMS templates. Wang and co-workers have controlled the pH value of the Mn2+ infusion solution to increase the adsorption capability of CMS templates and have synthesized Mn2O3 HoMSs with a tunable number of shells, porosity and shell thickness (Fig. 4k–m).20 Additional research shows that CMS templates can adsorb not only cations by electrostatic interactions but also anions.4 And a new mechanism has been proposed. The CMS templates contain abundant functional groups including –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C–O–C groups which can adsorb ions with similar elemental compositions. Hence CMS templates can adsorb OH− or other oxygen-containing groups. As a consequence, the CMSs become negatively charged and can adsorb cations by electrostatic interactions. This proposed mechanism is supported by modelling and calculations. V2O5 HoMSs have been synthesized by adsorbing VO3− anions. Additional extended experiments have been conducted to synthesize MoO3, Cr2O3 and WO3 HoMSs, which further confirm the proposed mechanism. Based on the anion-adsorption process, Chen and co-workers have successfully synthesized MnO2 HoMSs by the STA under hydrothermal conditions.39
O and –C–O–C groups which can adsorb ions with similar elemental compositions. Hence CMS templates can adsorb OH− or other oxygen-containing groups. As a consequence, the CMSs become negatively charged and can adsorb cations by electrostatic interactions. This proposed mechanism is supported by modelling and calculations. V2O5 HoMSs have been synthesized by adsorbing VO3− anions. Additional extended experiments have been conducted to synthesize MoO3, Cr2O3 and WO3 HoMSs, which further confirm the proposed mechanism. Based on the anion-adsorption process, Chen and co-workers have successfully synthesized MnO2 HoMSs by the STA under hydrothermal conditions.39
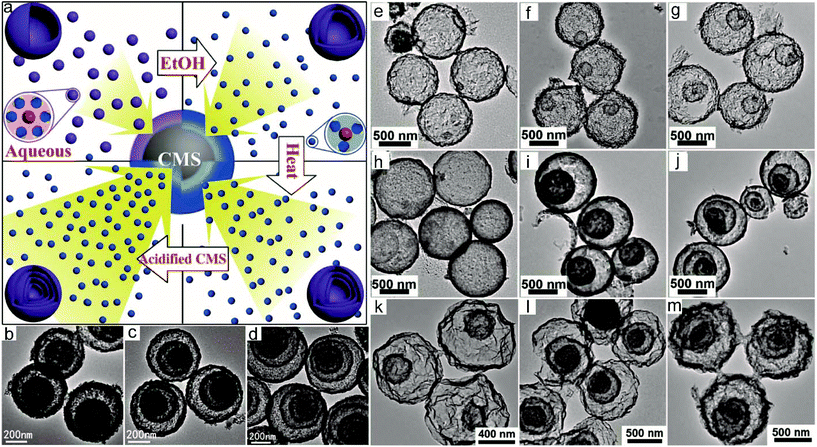 | ||
| Fig. 4 (a) Illustration of the synthesis of Co3O4 HoMSs by controlling the adsorption conditions. TEM images of (b) double-, (c) triple-, and (d) quadruple-shelled Co3O4 HoMSs. Adapted with permission from ref. 3. Copyright 2013, John Wiley & Sons. TEM images of α-Fe2O3 HoMSs: (e) thin single-shell, (f) thin double-shell, (g) thin triple-shell, (h) thick single-shell, (i) thick double-shell and (j) thick triple-shell. Adapted from ref. 17 with permission from The Royal Society of Chemistry. TEM images of (k) double-, (l) triple-, and (m) quadruple-shelled Mn2O3 HoMSs.20 | ||
For the STA to synthesize HoMSs, it should be noted that the metal precursors have to be adsorbed not only on the CMS surface but also deep inside to ensure the sequential formation of multiple shells. This idea can be extended considering that metal–carbon composites with radially distributed metal precursors are necessary while it can be met by other methods. A hydrothermal process can also produce carbonaceous spheres containing metal precursors which are similar to the as-adsorbed CMSs obtained by the STA. After calcination treatment, HoMSs may be achieved. Although this method is claimed as a template-free method, the formation process of HoMSs is quite similar to the STA. Qi and co-workers have synthesized carbon microspheres using glucose, urea and CeCl3 by a hydrothermal process.66 A subsequent annealing process leads to the formation of AxBy type CeO2 HoMSs. This method can also be used to synthesize other AxBy type metal oxide materials with HoMSs such as ZnO, La2O3, NiO and so on.67–70 Another way is the spray drying method which can produce metal–carbon composite spheres. Zhou and co-workers have synthesized α-Fe2O3 HoMSs by the spray drying method using iron nitrate and sucrose as precursors followed by the calcination process.71 Other metal sources can also be used as precursors to synthesize AxBy type HoMSs by the spray drying method.72–74 Similar to the spray drying method, the electrospinning method is also useful for HoMSs. This method can be used to synthesize some tubular HoMSs.75 The disadvantage of the methods to directly synthesize metal–carbon composites is the poor size distribution of the final HoMSs. However, the spray drying and electrospinning methods are relatively cheap and can be scalable. Metal–organic frameworks (MOFs) are unique promising materials containing metal elements and organic components, which meet the requirements of the STA for HoMS synthesis, theoretically. Hence it is possible to acquire HoMSs similar to the CMS-based STA. Wang and co-workers have realized the transformation from ZIF-67 to AxBy type Co3O4 dodecahedron HoMSs with an exposed (111) facet.11 As illustrated in Fig. 5, the Co atoms in the exposed (001) and (011) facets of the ZIF-67 dodecahedron show similar periodic arrangements to that in the (111) facet of Co3O4. The Co atoms can preferentially shift in ZIF-67 with enough space to transform to Co3O4 with an exposed (111) facet. This transformation is defined as “genetic inheritance”. The transformation and the controllable HoMSs are carefully tuned by controlling both the annealing process and the oxygen partial pressure. This method is an extension of the STA and it provides a new way to realize the synthesis of HoMSs. This great step may spur more precise control on the exposed crystal orientation of HoMSs in future.
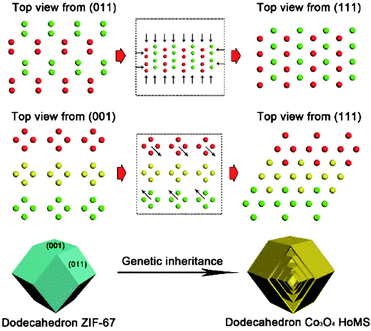 | ||
| Fig. 5 “Genetic inheritance” from MOFs to Co3O4 HoMS. All atoms are Co, and different colors (green, yellow, and red) indicate periodic units. Reproduced with permission from ref. 11. Copyright 2019 American Chemical Society. | ||
The soft template method is also a useful way to synthesize hollow structured materials. This method usually involves using some “elastic” templates such as bubbles, vesicles, micelles and emulsions.24,76,77 The soft template interface can act as specific sites for material growth and solidification, and direct the shell formation of target materials. When the soft templates exist as multi-shelled structures, it is possible to synthesize HoMSs. For example, Xu and co-workers have synthesized AxBy type Cu2O HoMSs by using cetyltrimethylammonium bromide (CTAB) multi-lamellar vesicles as soft templates.24 As illustrated in Fig. 6a, the self-assembly of the CTAB surfactant leads to the formation of micelles when heated in aqueous solution. The electrostatic interaction of Cu2+ and Br− results in Cu–Br moieties thus accumulating Cu2+ at the interface around the CTAB micelles. The Cu2O shell forms when the Cu2+ ions are reduced. By controlling the concentration of CTAB, multi-lamellar vesicles can be adjusted; hence Cu2O can grow on each soft template layer and finally Cu2O HoMSs with 1–4 shells can be synthesized as illustrated in Fig. 6c. Similarly, Wang and co-workers have used PVP micelles as soft templates to synthesize Co3O4 HoMSs.40 The concentration of PVP is adjusted to control the formation of multi-lamellar PVP micelles thus directing the formation of single-shelled (S-Co), double-shelled (D-Co) and triple-shelled Co3O4 (T-Co) hollow spheres. Other similar multi-lamellar micelles or vesicles as templates have also achieved the synthesis of AxBy type HoMSs, such as Cu2O, CuO and SiO2 HoMSs.21,24,78
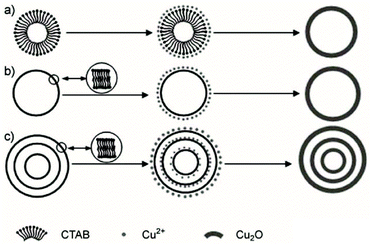 | ||
| Fig. 6 The formation of different Cu2O hollow structures in the presence of a CTAB template: (a) micelle, (b) single-lamellar vesicle, and (c) multi-lamellar vesicle. Adapted with permission from ref. 24. Copyright 2007, John Wiley & Sons. | ||
Compared to the template methods, the template-free method does not rely on templates. Several mechanisms have been proposed to elucidate the formation of HoMSs by the template-free method, such as the Kirkendall effect, Ostwald ripening process, and ion exchange.25,79 The Ostwald ripening process involves the dissolution of small particles and re-deposition of the dissolved particles onto larger particles.80 Lots of hollow structures have been realized via the Ostwald ripening process.81,82 Besides, it is also possible to synthesize HoMSs via this method. Through a two-step Ostwald ripening process, Zhang and co-workers have carried out the synthesis of Cu2O double-layer nanoshells.25 Firstly, Cu2O single-layer nanoshells are synthesized by a one-step Ostwald ripening process.83 Secondly, as illustrated in Fig. 7a, new thicker Cu2O shells are quickly grown onto the Cu2O single-layer nanoshells followed by the second Ostwald ripening process. With the increased ripening time, Cu2O double-layer nanoshells can be gradually produced as shown in Fig. 7b–e. This method can be extended to the multi-step Ostwald ripening process to control the shell number, generating Cu2O triple-layer and quadruple-layer nanoshells. Besides, the geometrical parameters including the shell thickness and the intershell spacing can also be controlled. The nanoscale Kirkendall effect was firstly proposed by Yin and co-workers to explain the formation of hollow nanocrystals.84 In a diffusion couple, due to the different diffusion rates between two components, pores are possible to form, resulting in hollow nanocrystals or even core–shell hollow nanocrystals. However, HoMSs via the Kirkendall effect are seldom seen. Ostwald ripening and ion exchange can also achieve the synthesis of some AxBy type HoMSs such as Cu2O, Cu2S, ZnO and CeO2.41,42,85,86 However, the soft template method and template-free method are sensitive to more parameters, which is a limitation to extend them to more materials. It should be noted that phosphidation and sulphidation processes can also transform some metal oxide HoMSs to AxBy type metal phosphide or metal sulphide HoMSs easily.8,87,88
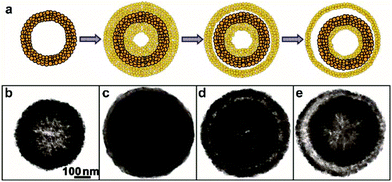 | ||
| Fig. 7 (a) Schematics illustrating the formation of Cu2O double-layer nanoshells through a two-step Ostwald ripening process, and corresponding TEM images of individual spheres obtained at (b) 40 min during the first-step Ostwald ripening, and (c) 1, (d) 40 and (e) 60 min during the second-step Ostwald ripening. Adapted with permission from ref. 25. Copyright 2011 American Chemical Society. | ||
2.2 AxBy–CxDy composites
