A ligand conformation preorganization approach to construct a copper–hexacarboxylate framework with a novel topology for selective gas adsorption†
Yao
Wang
a,
Minghui
He
a,
Xiaoxia
Gao
a,
Xia
Wang
a,
Guohai
Xu
*b,
Zhengyi
Zhang
c and
Yabing
He
 *a
*a
aKey Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Life Sciences, Zhejiang Normal University, Jinhua 321004, China. E-mail: heyabing@zjnu.cn
bKey Laboratory of Jiangxi University for Functional Materials Chemistry, School of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou, Jiangxi 341000, China. E-mail: xugh308@gnnu.cn
cBruker (Beijing) Scientific Technology Co., Ltd, Beijing 100192, China
First published on 27th November 2018
Abstract
The development of porous MOFs with novel structures and functional properties is a key research topic in the MOF chemistry and materials field. In this article, by employing a ligand conformation preorganization strategy, we designed a nonplanar methyl-substituted triisophthalate ligand, which was used to successfully construct a copper–hexacarboxylate framework with a novel topological structure. More importantly, the obtained MOF not only exhibits good hydrolytic stability but also shows utility as an adsorbent for efficient separation and purification of C2H2 and natural gas under ambient conditions. At ambient temperature and atmospheric pressure, the C2H2 and CO2 uptake capacities reach up to 149.1 and 83.1 cm3 (STP) g−1, while the IAST-predicted C2H2/CH4 (50/50, v/v), C2H2/CO2 (50/50, v/v), and CO2/CH4 (50/50, v/v) adsorption selectivities are as high as 22.2, 3.81, and 5.13.
1. Introduction
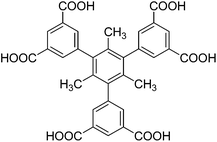
Porous metal–organic frameworks (MOFs) are emerging as a family of advanced organic−inorganic hybrid crystalline porous materials constructed by assembling inorganic metal ions or clusters and organic linkers via metal–organic coordination bonds. Such materials have found a large variety of potential applications including but not limited to selective gas/molecule adsorption and separation,1 catalysis,2 sensing,3 and drug delivery,4 most likely because they offer a high degree of diversity and tunability in terms of structure, chemical composition, porosity, and functionality compared to traditional porous materials. Up to now, design and construction of porous MOFs with intriguing structures and functional properties is a key research topic in the realm of MOF chemistry and materials.It is well known that the final structures of MOFs commonly rely on a number of factors such as the organic ligands, the metal ions, the metal–ligand ratio, solvent, reaction temperature and time, and pH value, in which the judicious choice of organic ligands and metal ions is very critical. We recently became particularly interested in employing isophthalate-containing multicarboxylate, including diisophthalate and triisophthalate, as organic ligands to fabricate copper-based MOFs, primarily because of the strong coordination ability of the isophthalate moiety and its propensity to engender metal–organic polyhedral cages with predesigned open copper sites upon self-assembly with copper ions, which is beneficial for gas adsorption.5 During our studies, it has been found that the final structures of the resultant MOFs are largely dictated by the ligand's conformations. For instance, for copper-bent diisophthalate systems, they are prone to form MOFs with variable topologies which are heavily dependent on the ligand's conformations. By capitalizing on conformational flexibility, we very recently fabricated a pair of supramolecular isomers using a bent diisophthalate ligand.6 When the bent diisophthalate building block becomes more preorganized, the self-assembly pathway and product become significantly more predictable. By employing steric hindrance imposed by methyl groups to preorganize the conformation of a bent diisophthalate ligand, we more recently successfully targeted an ssa-type MOF in which the organic building blocks only adopt one conformation.7 When shifting our attention from copper–diisophthalate systems to copper–triisophthalate ones, we note that it is required that the terminal three isophthalate units of the C3-symmetric triisophthalate ligand employed must be coplanar for the formation of rht-type MOFs, as reported in the literature.8 Thus, it is reasonable to speculate that the generation of interesting MOFs with novel architectures and topologies will occur if the peripheral isophthalate units of the triisophthalate ligands are not in a plane. For this purpose, we employed the ligand conformation preorganization strategy to design a nonplanar methyl-substituted triisophthalate ligand, namely, (1,3,5-trimethylbenzene-2,4,6-triyl) triisophthalate (H6L, Scheme 1). The introduction of methyl groups in the central benzene unit of the ligand is expected to help fix the ligand's conformation in which the terminal isophthalate moieties are almost perfectly perpendicular to the central benzene unit. It should be pointed out that this ligand has been utilized to construct Zn2+/Co2+-based MOFs by other research groups,9 but copper-based MOFs based on this ligand have been not reported so far. In addition, a structurally similar BHB ligand but without methyl groups on the central phenyl ring has been used to construct a copper-based MOF UTSA-20 with a novel zyg topology.10 However, different with our ligand, the terminal isophthalate moieties of the BHB ligand are not perpendicular to the central phenyl ring with a torsion angle of ca. 34° due to the absence of the steric hindrance effect induced by methyl groups. With the ligand H6L in hand, we successfully constructed a copper-based MOF under solvothermal conditions, which features a novel topological structure instead of rht-type and zyg-type ones.