Each shell of the AxBy–CxDy type HoMSs is composed of two mixed substances. It is more complicated compared to the AxBy type HoMSs since more than one material have to be taken into consideration.89 The conventional layer-by-layer hard template method still works for some materials. One typical and simple example is double-shelled SiO2–TiO2 HoMSs synthesized by a layer-by-layer method followed by partial etching of SiO2 templates.43,90 The rest of SiO2 co-exists with TiO2 within the shells forming composites. In comparison with the layer-by-layer method, more composites with HoMSs have been synthesized by the STA and STA-derivative methods. Waqas and co-workers have prepared multi-shelled TiO2/Fe2TiO5 composite hollow microspheres with controllable shell numbers, shell spacing and Fe/Ti molar ratios by the STA.28 The formation of the composite composed of two substances is fulfilled by co-adsorbing Fe and Ti salts followed by one-step annealing treatment. The adsorption process can also be performed under hydrothermal conditions. Ma and co-workers have produced CeO2–MnOx HoMSs by the STA where the adsorption step by CMS templates is carried out by a hydrothermal treatment.44 However, by using the STA, the molar ratios of two metal elements in the final HoMS products are usually difficult or complicate to be adjusted in a large range since the adsorption capability of different metal precursors by the CMS templates varies a lot. In contrast, the molar ratios can be easily controlled by the spray method due to the homogeneously distributed metal ions in the precursor droplets driven by high electrostatic voltage. By the electrostatic spray method and the subsequent calcination process, Wang and co-workers have synthesized Co3O4–CeO2−x nanocomposite HoMSs with tunable Co/Ce ratios (Fig. 8).45 The composite composition of Co3O4 and CeO2 is confirmed by the high-resolution TEM (HRTEM) image, selected-area electron diffraction (SAED) patterns and X-ray diffraction (XRD) patterns as shown in Fig. 8d and e. The elements are uniformly distributed within each shell as indicated by the elemental mapping images in Fig. 8c. The spray pyrolysis method is similar to the electrostatic spray method. Choi and co-workers have synthesized ZnO–Mn3O4 HoMSs with this method. The molar ratio of Zn to Mn can also be manipulated easily.91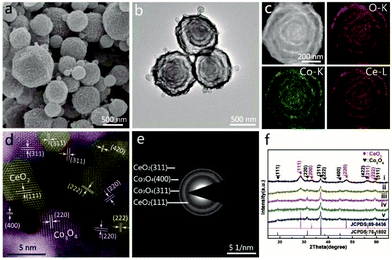 | ||
| Fig. 8 Characterization of Co3O4–CeO2−x (Co/Ce = 4/1) HoMS: (a) SEM image, (b) TEM image, (c) HAADF-STEM image, (d) HRTEM image, and (e) SAED image. (f) XRD patterns (i. Co/Ce = 2/1, ii. Co/Ce = 4/1, iii. Co/Ce = 8/1, iv. Co/Ce = 12/1, v. Co/Ce = 16/1). (Co3O4: purple section, CeO2: yellow section). Reproduced with permission from ref. 45. Copyright 2007, John Wiley & Sons. | ||
As aforementioned, MOFs can be used to synthesize hollow structures or even HoMSs. The MOF-based STA sometimes also works to acquire AxBy–CxDy type HoMSs. Jiao and co-workers have achieved the synthesis of multi-shelled manganese–cobalt oxide hollow dodecahedra (Co/Mn-HD) by using the Mn-doped zeolitic imidazolate framework (ZIF-67) as the precursor (Co/Mn-ZIF) through a continuous two-step calcination process.19 As illustrated in Fig. 9a, the Co/Mn-ZIF is firstly synthesized by substituting partial Co in ZIF-67 with Mn by a co-precipitation method in a methanol solution. Then the Co/Mn-ZIF is converted to single-, double- and triple-shelled Co/Mn-HD by carefully controlling the calcination process (Fig. 9b–d). During the calcination process, the organic composition decomposes and shrinks inwards while the formed grains contract and grow with the precursors. The calcination process plays the key role in determining the formation of HoMSs since the nucleation rate has to be matched with the combustion rate. Song and co-workers have synthesized ZnO–ZnFe2O4 HoMSs by using crystalline Prussian Blue analogues as templates, while Zhan and co-workers have used amorphous ZIF-90 to synthesize CuO/ZnO HoMSs with the aid of copper.46,92
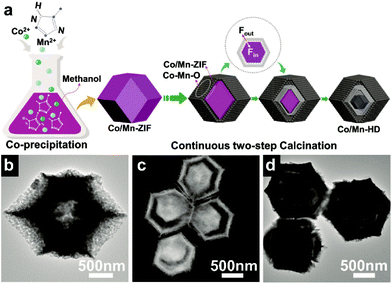 | ||
| Fig. 9 (a) The synthesis mechanism of triple-shelled Co/Mn-HD. (b) TEM image of single-shelled Co/Mn-HD. (c) Scanning TEM (STEM) image of double-shelled Co/Mn-HD. (d) TEM image of triple-shelled Co/Mn-HD. Reproduced with permission from ref. 19. Copyright 2018, John Wiley & Sons. | ||
Generally speaking, the STA performs great for the synthesis of AxBy–CxDy type HoMSs. However, for some composites, during the calcination process, the two substances may react to generate only one substance or other impurities. Or some materials can only be achieved at higher annealing temperature while the HoMSs may collapse to some extent. To avoid impure by-products within SrTiO3–TiO2 HoMSs, Wei and co-workers have firstly synthesized TiO2 HoMSs by the STA.7 Then a hydrothermal synthesis is performed using the TiO2 HoMSs as reagents and directors. During the hydrothermal process, the Sr4+ ions released from Sr(OH)2 can react with TiO2 to generate SrTiO3via a dissolution-precipitation process, which results in HoMSs, as shown in Fig. 10. The exposed facets of the SrTiO3 are also precisely tailored by using 1,2-propanediol as a surfactant. This is a good example using AxBy type HoMSs as templates to synthesize AxBy–CxDy type HoMSs. The second-step loading process surely can also generate this type of HoMS such as Au/CeO2, SnO2–Fe2O3 and SnO2–TiO2 composite HoMSs.14,93,94 It should be mentioned that the partial sulphidation and phosphidation process can also produce AxBy–CxDy type HoMSs, for example, NiS–NiO, CoO–Co9S8 and CoP–Cu3P HoMSs.47–49 Most AxBy–CxDy type HoMSs are synthesized by the above-mentioned methods while the soft template method or template-free method is barely seen.
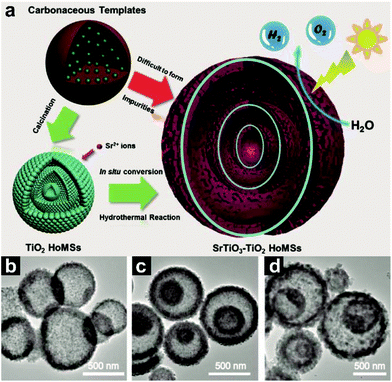 | ||
| Fig. 10 (a) Synthesis route of SrTiO3–TiO2 hollow multi-shelled structures from the hydrothermal reaction. TEM images of (b) single (1S-), (c) double (2S-), and (d) triple (3S-) shelled STHoMSs. Reproduced with permission from ref. 7. Copyright 2019, John Wiley & Sons. | ||
2.3 AxBy@CxDy
For this type, the inner shell composition and the outer shell composition are different. The STA may not work well for this type since the used CMS usually adsorbs metal ions simultaneously without separation between two or more layers. The most effective way is the conventional layer-by-layer hard template method. The template layers could effectively separate each precursor layer; hence the composition of each shell is independent and can be manipulated easily. One typical synthesis is based on the SiO2 template. For instance, Li and co-workers have synthesized double-shell anatase-rutile TiO2 hollow spheres, of which the composition of the inner shell is anatase while the composition of the outer shell is rutile.10 Titanium butoxide (TBOT) is firstly deposited onto silica templates by a sol–gel process with hydroxypropyl cellulose (HPC) as the surfactant, forming SiO2@TiO2 spheres. Then another SiO2 layer is deposited onto the surface of the SiO2@TiO2 spheres, followed by the deposition of the second TiO2 layer forming SiO2@TiO2@SiO2@TiO2 spheres. A crystallization process by calcination makes the deposited amorphous TiO2 transform to anatase and rutile phases. After removing the SiO2 layer by an etching method with an aqueous NaOH solution, double-shell anatase-rutile TiO2 hollow spheres are achieved. The different phases of the inner shell and the outer shell are attributed to the protection of the SiO2 layer. The outer TiO2 layer can be transformed to a rutile phase more easily without the protection of a SiO2 layer while the transformation of the inner TiO2 layer is inhibited by the SiO2 layer resulting in anatase only. This method realizes the synthesis of HoMSs with different phases of the inner shell and the outer shell. Joo and co-workers have used a similar layer-by-layer method to synthesize SiO2@TiO2@SiO2 spheres.9 However, both the inner and outer SiO2 layers are partially etched with a NaOH solution. SiO2@TiO2@SiO2 HoMSs are achieved after the etching process.Polymers can also be used as templates to control the composition of the inner shell and the outer shell of HoMSs. Zhang and co-workers have synthesized polymer/SiO2/polymer/TiO2 tetra-layer microspheres and corresponding double-walled hollow SiO2/TiO2 microspheres after removing the polymer composition.95 The composition of the inner shell of the double-walled hollow SiO2/TiO2 microspheres is SiO2 while the outer shell is composed of TiO2. Similarly, Qin and co-workers have synthesized tetra-layer poly(N,N′-methylenebisacryl amide-comethacrylic acid) (P(MBA-co-MAA))/Zr(OH)4/poly(ethyleneglycol dimethacrylate-co-methacrylic acid) (P(EGDMA-co-MAA))/TiO2 hybrid microspheres and corresponding double-shelled zirconia/titania (ZrO2/TiO2) hollow microspheres.50 Resorcinol-formaldehyde (RF) is also an alternative to realize the synthesis of AxBy@CxDy type HoMSs. Fang and co-workers have synthesized double-shelled TiO2/SiO2 hollow spheres with different shell compositions using RF as the template via a layer-by-layer method.51 Au nanoparticles can further be confined in the double-shelled TiO2/SiO2 hollow spheres afterwards. By using carbonaceous spheres as templates followed by a layer-by-layer coating process, HoMSs with different shell compositions are also possible to be synthesized. Yuan and co-workers have firstly coated SnO2 onto carbonaceous spheres and then coated TiO2 subsequently.96 After a calcination treatment, double-shelled TiO2/SnO2 hollow spheres are produced. Although it is claimed that the inner shell is SnO2 and the outer shell is TiO2, it is still possible that both shells might have mixed compositions considering the direct contact of the SnO2 shell with the TiO2 shell before calcination. It is also a possible similar issue for multi-shelled Fe3O4@MnOx hollow microspheres.97
Coupling ion exchange with the MOF-based method to synthesize AxBy@CxDy type HoMSs is also a possible way. Lu and co-workers have constructed Co3O4@Co3V2O8 hollow structures by a MOF-engaged strategy.52 As shown by route I illustrated in Fig. 11a, ZIF-67 nanocubes are firstly synthesized as the initial templates. Then the nanocubes are dispersed in a vanadium oxytriisopropoxide (VOT) solution for a hydrothermal treatment. An ion exchange reaction between VO43− released from VOT and 2-methylimidazole anions in ZIF-67 occurs, which results in the formation of an amorphous Co3V2O8 (a-Co3V2O8) shell. At the same time, a gap space forms between the shell and the remaining inner ZIF-67 core due to some consumption, as shown by step I in Fig. 11a. During the calcination process (step II), the remaining ZIF-67 core can gradually evolve into an inner Co3O4 hollow structure while the a-Co3V2O8 shell crystallizes, leading to the formation of a triple-shelled Co3O4@Co3V2O8 hollow structure. The elemental mapping analysis of the triple-shelled Co3O4@Co3V2O8 hollow structure (Fig. 11b–f) shows that vanadium is only detected in the outmost shell while the rest of the elements distribute in all shells. Hence the outmost shell should be Co3V2O8 while the inner two shells are Co3O4. Moreover, the amount of VOT can be tuned to control the hollow structures. Another successful example using a MOF as a template is Co3O4/NiCo2O4 double-shelled nanocages (DSNCs) synthesized by Hu and co-workers.98 When ZIF-67 is dispersed in Ni(NO3)2 solution, Ni2+ ions hydrolyze and generate protons. The protons can etch the ZIF-67 releasing Co2+ ions which can be further oxidized to Co3+. The cobalt ions and the nickel ions can react to generate Ni–Co layered double hydroxide (LDH) around the ZIF-67 core forming a ZIF-67@Ni–Co LDH yolk–shell structure. Further calcination treatment in air can oxidize the core–shell structure and form Co3O4/NiCo2O4 DSNCs. Park and co-workers have used the same method to synthesize a ZIF-67@Ni–Co LDH yolk–shell structure.99 Instead of calcination in air, a selenization process is performed to form CoSe2/(NiCo)Se2 box-in-box hollow nanocubes with different shell compositions. A new layer of another material can also be coated onto the AxBy@CxDy type HoMSs.100 However, the new introduced material may also be present on the inner shells. Hence the coating method should be well selected and designed.
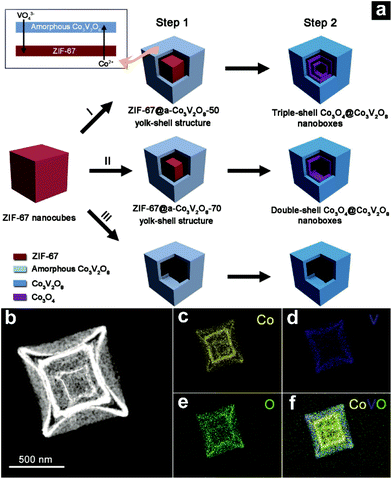 | ||
| Fig. 11 (a) Illustration of the formation of complex Co3O4@Co3V2O8 hollow structures. Step 1 involves the solvothermal reactions with different amounts of VOT: (I) 50 μL, (II) 70 μL, and (III) 120 μL. Step 2 is simple annealing in air. (b–f) Elemental distributions for an individual T-Co3O4@Co3V2O8 nanobox. (b) HAADF-STEM image, (c) Co mapping, (d) V mapping, (e) O mapping, and (f) overlay mapping. Reproduced with permission from ref. 52. Copyright 2017, John Wiley & Sons. | ||
2.4 AxByCz
The synthesis of the AxByCz type HoMSs is relatively difficult compared to that of the AxBy or AxBy–CxDy type since mixed two or more substances may be easily formed during the synthesis process. As reviewed above, by using the STA, AxBy–CxDy type HoMSs can be easily synthesized. In fact, by carefully controlling the synthesis procedure, the STA can also be used to produce AxByCz type HoMSs. Wang and co-workers have introduced Li+ into the infusion solution of Mn2+ to synthesize LiMn2O4 HoMSs via the STA.53 The molar ratio of the Li/Mn precursor has to be 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to achieve the final formation of LiMn2O4 HoMSs since Mn2+ can be more easily adsorbed by CMSs considering the higher charges which can form stronger electrostatic interaction with CMSs with negative charge. Through the STA, Zhao and co-workers have synthesized sextuple and septuple-shelled (Co2/3Mn1/3)(Co5/6Mn1/6)2O4 HoMSs with thin shells and a uniform gap between adjacent shells by co-adsorbing Co and Mn precursors using CMSs as templates.29 As illustrated in Fig. 12a, the Co/Mn ratio is found to affect the final structures of the HoMSs. This originates from the difference between the crystallization rate of the exterior metal oxide and the burning shrinking rate of the inner CMS. The doping amount of Mn into Co3O4 can change the crystallization rate of the metal oxide shell. When the Co/Mn ratio is 3.75, the crystallization and growth of metal oxide particles to form a shell are fast during the annealing treatment, leading to the formation of sextuple-shelled and septuple-shelled HoMSs, as shown in Fig. 12b and c. However, for some AxByCz type HoMSs synthesized by the STA, directly using two precursors in the infusion solution may lead to a direct reaction between the precursors. For instance, Y3+ and VO3− ions can react to form YVO4 directly. Zong and co-workers have adopted a two-step adsorption process to synthesize YVO4 HoMSs by the STA.54 Y3+ is firstly adsorbed by CMSs to form Y-CMSs through a hydrothermal treatment process. Afterwards, VO3− ions are adsorbed by the Y-CMSs via another hydrothermal treatment. YVO4 HoMSs can be acquired after an annealing treatment (Fig. 12d). Different from the two-step adsorption method, Zhao and co-workers have introduced citric acid to restrain the precipitation reaction between cations and anions, and have realized the synthesis of multi-shelled binary metal oxides by the STA.18 The citric acid added into the infusion solution can chelate with not only anions like MoO42− but also cations like Fe3+ suppressing the hydrolysis and precipitation reaction. The smaller free Fe3+ and MoO42− ions with more charge can be adsorbed by CMS templates. Meanwhile, the chelated coordination ions can act as a reservoir releasing more free Fe3+ and MoO42− ions. Consequently, a series of binary metal oxides including Fe2(MoO4)3 (Fig. 12e and f), NiMoO4, MnMoO4, CoWO4, and MnWO4 HoMSs have been obtained after a calcination process. Another way of using ethylene glycol as the solvent for the infusion solution can also prevent the precipitation reaction and lead to the formation of Bi2WO6 HoMSs by the STA.101 Zhang and co-workers have carried out the penetration and solidification process in an ethylene glycol solution of metal acetate precursors at high temperature by the STA, and have synthesized HoMSs composed of various mixed metal oxides.102
1 to achieve the final formation of LiMn2O4 HoMSs since Mn2+ can be more easily adsorbed by CMSs considering the higher charges which can form stronger electrostatic interaction with CMSs with negative charge. Through the STA, Zhao and co-workers have synthesized sextuple and septuple-shelled (Co2/3Mn1/3)(Co5/6Mn1/6)2O4 HoMSs with thin shells and a uniform gap between adjacent shells by co-adsorbing Co and Mn precursors using CMSs as templates.29 As illustrated in Fig. 12a, the Co/Mn ratio is found to affect the final structures of the HoMSs. This originates from the difference between the crystallization rate of the exterior metal oxide and the burning shrinking rate of the inner CMS. The doping amount of Mn into Co3O4 can change the crystallization rate of the metal oxide shell. When the Co/Mn ratio is 3.75, the crystallization and growth of metal oxide particles to form a shell are fast during the annealing treatment, leading to the formation of sextuple-shelled and septuple-shelled HoMSs, as shown in Fig. 12b and c. However, for some AxByCz type HoMSs synthesized by the STA, directly using two precursors in the infusion solution may lead to a direct reaction between the precursors. For instance, Y3+ and VO3− ions can react to form YVO4 directly. Zong and co-workers have adopted a two-step adsorption process to synthesize YVO4 HoMSs by the STA.54 Y3+ is firstly adsorbed by CMSs to form Y-CMSs through a hydrothermal treatment process. Afterwards, VO3− ions are adsorbed by the Y-CMSs via another hydrothermal treatment. YVO4 HoMSs can be acquired after an annealing treatment (Fig. 12d). Different from the two-step adsorption method, Zhao and co-workers have introduced citric acid to restrain the precipitation reaction between cations and anions, and have realized the synthesis of multi-shelled binary metal oxides by the STA.18 The citric acid added into the infusion solution can chelate with not only anions like MoO42− but also cations like Fe3+ suppressing the hydrolysis and precipitation reaction. The smaller free Fe3+ and MoO42− ions with more charge can be adsorbed by CMS templates. Meanwhile, the chelated coordination ions can act as a reservoir releasing more free Fe3+ and MoO42− ions. Consequently, a series of binary metal oxides including Fe2(MoO4)3 (Fig. 12e and f), NiMoO4, MnMoO4, CoWO4, and MnWO4 HoMSs have been obtained after a calcination process. Another way of using ethylene glycol as the solvent for the infusion solution can also prevent the precipitation reaction and lead to the formation of Bi2WO6 HoMSs by the STA.101 Zhang and co-workers have carried out the penetration and solidification process in an ethylene glycol solution of metal acetate precursors at high temperature by the STA, and have synthesized HoMSs composed of various mixed metal oxides.102
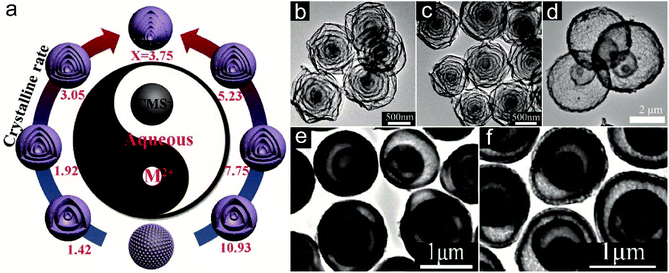 | ||
| Fig. 12 (a) Illustration of the synthesis of septuple-shelled (Co2/3Mn1/3)(Co5/6Mn1/6)2O4 HoMSs with a thin shell, uniform gap between adjacent shells by finely controlling the Co/Mn molar ratio using the STA (M2+: Co2+ and Mn2+; X: the molar ratio of Co/Mn). TEM images of (b) sextuple-shelled and (c) septuple-shelled (Co2/3Mn1/3)(Co5/6Mn1/6)2O4 HoMSs. Reproduced with permission from ref. 29. Copyright 2017, John Wiley & Sons. (d) TEM image of triple-shell YVO4 HoMSs. Reproduced with permission from ref. 54. Copyright 2016, John Wiley & Sons. (e) and (f) TEM images of Fe2(MoO4)3 HoMSs. Adapted with permission from ref. 18. Copyright 2018 American Chemical Society. | ||
The STA-derivative methods also work for the synthesis of AxByCz type HoMSs. The spray pyrolysis method can be used to prepare HoMSs on a large scale and the composition of the final products is easier to control sometimes. Materials with HoMSs such as LiV3O8, CoMn2O4, LiMn2O4 and NiCo2O4 have been successfully synthesized by the spray pyrolysis method.103–107 However, much effort still has to be made to precisely control the HoMS parameters by this method. Shen and co-workers have used a hydrothermal method to synthesize NiCo-glycolate solid spheres which are similar to the as-adsorbed CMSs obtained by the STA.55 These solid spheres can evolve into NiCo2O4 HoMSs by annealing treatment in air. By using this method, HoMSs including ZnMn2O4, MnCo2O4, ZnFe2O4 and NiMn2O4 have been synthesized.108–111
Ion exchange is also a way to synthesize HoMSs. Shen and co-workers have synthesized a ternary nickel cobalt sulfide (NiCo2S4) ball-in-ball hollow structure by an anion exchange method.112 As illustrated in Fig. 13a, at stage I, an anion exchange reaction between the S2− ions released from thioacetamide (TAA) and the hydrothermally synthesized NiCo-glycerate (Fig. 13b) occurs to form a NiCo-glycerate@NiCo2S4 core–shell sphere (Fig. 13c). The ongoing anion exchange continues until the metal cations cannot diffuse to the outer shell via the large empty gap, thus forming the second NiCo2S4 shell at stage II (Fig. 13d). The NiCo2S4 ball-in-ball hollow spheres are acquired at stage III when the anion exchange reaction ends (Fig. 13e). This method can also be extended to other sulfides. Recently, it was found that metal oxide HoMSs can also act as templates to form AxByCz type HoMSs. Guan and co-workers have used onion-like Co3O4 HoMSs to synthesize NiCo2S4 HoMSs by a sequential ion-exchange method.56 Firstly, an anion-exchange reaction between Co3O4 and S2− ions takes place forming onion-like Co4S3 HoMSs. A subsequent cation-exchange reaction with Ni2+ results in the formation of NiCo2S4 HoMSs. Beyond the above method, other AxByCz type HoMSs can also be achieved by other methods.113,114
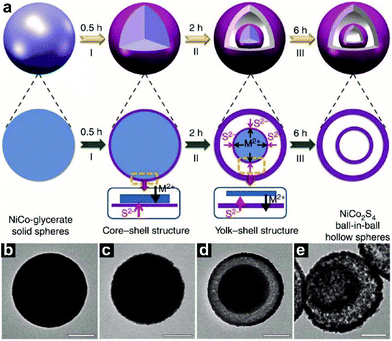 | ||
| Fig. 13 (a) Schematic illustration of the formation process of NiCo2S4 ball-in-ball hollow spheres. M2+ refers to metal cations including Ni2+ and Co2+ ions. TEM images of (b) NiCo-glycerate solid spheres, and corresponding products obtained after the sulfidation process at 160 °C for different durations: (c) 0.5 h; (d) 2 h; (e) 6 h. Scale bars, 200 nm. Reprinted with permission from ref. 112, copyright 2015 Springer Nature. | ||
2.5 Others
Except for the above types of HoMSs, there are still some other types, such as polymers, carbon, MOF HoMSs and so on. Bai and co-worker have synthesized double-shelled polypyrrole (PPy) HoMSs by using polystyrene (PS) spheres as templates.57 PS hollow spheres with holes are firstly prepared by swelling solid PS spheres with toluene.115 The small hole on the PS surface is the key to coat the PPy layer on both the inner and outer surface of the prepared PS templates, as illustrated in Fig. 14a. After removing the PS templates with tetrahydrofuran (THF), double-shelled PPy HoMSs are acquired as shown in Fig. 14b–d. PVP is found to play a key role in the porosity of the shells as illustrated in Fig. 14a. A similar way of using Fe3O4 hollow or core–shell templates can also produce polymer HoMSs.116p-Toluenesulfonic acid (p-TSA) is used to gradually etch the Fe3O4 hollow templates forming pores thus contributing to the penetration of monomers into the inner surface. When the templates are finally dissolved, poly(3,4-ethylenedioxythiophene) (PEDOT) and PPy HoMSs are formed. Mesoporous silica hollow spheres can also be used as templates to coat materials on both inner and outer surfaces of the silica templates. Sun and co-workers have synthesized carbon HoMSs by coating carbon on mesoporous silica hollow spheres and a subsequent etching process to remove the silica templates.117 Double-shelled mesoporous silica hollow spheres are also further used to prepare quadruple-shelled carbon HoMSs. To obtain carbon HoMSs, Gu and co-workers have firstly used an aqueous emulsion route to synthesize mesoporous phenolic resin-silica multi-layer vesicle structures.118 By a calcination treatment of the vesicle structured composites, onion-like mesoporous multi-layered carbon and carbon-silica composite hollow spheres have been synthesized. The multi-layered carbon hollow spheres can be further used to load sulphur forming multi-shelled hollow carbon sphere-encapsulated sulfur composites.5 Inorganic–organic HoMSs are also reported. Teng and co-workers have developed a “multi-interface transformation” approach to synthesize monodisperse multi-shelled periodic mesoporous organosilica (PMO) hollow spheres.58 As illustrated in Fig. 15, the hydrolysis and condensation reaction of the added silane precursors contributes to the formation of particles on the CTAB micelles through a sol–gel process. When the silicate species are exhausted, the growth of organosilica/CTAB spheres ends while the Si–OH and Si–OC2H5 groups on the composite surface can still be hydrolyzed and cross-linked forming a highly condensed layer. Repeating the process by adding new silane precursors can lead to additional growth of the composite and more highly condensed layers to form multiple cross-linked interfaces. During the hydrothermal process, the low condensed organosilica/CTAB species are dissolved while the highly condensed multiple interfaces can be the nucleation sites for re-growth of the dissolved species. The shrinkage towards the multiple interfaces finally results in the formation of HoMSs. The diameter, shell thickness, inter-shell spacing, shell number and the organic group of the multi-shelled PMO can be further precisely controlled. MOFs with HoMSs are very novel and interesting materials. Liu and co-workers have rationally fabricated multi-shelled hollow chromium(III) terephthalate MOFs (MIL-101) with single-crystalline shells by step-by-step crystal growth and subsequent etching processes (Fig. 16).59 An inhomogeneous MIL-101 crystal (Fig. 16b) synthesized by a hydrothermal method is the key to the formation of hollow structured MOFs. As illustrated in Fig. 16a, the inner section of the MIL-101 is less stable, which is easier to etch with acetic acid, while the outer section is more stable in the acetic acid. As a consequence, single-shelled hollow MIL-101 (Fig. 16c) is obtained by a selective etching process. Second and third growth of new MIL-101 layers with both less stable and more stable sections is achievable. Hence corresponding double-shelled and triple-shelled hollow MIL-101 can be acquired by a selective etching process (Fig. 16d and e). Moreover, the size of the cavity and the thickness of each shell can be tuned. Other ways including a sequential self-assembly strategy and de novo approach can also achieve MOF HoMSs.119,120 Besides these, HoMSs containing more than three elements are also reported.121–126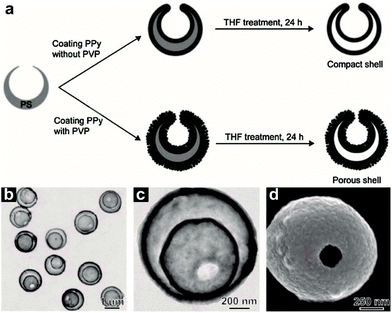 | ||
| Fig. 14 (a) Schematic illustrating the synthetic procedures for generating double-shelled PPy HoMSs in the absence or presence of PVP. (b and c) TEM images and (d) SEM image of double-shelled PPy HoMSs. Reproduced with permission from ref. 57. Copyright 2010, John Wiley & Sons. | ||
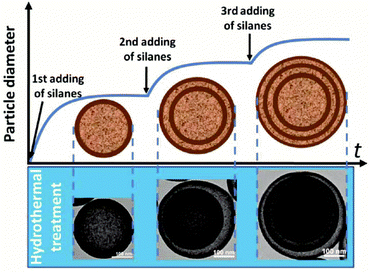 | ||
| Fig. 15 Schematic illustration of the successive growth process of the organosilica/CTAB composite spheres in ammonia and ethanol solution and the corresponding multi-shelled products after the hydrothermal treatment. Adapted with permission from ref. 58. Copyright 2015 American Chemical Society. | ||
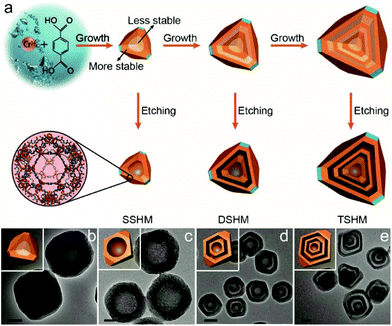 | ||
| Fig. 16 (a) Fabrication of single-, double-, and triple-shelled hollow MIL-101, which are denoted as SSHM, DSHM and TSHM, respectively. TEM images of (b) solid MIL-101, (c) SSHM, (d) DSHM, and (e) TSHM. Scale bar: (b and c) 50 nm; (d and e) 200 nm. Reproduced with permission from ref. 59. Copyright 2017, John Wiley & Sons. | ||
3. Applications of HoMSs
3.1 Energy conversion applications
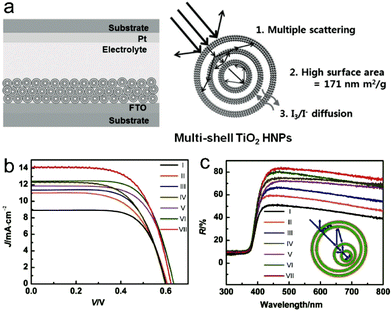 | ||
| Fig. 17 (a) Schematic illustration of assembled DSSCs based on multi-shell porous TiO2 hollow nanoparticles (MS-TiO2-HNPs) and the magnified structure of MS-TiO2-HNPs with multifunction capabilities. Reproduced with permission from ref. 16. Copyright 2014, John Wiley & Sons. (b) J–V curves and (c) corresponding UV/vis diffuse reflectance spectra of DSSCs based on ZnO HoMSs: (I) single-shelled, (II) double-shelled, (III) triple-shelled, (IV) quadruple-shelled, (V) double-shelled (with close double shells in the exterior), (VI) triple-shelled (with close double shells in the exterior and smaller hollow core) and (VII) quadruple-shelled (with close double shells in the exterior and a double-shelled hollow core). The inset in c is the schematic drawing for quadruple-shelled (with close double shells in the exterior and a double-shelled hollow core). Reproduced with permission from ref. 15. Copyright 2012, John Wiley & Sons. | ||
Dong and co-workers have used AxBy type ZnO HoMSs as photoanodes for DSSCs and investigated the effect of the shell number on the power conversion efficiency.15 It is found that with the increase of the shell number, the short-circuit current density and the power conversion efficiency of the DSSCs increase, as depicted in Fig. 17b. One reason is the increased surface area which can adsorb more dye when the number of shells is increased. The other reason is the multiple light scattering and reflection effect between the multiple shells which can enhance the light capture as shown in Fig. 17c. An interesting result is the great influence of the inter-shell distance. The ZnO HoMSs with close exterior double shells show 20% higher power conversion efficiency as compared with the ZnO HoMSs with homogeneous structures. A high power conversion efficiency of 5.6% can be achieved by using quadruple-shelled ZnO HoMSs with close exterior double shells. AxBy type SnO2 HoMSs have also been adopted as photoanodes for DSSCs by Dong and co-workers.38 The power conversion efficiency of the DSSCs also increases with the shell number. After TiCl4 treatment, the quintuple-shelled SnO2 HoMSs with closed exterior double shells show the highest power conversion efficiency of 7.18%. Moreover, the quintuple-shelled SnO2 HoMSs can also be used as a scattering layer to improve the light harvesting capability on the top of a TiO2 particle (P25) layer. A high power conversion efficiency of 9.53% is achieved, which is much higher as compared to that of the P25-based DSSC (7.29%). TiO2 coating is very useful for SnO2 HoMSs. Qian and co-workers have prepared AxBy–CxDy type TiO2-coated multi-layered SnO2 hollow microspheres (TiO2–SnO2 MHSs) and used them as photoanodes for DSSCs.14 The power conversion efficiency is greatly improved to 5.65%, which is higher than that of SnO2 MHSs (1.4%) and TiO2–SnO2 nanoparticles (4.22%). The mixed AxBy–CxDy composition of the shells apparently improved the performance of the DSSCs.
Compared to ZnO and SnO2, TiO2 is more attractive as a photoanode for DSSCs due to its wide band gap, high stability and environmental friendliness. Qiu and co-workers have synthesized coaxial multi-shelled TiO2 nanotube arrays (NTAs) by a template method and investigated their DSSC performance.60 The power conversion efficiency can be enhanced with the increase of the shell number. The DSSC using quintuple-shelled TiO2 NTAs can realize the highest power conversion efficiency of 6.2%. The surface area is an important parameter for photoanodes, which can affect the amount of adsorbed dyes and the contact with the electrolyte. Hwang and co-workers have prepared multi-shell porous TiO2 hollow nanoparticles (MS-TiO2-HNPs) with a high surface area (ca. 171 m2 g−1) by a layer-by-layer method.16 Even the DSSC using single-shell-TiO2-HNPs as a photoanode can achieve a high power conversion efficiency of 8.0%. When the shell number is increased, the power conversion efficiency is apparently improved. The optimized power conversion efficiency of the DSSC using MS-TiO2-HNPs as a photoanode can be as high as 9.4%, which is 17.5% higher compared to that of the single-shell-TiO2-HNPs.
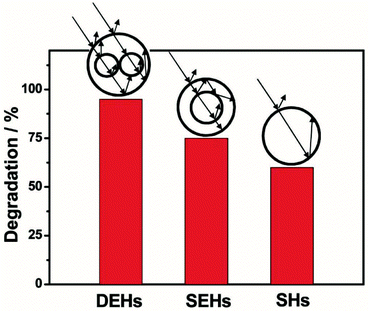 | ||
| Fig. 18 Comparison of photocatalytic activities of ZnO hollow spheres with different morphologies: DEHs, SEHs, and SHs. The insets show a schematic illustration of the light multi-reflections within all three structures. Reproduced with permission from ref. 76. Copyright 2012, John Wiley & Sons. | ||
Solar water splitting is a desirable way to convert solar energy, which can directly produce hydrogen and oxygen. The oxygen evolution process is usually harder as compared to the hydrogen evolution process due to the requirement of more photo-induced holes. Qi and co-workers have applied CeO2 HoMSs as photocatalysts for water oxidation.66 The HoMSs can capture more incident light through the multiple reflection effect. Meanwhile, the large surface area and more active sites can separate electrons and holes more efficiently. The average oxygen evolution rates of the commercial CeO2 nanoparticles, and single-shelled, double-shelled, and triple-shelled CeO2 HoMSs are 14, 30, 46, and 78 μmol g−1 h−1, respectively. The best triple-shelled CeO2 HoMSs also show the best photocatalytic stability. The oxygen evolution rate can be retained as 51.3% of the initial rate after three cycles of catalysis for 4 h each cycle. Except for water oxidation, Wei and co-workers have used AxBy–CxDy type SrTiO3–TiO2 heterogeneous HoMSs (STHoMSs) for photocatalytic overall water splitting (Fig. 19).7 After photo-depositing Pt onto the STHoMSs, the photocatalytic performance has been evaluated under 300 W Xe lamp irradiation. As shown in Fig. 19a, the ratio of the produced hydrogen to oxygen is approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The triple-shelled STHoMSs (3S-STHoMSs) show the highest photocatalytic activity with the hydrogen and oxygen production rates of 10.6 μmol h−1 and 5.1 μmol h−1, respectively. There is no obvious fading when continuously performing the photocatalysis as shown in Fig. 19b. Furthermore, the 3S-STHoMSs show excellent durability for 5 cycles, indicating their superior stability (Fig. 19c). Their excellent catalytic performance is ascribed to several aspects. The SrTiO3 and Pt on the surface of the shells can directly photocatalyze the water splitting process. Meanwhile, as illustrated in Fig. 19d, the AxBy–CxDy type HoMSs with a mixed composition forming abundant SrTiO3–TiO2 junctions are favorable for the electron transfer from the conduction band (CB) of SrTiO3 to the CB of TiO2 while the holes can be transferred from the valence band (VB) of TiO2 to the VB of SrTiO3, which can improve the charge separation efficiency. Moreover, the HoMSs composed of nanoparticles and selected facets can increase the incident light harvesting and provide more active sites. Photocatalytic reduction of CO2 is another way to take advantage of solar energy. Wang and co-workers have used MOF-derived multi-shelled Co3O4 hollow dodecahedra as photocatalysts to reduce CO2 to produce CO and O2.11 The ratio of the generated CO to O2 is 1
1. The triple-shelled STHoMSs (3S-STHoMSs) show the highest photocatalytic activity with the hydrogen and oxygen production rates of 10.6 μmol h−1 and 5.1 μmol h−1, respectively. There is no obvious fading when continuously performing the photocatalysis as shown in Fig. 19b. Furthermore, the 3S-STHoMSs show excellent durability for 5 cycles, indicating their superior stability (Fig. 19c). Their excellent catalytic performance is ascribed to several aspects. The SrTiO3 and Pt on the surface of the shells can directly photocatalyze the water splitting process. Meanwhile, as illustrated in Fig. 19d, the AxBy–CxDy type HoMSs with a mixed composition forming abundant SrTiO3–TiO2 junctions are favorable for the electron transfer from the conduction band (CB) of SrTiO3 to the CB of TiO2 while the holes can be transferred from the valence band (VB) of TiO2 to the VB of SrTiO3, which can improve the charge separation efficiency. Moreover, the HoMSs composed of nanoparticles and selected facets can increase the incident light harvesting and provide more active sites. Photocatalytic reduction of CO2 is another way to take advantage of solar energy. Wang and co-workers have used MOF-derived multi-shelled Co3O4 hollow dodecahedra as photocatalysts to reduce CO2 to produce CO and O2.11 The ratio of the generated CO to O2 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The yield of CO increases with the shell number until it reaches 46.3 μmol g−1 h−1 by using quadruple-shelled (QS) Co3O4 HoMSs as catalysts, which is approximately 5 times higher than that of Co3O4 nanoparticles. The catalytic activity can be maintained after four cycles by using QS-Co3O4 HoMSs as photocatalysts, which indicates their excellent catalytic stability. The MOF-derived QS-CO3O4 HoMSs show better charge-transfer ability as compared to quadruple-shelled Co3O4 hollow microspheres synthesized by the STA. The oriented exposed (111) facets are found to be a key factor for the enhanced photocatalytic activity by electrochemical impedance spectroscopy and transient photocurrent response evaluation. The HoMSs are also an advantage to provide multiple interfaces and strong solar light harvesting.
2. The yield of CO increases with the shell number until it reaches 46.3 μmol g−1 h−1 by using quadruple-shelled (QS) Co3O4 HoMSs as catalysts, which is approximately 5 times higher than that of Co3O4 nanoparticles. The catalytic activity can be maintained after four cycles by using QS-Co3O4 HoMSs as photocatalysts, which indicates their excellent catalytic stability. The MOF-derived QS-CO3O4 HoMSs show better charge-transfer ability as compared to quadruple-shelled Co3O4 hollow microspheres synthesized by the STA. The oriented exposed (111) facets are found to be a key factor for the enhanced photocatalytic activity by electrochemical impedance spectroscopy and transient photocurrent response evaluation. The HoMSs are also an advantage to provide multiple interfaces and strong solar light harvesting.
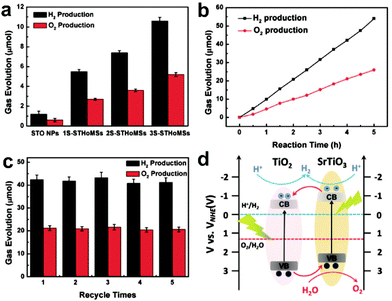 | ||
| Fig. 19 (a) Photocatalytic overall water splitting performance of 1S-, 2S-, and 3S-STHoMSs with a hydrothermal reaction time of 12 h. (b) Time course of H2 and O2 production from pure water under 300 W Xe lamp irradiation with 50 mg 3S-STHoMS photocatalyst. (c) The durability of 3S-STHoMSs in overall water splitting after each 5 h and (d) possible band structure diagram of STHoMSs. Reproduced with permission from ref. 7. Copyright 2019, John Wiley & Sons. | ||
Electrocatalytic water splitting to produce hydrogen and oxygen is an alternative to acquire clean energy. HoMSs can provide abundant active sites for fast diffusion and rapid kinetics. Sun and co-workers have achieved excellent hydrogen and oxygen evolution performance by using multi-shelled Ni2P hollow microspheres as electrocatalysts.8 In a 1 M KOH electrolyte, the overpotential for the hydrogen evolution reaction (HER) to acquire a current density of 10 mA cm−2 is only 98 mV and the corresponding Tafel slope is 86.4 mV dec−1, which are much better than those of hierarchical Ni2P and nanostructured Ni2P. It is among the top electrocatalysts for the HER.131–133 The overpotential for the oxygen evolution reaction (OER) is only 270 mV to achieve a current density of 10 mA cm−2, and the corresponding Tafel slope is only 40.4 mV dec−1 indicating the rapid kinetics. The multi-shelled Ni2P hollow microspheres can enhance the charge-transfer ability, which is proved by electrochemical impedance spectroscopy. The overall water splitting by using the Ni2P HoMSs on carbon fiber paper as both the anode and cathode is conducted. A current density of 6.0 mA cm−2 can be acquired at about 1.5 V, which is even better than that obtained using commercial Pt/C and RuO2 on carbon fiber paper as the anode and cathode. Besides the above catalytic applications, materials with HoMSs can also be used in other catalysis areas such as selective catalytic reduction of NO with NH3, catalytic oxidation of CO, catalytic reduction of p-nitrophenol, oxidation of styrene, and so on.44,45,59,93,134
3.2 Energy storage applications
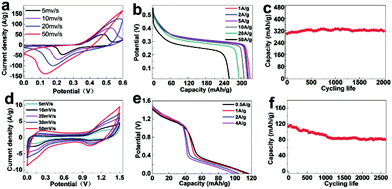
Wang and co-workers have synthesized AxBy type Co3O4 HoMSs by the STA and investigated their lithium storage properties as anode materials.3 The discharge capacities of the single-shelled, double-shelled, triple-shelled and quadruple-shelled Co3O4 HoMSs after 30 cycles at a current density of 50 mA g−1 can still be maintained as 792.7, 1303, 1615.8, and 1011.5 mA h g−1, respectively, which are much higher than that of commercial Co3O4. Moreover, the triple-shelled Co3O4 HoMSs can achieve a high specific capacity of 1117.3 mA h g−1 even at a high current density of 2000 mA g−1. The triple-shelled Co3O4 HoMSs show the best performance among the estimated HoMSs, which is ascribed to the appropriated volume-occupying rate guaranteeing a high volumetric specific capacity and fair structure stability. The superior performance is attributed to the unique HoMSs with porous structures composed of nanoparticles. The nanoparticles can effectively shorten the Li+ diffusion paths and the porous structures can increase the contact area between the electrolyte and the anode material. Furthermore, the interior cavities can provide extra space for lithium storage and buffer volumetric change derived from lithium ion insertion/extraction processes, thus improving the performance. TiO2 is also a promising anode candidate for LIBs because of its excellent high rate performance, high safety and small volumetric change during lithium ion insertion/extraction processes. Ren and co-workers have investigated the LIB performance of multi-shelled anatase-rutile TiO2 hollow microspheres (MS-TiO2-HMS) and multi-shelled TiO2 hollow microspheres with close exterior double shells (MS-TiO2-HMS-CDS).27 As shown in Fig. 20a, the 1S-, 2S-, 3S-TiO2-HMS, and 2S-, 3S-, 4S-TiO2-HMS-CDS at a current rate of 1 C (167.5 mA g−1) show the initial discharge capacities of 172, 204, 260, 97, 122, and 154 mA h g−1, respectively. All of the TiO2 hollow spheres show superior cycling performance as compared to commercial TiO2 (P25), as presented in Fig. 20b. For both MS-TiO2-HMS and MS-TiO2-HMS-CDS, the capacity increases with the number of shells, which is attributed to the higher specific surface area and larger pores improving electrolyte transport and Li+ diffusion. Among the hollow structures, the 3S-TiO2-HMS with thin shells show the highest discharge capacity of 237 mA h g−1 at a current rate of 1 C, which is better than that of triple-shelled TiO2 HoMSs composed of pure anatase. The mixed anatase-rutile composition shows a positive effect on the performance. Besides, high capacities of 159 mA h g−1 and 119 mA h g−1 can be achieved at high current rates of 5 C and 10 C, respectively (Fig. 20c). Furthermore, Ren and co-workers have introduced TiO2(B) into HoMSs forming AxBy–CxDy type multi-shelled heterostructured anatase/TiO2(B) hollow microspheres as anode materials for LIBs.6 The TiO2(B) can improve both the capacity and the rate capability. Besides, benefiting from the two-phase composition, charge separation at the two-phase boundaries can be enhanced, which contributes additional lithium-ion storage sites. A high specific capacity of 215.4 mA h g−1 is achieved at a current rate of 1 C (335 mA g−1) after 100 cycles. After cycling 1000 times at high rates of 10 C and 20 C, the specific capacities of 141.6 and 125.7 mA h g−1 are retained, respectively. Some electrode materials suffer from a large volume change during the charge/discharge processes. Zhang and co-workers have designed SnO2@Fe2O3(MOF)-HoMSs by growing MOF layers on the shells of SnO2 HoMSs followed by transformation of the MOF layers to metal oxides.94 The MOF-derived coatings can effectively protect the SnO2 from destruction leading to better performance as anode materials for LIBs. Other anode and cathode materials with HoMSs also show outstanding lithium storage properties indicating the advantages of the unique morphology.4,17,53,65
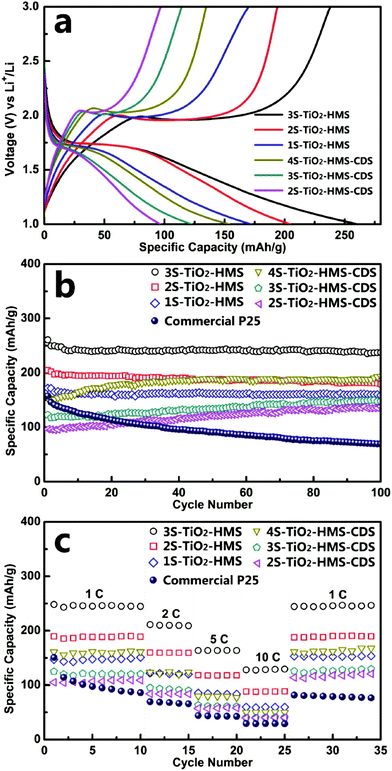 | ||
| Fig. 20 (a) Initial charge–discharge voltage profiles and (b) cycling performance (discharge capacities) at a current rate of 1 C between 1.0 and 3.0 V. (c) Cycling performance (discharge capacities) at various charge–discharge current rates of the MS-TiO2-HMS and MS-TiO2-HMS-CDS between 1.0 and 3.0 V. Adapted with permission from ref. 27. Copyright 2014 American Chemical Society. | ||
In terms of the abundant reserves of sodium, sodium-ion batteries are an alternative for energy storage. Zhao and co-workers have used AxByCz type binary metal oxide (Fe2(MoO4)3) HoMSs as cathode materials for sodium-ion batteries.18 The HoMSs composed of nanoparticles can provide a large electrode/electrolyte contact area to shorten the diffusion length of electrons and sodium ions, thus amending the sluggish electrochemical kinetics. The specific capacities of the nanosheets and single-shelled, double-shelled, triple-shelled, quadruple-shelled, and quintuple-shelled Fe2(MoO4)3 HoMSs for the second cycle at a current rate of 0.1 C (91 mA g−1) are 90.46, 92.21, 93.96, 94.73, 95.10, and 99.03 mA h g−1, respectively, which indicates the improved performance as compared to the nanosheets. At a current rate of 2.2 C, a high discharge capacity of 85.6 mA h g−1 can be acquired for the quintuple-shelled Fe2(MoO4)3 HoMSs after 100 cycles. Furthermore, the quintuple-shelled Fe2(MoO4)3 HoMSs can still achieve a high discharge capacity of 67.4 mA h g−1 even at a high current rate of 10 C.
Compared to LIBs and sodium-ion batteries, alkaline rechargeable batteries are much safer. Meanwhile, large specific capacity and excellent cycling stability can be achieved by using some promising materials as electrodes. HoMSs can provide more electrochemically active sites since the exterior shells can protect the active sites from being occupied by binders while fabricating batteries. Besides, HoMSs are more stable in terms of the structures, which can improve the cycling stability. Jiao and co-workers have prepared Co/Mn-HD derived from MOFs and used them as cathode materials for alkaline rechargeable batteries.19 As shown in Fig. 21a, the redox peaks are present in the cyclic voltammetry (CV) curves, indicating a typical faradaic reaction of Co/Mn-HD in a three-electrode system. A high specific capacity of 331.94 mA h g−1 for the triple-shelled Co/Mn-HD is achieved at a current density of 1 A g−1 as indicated in Fig. 21b. The capacities remained high when the current density is increased even as high as 50 A g−1. The cycling stability is excellent even for 2000 cycles at a current density of 10 A g−1 with a high capacity retention of 96% (Fig. 21c). The superior performance is ascribed to the unique HoMSs with MOF-derived pores guaranteeing better contact with the electrolyte, and residual C and N improving the conductivity and the structural stability. Button batteries with the triple-shelled Co/Mn-HD and commercial activated carbon as electrode materials still show excellent performance as shown in Fig. 21d–f. The specific capacity can be up to 117 mA h g−1 at a current density of 0.5 A g−1, and 72% of the capacity can be retained even after 2500 cycles. Beyond the above rechargeable batteries, HoMSs can also be used as hosts of S for Li–S rechargeable batteries, which also show outstanding performance.5,137 Salhabi and co-workers have used TiO2−x HoMSs as sulfur carriers.137 The suitable interlayer space can confine sulfur, which can buffer the volume expansion and limit the shuttle of polysulfides. A high capacity of 903 mA h g−1 is achieved using S@triple-shelled TiO2−x HoMSs as the cathode for Li–S batteries with a capacity retention of 79% at 0.5 C for 1000 cycles.
 | ||
| Fig. 21 The electrochemical performance of the triple-shelled Co/Mn-HD as electrode material in a three-electrode system: (a) cyclic voltammetry; (b) galvanostatic charge–discharge voltage; (c) cycling life. Co/Mn-HD as the electrode material in an alkaline rechargeable battery: (d) cyclic voltammetry curves; (e) galvanostatic charge–discharge curves; (f) cycling stability. Reproduced with permission from ref. 19. Copyright 2018, John Wiley & Sons. | ||
Wang and co-workers have synthesized AxBy type Mn2O3 HoMSs as electrodes for supercapacitors.20 At a discharge current density of 0.5 A g−1, the triple-shelled Mn2O3 HoMSs can achieve a high specific capacitance of 1651 F g−1 in a three-electrode system. As shown in Fig. 22, at a high current density of 10 A g−1, a high specific capacitance of 1422 F g−1 can still be acquired. All the Mn2O3 HoMSs show higher specific capacitance than Mn2O3 nanoparticles indicating the advantages of HoMSs. The triple-shelled Mn2O3 HoMSs show the best performance among the HoMSs due to the thin shells and larger specific surface area. The Mn2O3 HoMSs also show excellent cycling stability. At a current density of 1 A g−1, the triple-shelled Mn2O3 HoMSs can still achieve a high specific capacitance of 1517 F g−1 with a small decay of 7.8% after 2000 cycles, which is much higher than that of 376.5 F g−1 for Mn2O3 nanoparticles. The excellent stability is ascribed to the protection effect of the exterior shell suppressing the dissolution of the interior shells. Chen and co-workers have evaluated the capacitor performance of AxBy type MnO2 HoMSs.39 The specific capacitance increases with the shell number. The quadruple-shelled MnO2 HoMSs show the highest specific capacitance of 1457 F g−1 at a current density of 0.5 A g−1, and 91.2% of the capacitance is retained after 4000 cycles. A high specific capacitance of 1037 F g−1 can still be achieved even at a high current density of 10 A g−1. Both the Mn2O3 and MnO2 HoMSs are among the best electrode materials for supercapacitors.
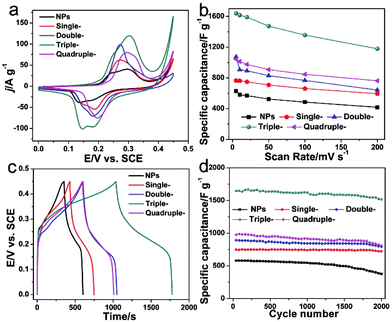 | ||
| Fig. 22 (a) Comparison of CV curves of Mn2O3 nanoparticles, and single-, double-, triple- and quadruple-shelled Mn2O3 HoMSs at a scan rate of 20 mV s−1. (b) Specific capacitances of Mn2O3 nanoparticles, and single-, double-, triple- and quadruple-shelled Mn2O3 HoMSs at various scan rates. (c) Comparison of galvanostatic charge/discharge curves of Mn2O3 nanoparticles, and single-, double-, triple- and quadruple-shelled Mn2O3 HoMSs at a current density of 1 A g−1 in the voltage range of 0–0.45 V. (d) Cycling stability of Mn2O3 nanoparticles, and single-, double-, triple- and quadruple-shelled HoMSs for 2000 cycles at a current density of 1 A g−1.20 | ||
Some AxBy–CxDy type HoMSs as electrodes for supercapacitors have also been reported. Wang and co-workers have used a sulphidation process to prepare multi-shelled CoO/Co9S8 hollow microspheres and multi-shelled NiS/NiO hollow microspheres with excellent performance for supercapacitors.47,48 Besides the high specific capacitances of these HoMS-based supercapacitors, asymmetric supercapacitors using these HoMSs as positive materials and activated carbon as the negative material also show excellent performance. The specific capacitances of the multi-shelled CoO/Co9S8 hollow microspheres and multi-shelled NiS/NiO hollow microspheres at a high current density of 2 A g−1 are 72 and 58.5 F g−1, respectively. The heterostructures are believed to enhance the interfacial charge transfer and surface reaction kinetics. Similarly, by a phosphidation process, CoP–Cu3P HoMSs have also been used as electrode materials for asymmetric supercapacitors where activated carbon is used as the negative electrode.49 Due to the advantages of HoMSs and high conductivity, an ultrahigh specific capacitance of 131 F g−1 is achieved at a current density of 33 mA cm−2 and 64% of the capacitance can be retained at an ultrahigh current density of 330 mA cm−2. A high energy density of 46.6 W h kg−1 and the corresponding high power density of 16 kW kg−1 are achieved. More electrode materials including AxByCz and AxBy@CxDy type HoMSs are also investigated for supercapacitors with superior performance.55,56,98,112
3.3 Other applications
Except for the above applications, the applications of HoMSs in other areas have also been explored. Lai and co-workers have investigated the gas-sensing properties of α-Fe2O3 HoMSs with different numbers of shells in the presence of ethanol.26 The sensitivity to ethanol is much better as compared with α-Fe2O3 nanoparticles. The triple-shelled α-Fe2O3 HoMSs show the highest sensitivity. This is attributed to the larger specific surface areas and porous shells. Other materials with HoMSs for gas-sensing also show excellent performance.22,23,140 ZnO HoMSs have been used for photodetectors with enhanced optoelectronic performance.76 The multiple reflection and scattering effect can trap and harvest light efficiently. The shell space also shows effects on the performance of photodetectors.141 Upconversion luminescent materials are also useful for some applications such as biological imaging, photodynamic therapy and clinical diagnosis.142 Zong and co-workers have doped Yb3+ and Er3+ into YVO4 HoMSs and investigated their upconversion luminescence properties. The triple-shelled YVO4:Yb3+/Er3+ HoMSs show the highest luminescence intensity. This might be ascribed to the multiple reflections which can improve the light harvesting efficiency of the near-infrared excitation light. Besides, there is no doubt that materials with HoMSs can also be used for gas adsorption, drug loading, dye adsorption and cancer immunotherapy due to their large specific surface area.21,58,126,143The aforementioned investigation on the applications of HoMSs apparently shows that HoMSs with more thin shells can usually achieve better performance. However, the effect of the shell composition on the performance of applications is not comprehensively investigated. But, there are still some examples indicating the influence of composition. For instance, the AxBy–CxDy type SrTiO3–TiO2 heterogeneous HoMSs show enhanced photocatalytic water splitting performance, where the heterogeneous junctions play an important role in efficient electron transfer. Similarly, AxBy@CxDy type double-shelled TiO2 hollow spheres with different phases of each shell show improved photocatalytic activity. The AxBy–CxDy type anatase/rutile TiO2 HoMSs and anatase/TiO2(B) HoMSs show improved lithium storage properties. The MOF-derived coatings on the shells of SnO2 can act as buffer layers to release the large volume change of SnO2 during the lithiation/delithiation processes. However, more research studies have to be conducted for comprehensive investigation. Moreover, although a variety of materials with HoMSs have already been investigated for applications in some fields, the applications in other areas have not been studied in detail. For example, the applications of TiO2 HoMSs synthesized by STA have not been explored for sodium-ion batteries, DSSCs or photocatalysis areas. The MnO2 HoMSs obtained by the STA may also be used as cathode materials for aqueous zinc-ion batteries but they are unexplored. More promising and interesting application areas may be extended besides the applications as listed in Table 1.
4. Summary and outlook
In summary, owing to their promising properties related to the unique structures, materials with HoMSs are considered as one kind of suitable candidate for energy conversion and storage applications to achieve a clean and sustainable future. In this review, we have firstly introduced the general methods including the hard template method, soft template method and template-free method to synthesize HoMSs. Based on the composition, in order to acquire different types of HoMSs including AxBy, AxBy–CxDy, AxBy@CxDy, AxByOz and other types, appropriate synthetic methods should be carefully considered. Detailed synthesis approaches for each type of HoMS have been comprehensively summarized. Afterwards, the promising applications of materials with HoMSs in energy conversion and energy storage fields such as DSSCs, catalysts, rechargeable batteries and supercapacitors have been reviewed.Although great progress and achievements have been made for both the synthetic methods and the promising applications of HoMSs, there are still some challenges in this field and extensive future research needs to be performed. Firstly, the present methods still cannot achieve HoMS products with high yield, which is an obstacle for practical widespread applications. The STA is a promising method considering its generality and easy manipulation of various parameters, regardless of yield. Hence a new method for high-yield CMS templates may be a key to high output of HoMSs based on the STA, which might make the STA more attractive. The spray drying method could be another promising way to high yield. Secondly, the MOF-based STA is a newborn while interesting and promising method to synthesize materials with HoMSs for energy conversion and storage applications. However, only a few accomplishments have been achieved. Considering the unique porous structure of MOFs, more investigations should be conducted for detailed control to achieve HoMSs with abundant pores. Thirdly, the composition of HoMSs can determine the properties of materials for energy conversion and storage applications, whereas the correlation between the composition and the performance is still not comprehensively investigated. The fact we can see is that some HoMSs with heterogeneous compositions can improve the charge-separation ability. This works not only for catalysts but also for batteries. More research on precise control of heterogeneous compositions for HoMSs is believed to be an effective way to optimize the application performance. Moreover, some exterior shell composed of materials with negligible/smaller volumetric change or high chemical stability can protect the interior shells from destruction or dissolution during reactions, especially for battery and capacitor applications. The realization of protective shells can be another direction. All in all, lots of extensive studies still have to be conducted to realize the final practical application of the promising HoMSs in the fields of energy conversion and storage.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (21671016 and 51872024).Notes and references
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2017, 16, 16–22 CrossRef PubMed.
- B. O'regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- J. Y. Wang, N. L. Yang, H. J. Tang, Z. H. Dong, Q. Jin, M. Yang, D. Kisailus, H. J. Zhao, Z. Y. Tang and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed.
- J. Y. Wang, H. J. Tang, L. J. Zhang, H. Ren, R. B. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. R. Liu, Y. Zhang, Y. R. Wen, L. Gu, G. H. Ma, Z. G. Su, Z. Y. Tang, H. J. Zhao and D. Wang, Nat. Energy, 2016, 1, 16050 CrossRef CAS.
- S. Q. Chen, X. D. Huang, B. Sun, J. Q. Zhang, H. Liu and G. X. Wang, J. Mater. Chem. A, 2014, 2, 16199–16207 RSC.
- H. Ren, R. B. Yu, J. Qi, L. J. Zhang, Q. Jin and D. Wang, Adv. Mater., 2019, 31, 1805754 CrossRef PubMed.
- Y. Z. Wei, J. Y. Wang, R. B. Yu, J. W. Wan and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 1422–1426 CrossRef CAS PubMed.
- H. M. Sun, X. B. Xu, Z. H. Yan, X. Chen, F. Y. Cheng, P. S. Weiss and J. Chen, Chem. Mater., 2017, 29, 8539–8547 CrossRef CAS.
- J. B. Joo, Q. Zhang, M. Dahl, I. Lee, J. Goebl, F. Zaera and Y. D. Yin, Energy Environ. Sci., 2012, 5, 6321–6327 RSC.
- S. X. Li, J. Chen, F. Y. Zheng, Y. C. Li and F. Y. Huang, Nanoscale, 2013, 5, 12150–12155 RSC.
- L. Wang, J. W. Wan, Y. S. Zhao, N. L. Yang and D. Wang, J. Am. Chem. Soc., 2019, 141, 2238–2241 CrossRef CAS PubMed.
- J. Qi, X. Y. Lai, J. Y. Wang, H. J. Tang, H. Ren, Y. Yang, Q. Jin, L. J. Zhang, R. B. Yu, G. H. Ma, Z. G. Su, H. J. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 RSC.
- J. F. Qian, P. Liu, Y. Xiao, Y. Jiang, Y. L. Cao, X. P. Ai and H. X. Yang, Adv. Mater., 2009, 21, 3663–3667 CrossRef CAS.
- Z. H. Dong, X. Y. Lai, J. E. Halpert, N. L. Yang, L. X. Yi, J. Zhai, D. Wang, Z. Y. Tang and L. Jiang, Adv. Mater., 2012, 24, 1046–1049 CrossRef CAS PubMed.
- S. H. Hwang, J. Yun and J. Jang, Adv. Funct. Mater., 2014, 24, 7619–7626 CrossRef CAS.
- S. M. Xu, C. M. Hessel, H. Ren, R. B. Yu, Q. Jin, M. Yang, H. J. Zhao and D. Wang, Energy Environ. Sci., 2014, 7, 632–637 RSC.
- X. X. Zhao, J. Y. Wang, R. B. Yu and D. Wang, J. Am. Chem. Soc., 2018, 140, 17114–17119 CrossRef CAS PubMed.
- C. W. Jiao, Z. M. Wang, X. X. Zhao, H. Wang, J. Wang, R. B. Yu and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 996–1001 CrossRef CAS PubMed.
- J. Y. Wang, H. J. Tang, H. Ren, R. B. Yu, J. Qi, D. Mao, H. J. Zhao and D. Wang, Adv. Sci., 2014, 1, 1400011 CrossRef PubMed.
- J. Liu, S. B. Hartono, Y. G. Jin, Z. Li, G. Q. Lu and S. Z. Qiao, J. Mater. Chem., 2010, 20, 4595–4601 RSC.
- H. Zhang, Q. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766–2771 CrossRef CAS.
- R. Zhang, T. Zhang, T. T. Zhou and L. L. Wang, Sens. Actuators, B, 2018, 256, 479–487 CrossRef CAS.
- H. L. Xu and W. Z. Wang, Angew. Chem., Int. Ed., 2007, 46, 1489–1492 CrossRef CAS PubMed.
- L. Zhang and H. Wang, J. Phys. Chem. C, 2011, 115, 18479–18485 CrossRef CAS.
- X. Y. Lai, J. Li, B. A. Korgel, Z. H. Dong, Z. M. Li, F. B. Su, J. A. Du and D. Wang, Angew. Chem., Int. Ed., 2011, 50, 2738–2741 CrossRef CAS PubMed.
- H. Ren, R. B. Yu, J. Y. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. J. Zhao and D. Wang, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed.
- M. Waqas, Y. Z. Wei, D. Mao, J. Qi, Y. Yang, B. Wang and D. Wang, Nano Res., 2017, 10, 3920–3928 CrossRef CAS.
- X. X. Zhao, R. B. Yu, H. J. Tang, D. Mao, J. Qi, B. Wang, Y. Zhang, H. J. Zhao, W. P. Hu and D. Wang, Adv. Mater., 2017, 29, 1700550 CrossRef PubMed.
- J. Y. Wang, Y. Cui and D. Wang, Adv. Mater., 2018, 1801993 CrossRef PubMed.
- X. J. Wang, J. Feng, Y. C. Bai, Q. Zhang and Y. D. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- J. Y. Wang, H. J. Tang, H. Wang, R. B. Yu and D. Wang, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111–1114 CrossRef CAS PubMed.
- D. P. Wang and H. C. Zeng, Chem. Mater., 2009, 21, 4811–4823 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- C. C. Huang, W. Huang and C. S. Yeh, Biomaterials, 2011, 32, 556–564 CrossRef CAS PubMed.
- X. W. Lou, C. L. Yuan and L. A. Archer, Small, 2007, 3, 261–265 CrossRef CAS PubMed.
- Z. H. Dong, H. Ren, C. M. Hessel, J. Y. Wang, R. B. Yu, Q. Jin, M. Yang, Z. D. Hu, Y. F. Chen, Z. Y. Tang, H. J. Zhao and D. Wang, Adv. Mater., 2014, 26, 905–909 CrossRef CAS PubMed.
- M. J. Chen, J. Y. Wang, H. J. Tang, Y. Yang, B. Wang, H. J. Zhao and D. Wang, Inorg. Chem. Front., 2016, 3, 1065–1070 RSC.
- X. Wang, X. L. Wu, Y. G. Guo, Y. T. Zhong, X. Q. Cao, Y. Ma and J. N. Yao, Adv. Funct. Mater., 2010, 20, 1680–1686 CrossRef CAS.
- C. C. Yec and H. C. Zeng, Chem. Mater., 2012, 24, 1917–1929 CrossRef CAS.
- S. L. Xiong and H. C. Zeng, Angew. Chem., Int. Ed., 2012, 51, 949–952 CrossRef CAS PubMed.
- J. Lee, S. H. Hwang, J. Yun and J. Jang, ACS Appl. Mater. Interfaces, 2014, 6, 15420–15426 CrossRef CAS PubMed.
- K. L. Ma, W. X. Zou, L. Zhang, L. L. Li, S. H. Yu, C. J. Tang, F. Gao and L. Dong, RSC Adv., 2017, 7, 5989–5999 RSC.
- H. Wang, D. Mao, J. Qi, Q. H. Zhang, X. H. Ma, S. Y. Song, L. Gu, R. B. Yu and D. Wang, Adv. Funct. Mater., 2019, 29, 1806588 CrossRef.
- X. Z. Song, L. Qiao, K. M. Sun, Z. Q. Tan, W. Ma, X. L. Kang, F. F. Sun, T. Huang and X. F. Wang, Sens. Actuators, B, 2018, 256, 374–382 CrossRef CAS.
- Y. P. Wang, A. Q. Pan, Y. F. Zhang, J. R. Shi, J. D. Lin, S. Q. Liang and G. Z. Cao, J. Mater. Chem. A, 2018, 6, 9153–9160 RSC.
- Y. P. Wang, T. Zhu, Y. F. Zhang, X. Z. Kong, S. Q. Liang, G. Z. Cao and A. Q. Pan, J. Mater. Chem. A, 2017, 5, 18448–18456 RSC.
- S. E. Moosavifard, S. K. Kaverlavani, J. Shamsi and A. Bakouei, J. Mater. Chem. A, 2017, 5, 18429–18433 RSC.
- D. B. Qin, J. H. Yu, G. M. Bian, Y. L. Qi, D. W. Zhang and X. L. Yang, Chin. J. Chem., 2014, 32, 163–171 CrossRef CAS.
- J. S. Fang, Y. W. Zhang, Y. M. Zhou, C. Zhang, S. Zhao, H. X. Zhang and X. L. Sheng, Appl. Surf. Sci., 2017, 392, 36–45 CrossRef CAS.
- Y. Lu, L. Yu, M. H. Wu, Y. Wang and X. W. Lou, Adv. Mater., 2018, 30, 1702875 CrossRef PubMed.
- F. Wang, J. Y. Wang, H. Ren, H. J. Tang, R. B. Yu and D. Wang, Inorg. Chem. Front., 2016, 3, 365–369 RSC.
- L. B. Zong, J. Xu, S. Y. Jiang, K. Zhao, Z. M. Wang, P. R. Liu, H. J. Zhao, J. Chen, X. R. Xing and R. B. Yu, Adv. Mater., 2017, 29, 1604377 CrossRef PubMed.
- L. F. Shen, L. Yu, X. Y. Yu, X. G. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 1868–1872 CrossRef CAS PubMed.
- B. Y. Guan, Y. Yu, X. Wang, S. Y. Song and X. W. Lou, Adv. Mater., 2017, 29, 1605051 CrossRef PubMed.
- M. Y. Bai and Y. N. Xia, Macromol. Rapid Commun., 2010, 31, 1863–1868 CrossRef CAS PubMed.
- Z. G. Teng, X. D. So, Y. Y. Zheng, J. J. Zhang, Y. Liu, S. J. Wang, J. Wu, G. T. Chen, J. D. Wang, D. Y. Zhao and G. M. Lu, J. Am. Chem. Soc., 2015, 137, 7935–7944 CrossRef CAS PubMed.
- W. X. Liu, J. J. Huang, Q. Yang, S. J. Wang, X. M. Sun, W. N. Zhang, J. F. Liu and F. W. Huo, Angew. Chem., Int. Ed., 2017, 56, 5512–5516 CrossRef CAS PubMed.
- J. J. Qiu, F. W. Zhuge, X. M. Li, X. D. Gao, X. Y. Gan, L. Li, B. B. Weng, Z. S. Shi and Y. H. Hwang, J. Mater. Chem., 2012, 22, 3549–3554 RSC.
- Z. M. Li, X. Y. Lai, H. Wang, D. Mao, C. J. Xing and D. Wang, J. Phys. Chem. C, 2009, 113, 2792–2797 CrossRef CAS.
- D. Mao, J. W. Wan, J. Y. Wang and D. Wang, Adv. Mater., 2018, 1802874 CrossRef PubMed.
- X. M. Sun and Y. D. Li, Angew. Chem., Int. Ed., 2004, 43, 3827–3831 CrossRef CAS PubMed.
- H. Li, H. R. Ma, M. Yang, S. Wang, H. Shao, L. Wang, R. B. Yu and D. Wang, Mater. Res. Bull., 2017, 87, 224–229 CrossRef CAS.
- J. Zhang, H. Ren, J. Y. Wang, J. Qi, R. B. Yu, D. Wang and Y. L. Liu, J. Mater. Chem. A, 2016, 4, 17673–17677 RSC.
- J. Qi, K. Zhao, G. D. Li, Y. Gao, H. J. Zhao, R. B. Yu and Z. Y. Tang, Nanoscale, 2014, 6, 4072–4077 RSC.
- G. L. Wu, Z. R. Jia, Y. H. Cheng, H. X. Zhang, X. F. Zhou and H. J. Wu, Appl. Surf. Sci., 2019, 464, 472–478 CrossRef CAS.
- S. H. Qu, Y. K. Yu, K. J. Lin, P. Y. Liu, C. H. Zheng, L. D. Wang, T. T. Xu, Z. D. Wang and H. J. Wu, J. Mater. Sci.: Mater. Electron., 2018, 29, 1232–1237 CrossRef CAS.
- H. J. Wu, Y. Q. Wang, C. H. Zheng, J. M. Zhu, G. L. Wu and X. H. Li, J. Alloys Compd., 2016, 685, 8–14 CrossRef CAS.
- Z. G. Wu, Y. J. Zhong, J. T. Li, X. D. Guo, L. Huang, B. H. Zhong and S. G. Sun, J. Mater. Chem. A, 2014, 2, 12361–12367 RSC.
- L. Zhou, H. Y. Xu, H. W. Zhang, J. Yang, S. B. Hartono, K. Qian, J. Zou and C. Z. Yu, Chem. Commun., 2013, 49, 8695–8697 RSC.
- Z. Padashbarmchi, A. H. Hamidian, H. W. Zhang, L. Zhou, N. Khorasani, M. Kazemzad and C. Z. Yu, RSC Adv., 2015, 5, 10304–10309 RSC.
- Y. J. Hong, M. Y. Son and Y. C. Kang, Adv. Mater., 2013, 25, 2279–2283 CrossRef CAS PubMed.
- S. H. Choi and Y. C. Kang, Chem. – Eur. J., 2014, 20, 5835–5839 CrossRef CAS PubMed.
- L. M. Lang, D. Wu and Z. Xu, Chem. – Eur. J., 2012, 18, 10661–10668 CrossRef CAS PubMed.
- X. Wang, M. Y. Liao, Y. T. Zhong, J. Y. Zheng, W. Tian, T. Y. Zhai, C. Y. Zhi, Y. Ma, J. N. Yao, Y. Bando and D. Golberg, Adv. Mater., 2012, 24, 3421–3425 CrossRef CAS PubMed.
- Z. C. Wu, M. Zhang, K. Yu, S. D. Zhang and Y. Xie, Chem. – Eur. J., 2008, 14, 5346–5352 CrossRef CAS PubMed.
- J. H. Ju and K. S. Ryu, J. Electrochem. Soc., 2011, 158, A814–A817 CrossRef CAS.
- E. Gonzalez, J. Arbiol and V. F. Puntes, Science, 2011, 334, 1377–1380 CrossRef CAS PubMed.
- H. G. Yang and H. C. Zeng, J. Phys. Chem. B, 2004, 108, 3492–3495 CrossRef CAS.
- H. G. Yu, J. G. Yu, S. W. Liu and S. Mann, Chem. Mater., 2007, 19, 4327–4334 CrossRef CAS.
- Y. Chang, J. J. Teo and H. C. Zeng, Langmuir, 2005, 21, 1074–1079 CrossRef CAS PubMed.
- L. Zhang and H. Wang, ACS Nano, 2011, 5, 3257–3267 CrossRef CAS PubMed.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711–714 CrossRef CAS PubMed.
- J. S. Wu and D. F. Xue, Nanosci. Nanotechnol. Lett., 2011, 3, 371–377 CrossRef CAS.
- L. J. Han, R. J. Liu, C. S. Li, H. H. Li, C. X. Li, G. J. Zhang and J. N. Yao, J. Mater. Chem., 2012, 22, 17079–17085 RSC.
- D. W. Li, X. X. Zhao, R. B. Yu, B. Wang, H. Wang and D. Wang, Inorg. Chem. Front., 2018, 5, 535–540 RSC.
- S. H. Choi and Y. C. Kang, Small, 2014, 10, 474–478 CrossRef CAS PubMed.
- L. B. Zong, P. Z. Cui, F. Y. Qin, K. Zhao, Z. M. Wang and R. B. Yu, Mater. Res. Bull., 2017, 86, 44–50 CrossRef CAS.
- S. Lee, J. Lee, S. H. Hwang, J. Yun and J. Jang, ACS Nano, 2015, 9, 4939–4949 CrossRef CAS PubMed.
- S. H. Choi and Y. C. Kang, Chem. – Eur. J., 2014, 20, 3014–3018 CrossRef CAS PubMed.
- G. W. Zhan and H. C. Zeng, Chem. Mater., 2017, 29, 10104–10112 CrossRef CAS.
- P. F. Xu, R. B. Yu, H. Ren, L. B. Zong, J. Chen and X. R. Xing, Chem. Sci., 2014, 5, 4221–4226 RSC.
- J. Zhang, J. W. Wan, J. Y. Wang, H. Ren, R. B. Yu, L. Gu, Y. L. Liu, S. H. Feng and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 5266–5271 CrossRef CAS PubMed.
- H. Zhang, X. Zhang and X. L. Yang, J. Colloid Interface Sci., 2010, 348, 431–440 CrossRef CAS PubMed.
- J. J. Yuan, X. K. Zhang, H. D. Li, K. Wang, S. Y. Gao, Z. Yin, H. J. Yu, X. R. Zhu, Z. Z. Xiong and Y. M. Xie, Catal. Commun., 2015, 60, 129–133 CrossRef CAS.
- S. Song, S. Zhang, S. Y. Huang, R. Zhang, L. Yin, Y. Z. Hu, T. Wen, L. Zhuang, B. W. Hu and X. K. Wang, Chem. Eng. J., 2019, 355, 697–709 CrossRef CAS.
- H. Hu, B. Y. Guan, B. Y. Xia and X. W. Lou, J. Am. Chem. Soc., 2015, 137, 5590–5595 CrossRef CAS PubMed.
- S. K. Park, J. K. Kim and Y. C. Kang, J. Mater. Chem. A, 2017, 5, 18823–18830 RSC.
- L. W. Ye, Y. F. Yuan, D. Zhang, M. Zhu, S. M. Yin, Y. B. Chen and S. Y. Guo, Mater. Lett., 2018, 232, 228–231 CrossRef CAS.
- F. Y. Qin, P. Z. Cui, L. Hu, Z. M. Wang, J. Chen, X. R. Xing, H. Wang and R. B. Yu, Mater. Res. Bull., 2018, 99, 331–335 CrossRef CAS.
- G. Q. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 9041–9044 CrossRef CAS PubMed.
- S. H. Choi and Y. C. Kang, Chem. – Eur. J., 2013, 19, 17305–17309 CrossRef CAS PubMed.
- M. H. Kim, Y. J. Hong and Y. C. Kang, RSC Adv., 2013, 3, 13110–13114 RSC.
- X. F. Niu, Y. F. Li, Y. J. Hu, H. Jiang, X. Y. Hou, W. G. Li, S. J. Qiu and C. Z. Li, New J. Chem., 2016, 40, 1839–1844 RSC.
- S. H. Choi, S. K. Park, J. K. Lee and Y. C. Kang, J. Power Sources, 2015, 284, 481–488 CrossRef CAS.
- J. Leng, Z. X. Wang, X. H. Li, H. J. Guo, H. K. Li, K. M. Shih, G. C. Yan and J. X. Wang, J. Mater. Chem. A, 2017, 5, 14996–15001 RSC.
- G. Q. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
- J. F. Li, J. Z. Wang, X. Liang, Z. J. Zhang, H. K. Liu, Y. T. Qian and S. L. Xiong, ACS Appl. Mater. Interfaces, 2014, 6, 24–30 CrossRef CAS PubMed.
- H. Hwang, H. Shin and W. J. Lee, Sci. Rep., 2017, 7, 46378 CrossRef CAS PubMed.
- Y. L. Han, J. C. Mu, X. Y. Li, J. S. Gao, S. Y. Fan, F. Tan and Q. D. Zhao, Chem. Commun., 2018, 54, 9797–9800 RSC.
- L. F. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. G. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- L. Zhou, D. Y. Zhao and X. W. Lou, Adv. Mater., 2012, 24, 745–748 CrossRef CAS PubMed.
- Y. Qi, B. Q. Liu, L. Y. Zhang, Y. Q. Huo, L. Li, H. M. Xie, C. G. Wang and Z. M. Su, J. Mater. Chem. A, 2017, 5, 21994–22003 RSC.
- S. H. Hyuk Im, U. Jeong and Y. N. Xia, Nat. Mater., 2005, 4, 671–675 CrossRef CAS PubMed.
- R. Pang, X. J. Hu, S. Y. Zhou, C. H. Sun, J. Yan, X. M. Sun, S. Z. Xiao and P. Chen, Chem. Commun., 2014, 50, 12493–12496 RSC.
- Z. Sun, X. F. Song, P. Zhang and L. Gao, RSC Adv., 2015, 5, 3657–3664 RSC.
- D. Gu, H. Bongard, Y. H. Deng, D. Feng, Z. X. Wu, Y. Fang, J. J. Mao, B. Tu, F. Schuth and D. Y. Zhao, Adv. Mater., 2010, 22, 833–837 CrossRef CAS PubMed.
- J. Lee, J. H. Kwak and W. Choe, Nat. Commun., 2017, 8, 14070 CrossRef CAS PubMed.
- X. Y. Liu, F. R. Zhang, T. W. Goh, Y. Li, Y. C. Shao, L. S. Luo, W. Y. Huang, Y. T. Long, L. Y. Chou and C. K. Tsung, Angew. Chem., Int. Ed., 2018, 57, 2110–2114 CrossRef CAS PubMed.
- Z. Y. Wang, Z. C. Wang, H. B. Wu and X. W. Lou, Sci. Rep., 2013, 3, 1391 CrossRef PubMed.
- Z. L. Zhang, Y. J. Ji, J. Li, Q. Q. Tan, Z. Y. Zhong and F. B. Su, ACS Appl. Mater. Interfaces, 2015, 7, 6300–6309 CrossRef CAS PubMed.
- Y. W. Wang, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 14668–14672 CrossRef CAS PubMed.
- N. I. Chandrasekaran, H. Muthukumar, A. D. Sekar and M. Manickam, Mater. Chem. Phys., 2017, 195, 247–258 CrossRef CAS.
- M. Rajabzadeh, R. Khalifeh, H. Eshghi and M. Bakavoli, J. Catal., 2018, 360, 261–269 CrossRef CAS.
- J. Q. Feng, H. X. Guo, S. P. Wang, Y. J. Zhao and X. B. Ma, Chem. Eng. J., 2017, 321, 401–411 CrossRef CAS.
- J. Du, J. Qi, D. Wang and Z. Y. Tang, Energy Environ. Sci., 2012, 5, 6914–6918 RSC.
- M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. D. Yang, Nat. Mater., 2005, 4, 455–459 CrossRef CAS PubMed.
- H. Ren, H. Shao, L. J. Zhang, D. Guo, Q. Jin, R. B. Yu, L. Wang, Y. L. Li, Y. Wang, H. J. Zhao and D. Wang, Adv. Energy Mater., 2015, 5, 1500296 CrossRef.
- P. Z. Cui, J. L. Wang, Z. M. Wang, J. Chen, X. R. Xing, L. Z. Wang and R. B. Yu, Nano Res., 2016, 9, 593–601 CrossRef CAS.
- J. X. Feng, J. Q. Wu, Y. X. Tong and G. R. Li, J. Am. Chem. Soc., 2018, 140, 610–617 CrossRef CAS PubMed.
- S. A. Shah, X. P. Shen, M. H. Xie, G. X. Zhu, Z. Y. Ji, H. B. Zhou, K. Q. Xu, X. Y. Yue, A. H. Yuan, J. Zhu and Y. Chen, Small, 2019, 15, 1804545 CrossRef PubMed.
- J. H. Chen, J. W. Liu, J. Q. Xie, H. Q. Ye, X. Z. Fu, R. Sun and C. P. Wong, Nano Energy, 2019, 56, 225–233 CrossRef CAS.
- S. Y. Jiang, K. Zhao, M. Al-Mamun, Y. L. Zhong, P. R. Liu, H. J. Yin, L. X. Jiang, S. Lowe, J. Qi, R. B. Yu, D. Wang and H. J. Zhao, Inorg. Chem. Front., 2019, 6, 1667–1674 RSC.
- H. Ren, J. J. Sun, R. B. Yu, M. Yang, L. Gu, P. R. Liu, H. J. Zhao, D. Kisailus and D. Wang, Chem. Sci., 2016, 7, 793–798 RSC.
- H. Ren, J. Zhao, L. Yang, Q. H. Liang, S. Madhavi and Q. Y. Yan, Nano Res., 2019, 12, 1347–1353 CrossRef CAS.
- E. H. M. Salhabi, J. L. Zhao, J. Y. Wang, M. Yang, B. Wang and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 9078–9082 CrossRef CAS PubMed.
- H. P. Feng, L. Tang, G. M. Zeng, J. Tang, Y. C. Deng, M. Yan, Y. N. Liu, Y. Y. Zhou, X. Y. Ren and S. Chen, J. Mater. Chem. A, 2018, 6, 7310–7337 RSC.
- A. Noori, M. F. El-Kady, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, Chem. Soc. Rev., 2019, 48, 1272–1341 RSC.
- J. Xu, X. Yao, W. Y. Wei, Z. M. Wang and R. B. Yu, Mater. Res. Bull., 2017, 87, 214–218 CrossRef CAS.
- D. H. Lien, Z. H. Dong, J. R. D. Retamal, H. P. Wang, T. C. Wei, D. Wang, J. H. He and Y. Cui, Adv. Mater., 2018, 30, 1801972 CrossRef PubMed.
- L. B. Zong, P. F. Xu, Y. J. Ding, K. Zhao, Z. M. Wang, X. C. Yan, R. B. Yu, J. Chen and X. R. Xing, Small, 2015, 11, 2768–2773 CrossRef CAS PubMed.
- Y. N. Yang, Y. Lu, P. L. Abbaraju, J. Zhang, M. Zhang, G. Y. Xiang and C. Z. Yu, Angew. Chem., Int. Ed., 2017, 56, 8446–8450 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2019 |