The acetylene/methane (C2H2/CH4), acetylene/carbon dioxide (C2H2/CO2), and carbon dioxide/methane (CO2/CH4) separations are of high importance in the petrochemical industry. On one hand, as an important fuel and chemical raw material, C2H2 is typically produced by partial oxidation of natural gas. In this process, CO2 is unavoidably generated. Therefore, removal of CO2 and unconverted CH4 from C2H2 is required for its practical applications. On the other hand, owing to its natural abundance and less adverse effects on the environment, natural gas is regarded as one of the most attractive energy carriers for resolving the current energy crisis and environmental issues caused by the massive consumption of fossil fuels. In addition to CH4 as the major composition, natural gas also contains variable amounts of other gases such as CO2, nitrogen (N2), and light hydrocarbons. The acidic CO2 not only reduces the energy content of natural gas, but also corrodes the equipment and pipeline especially in the presence of humidity. Additionally, landfill gas is also a very important source of CH4. However, landfill gas often contains an unacceptable level of CO2. Consequently, the capture and removal of CO2 from CH4 is very essential and particularly important in natural gas upgrading and landfill gas utilization. Among the strategies being developed to address the above-mentioned issues, adsorption-based separation using porous solids can greatly reduce the energy consumption associated with the classical separation techniques of cryogenic distillation and chemical scrubbing. In this context, development of porous solids capable of highly effective separation and purification of C2H2 and natural gas under ambient conditions is in urgent demand. To date, some experimental and computational studies have showed that many MOFs exhibit adsorption selectivity in C2H2/CH4,11 C2H2/CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and CO2/CH4
12 and CO2/CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 separations, surpassing most traditional adsorbents. Due to the same kinetic diameter (3.3 Å) of C2H2 and CO2, the C2H2–CO2 mixture is the most difficult to separate among the three pairs of gas mixtures. It should be mentioned that Lin et al. recently reported a flexible MOF UTSA-300 being capable of sieving CO2 from C2H2.14 Herein, we presented the synthesis, characterization, and selective C2H2/CH4, C2H2/CO2, and CO2/CH4 adsorption properties of a new MOF based on copper ions and a hexacarboxylate ligand featuring a novel topological structure, which was rationally designed by the above-mentioned ligand conformation preorganization strategy.
13 separations, surpassing most traditional adsorbents. Due to the same kinetic diameter (3.3 Å) of C2H2 and CO2, the C2H2–CO2 mixture is the most difficult to separate among the three pairs of gas mixtures. It should be mentioned that Lin et al. recently reported a flexible MOF UTSA-300 being capable of sieving CO2 from C2H2.14 Herein, we presented the synthesis, characterization, and selective C2H2/CH4, C2H2/CO2, and CO2/CH4 adsorption properties of a new MOF based on copper ions and a hexacarboxylate ligand featuring a novel topological structure, which was rationally designed by the above-mentioned ligand conformation preorganization strategy.
2. Experimental
2.1 Materials and instruments
Unless otherwise noted, all commercially available chemical reagents and solvents were used as received without further purification. Dimethyl-5-(pinacolboryl) isophthalate was prepared according to the literature protocol.15 Thin layer chromatography (TLC) was carried out on aluminium sheets pre-coated with silica gel 60 F254 purchased from Merck, using ultra-violet (UV) light as the visualizing agent. Column chromatography was performed using silica gel (100–200 mesh, Qingdao Haiyang Chemical Co., Ltd). Proton and carbon nuclear magnetic resonance (1H and 13C NMR) spectra were recorded on a Bruker AV 400 or AV600 spectrometer at room temperature. Residual protonated species in the deuterated solvents (CDCl3 and DMSO-d6) were used as internal references. Chemical shifts were reported in ppm (parts per million) and coupling constants were reported in hertz (Hz). Data were represented as follows: chemical shift, multiplicity (br = broad, s = singlet, d = doublet, and t = triplet), coupling constants, and integration. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet 5DX FTIR spectrometer in the 400–4000 cm−1 region using KBr pellets. Elemental analyses of C, H, and N were carried out with a PerkinElmer 240 CHN elemental analyser. Thermogravimetric analysis experiments were performed on a Netzsch STA 449C thermal analyser from room temperature to 1073 K under a N2 atmosphere at a heating rate of 5 K min−1. Powder X-ray diffraction (PXRD) patterns were collected using Cu-Kα radiation (λ = 1.5418 Å) on a Bruker D8 Advance powder diffractometer at ambient temperature in the angular range of 2θ = 5–45°. The simulated PXRD patterns were calculated using the single-crystal X-ray diffraction data and processed using the free Mercury program (version 1.4.1) provided by the Cambridge Crystallographic Data Center. Topological analyses were performed using the Topos 4.0 program. Gas adsorption isotherms were obtained with a Micromeritics ASAP 2020 HD88 surface area and pore analyser. Extra-high purity gases (N2, 99.9999%; C2H2, 99.9%; CO2, 99.999%; CH4, 99.99%) were used for the adsorption–desorption measurements. A temperature of 77 K was maintained with liquid N2, and other temperatures were controlled by a circulating water bath. The pore size distribution (PSD) was obtained from the N2 adsorption data using the density functional theory (DFT) method and assuming a slit pore model.2.2 Single-crystal X-ray diffraction
The single-crystal structure data for ZJNU-100 were collected on a Bruker SMART APEX II diffractometer using graphite-monochromated Cu-Kα (λ = 1.54178 Å) radiation. The structure was solved by direct methods using the SHELXS-2017 program and refined by full-matrix least squares on F2 using the SHELXL-2017 program. The non-hydrogen atoms were located in successive difference Fourier syntheses and refined with anisotropic thermal parameters. In the refinement, hydrogen atoms were treated as riding atoms using the SHELXL default parameters. The guest molecules were highly disordered and randomly dispersed, and thus their locations cannot be determined. To resolve this issue, the electron density peaks of guest molecules were removed by the SQUEEZE procedure of the PLATON program suite.16 The detailed structure refinement parameters are summarized in Table S1 in the ESI.†2.3 Synthesis and characterization of the organic ligand
A mixture containing 1,3,5-tribromo-2,4,6-trimethyl-benzene (1.00 g, 2.80 mmol), dimethyl 5-(pinacolboryl) isophthalate (2.96 g, 9.25 mmol), Cs2CO3 (4.11 g, 12.61 mmol), and Pd(PPh3)4 (0.24 g, 0.21 mmol) was introduced into 100 mL of anhydrous 1,4-dioxane in a 250 mL single-necked round-bottom flask equipped with a magnetic stirring bar and a refluxing condenser. The flask was evacuated and then back-filled with N2 three times in order to ensure that the reaction is carried out under a N2 atmosphere. The resulting mixture was allowed to reflux for 3 days with continuous stirring. After that, the reaction mixture was filtered while hot through a plug of Celite and washed with chloroform, tetrahydrofuran (THF), and 1,4-dioxane. After the organic solvent was removed under reduced pressure, the residue was purified with column chromatography on silica gel using petroleum ether and ethyl acetate (1/1, v/v) as an eluent to afford the hexamethyl ester as a white solid (0.88 g, 1.26 mmol, 45%). 1H NMR (CDCl3, 400.1 MHz) δ (ppm): 8.711 (t, J = 1.6 Hz, 3H), 8.121 (d, J = 1.6 Hz, 6H), 3.984 (s, 18H), 1.675 (s, 9H).The hexamethyl ester intermediate (0.88 g, 1.26 mmol) was suspended in a mixed solvent composed of THF and methanol (80 mL, v/v = 1/1), and then an aqueous solution of 3 mol L−1 NaOH (20 mL) was added. The resultant mixture was stirred and refluxed overnight. After the reaction mixture was allowed to cool to room temperature, the organic solvents were removed, and the remaining solution was acidified with concentrated hydrochloric acid in an ice-water bath to give a white precipitate, which was collected by filtration, washed with water, and dried in a vacuum oven at 343 K to produce 0.75 g of the target compound H6L (1.22 mmol, 97%). 1H NMR (DMSO-d6, 400.1 MHz) δ (ppm): 13.300 (br, 6H), 8.486 (t, J = 1.2 Hz, 3H), 8.020 (d, J = 1.2 Hz, 6H), 1.621 (s, 9H); 13C NMR (DMSO-d6, 150.9 MHz) δ (ppm): 167.001, 142.288, 138.408, 134.530, 133.155, 132.404, 129.106, 19.879; selected FTIR (KBr, cm−1): 1701, 1601, 1448, 1390, 1269, 1207, 1134, 1111, 922, 760, 690, 669, 606.
2.4 Synthesis and characterization of ZJNU-100
H6L (5.0 mg, 8.2 μmol), CuCl2·2H2O (10.0 mg, 58.6 μmol), deionized water (0.3 mL), N,N-dimethyl formamide (DMF, 1.5 mL), and 6 mol L−1 HCl (70 μL) were charged in a 20 mL glass vial. The mixture was heated at 363 K for 48 h. After being cooled down to room temperature, the blue block-shaped crystals of ZJNU-100 suitable for single-crystal X-ray diffraction (SCXRD) analysis were harvested by suction filtration, and washed with fresh DMF (yield: 65% based on H6L). ZJNU-100 is insoluble in common solvents such as DMF, THF, 1,4-dioxane, acetone, methanol, dichloromethane, chloroform, and water. Based on single-crystal X-ray determination, TGA and microanalyses, ZJNU-100 can be best formulated to be [Cu24(HL)6(L)3(H2O)24]·52DMF. Selected FTIR (KBr, cm−1): 3423, 1655, 1630, 1578, 1437, 1369, 1255, 1101, 779, 727, 700, 663, 611. Elemental analysis calcd (%) for C453H580N52O184Cu24: C, 48.48; H, 5.21; N, 6.49; Found: C, 48.38; H, 5.20; N, 6.54.3. Results and discussion
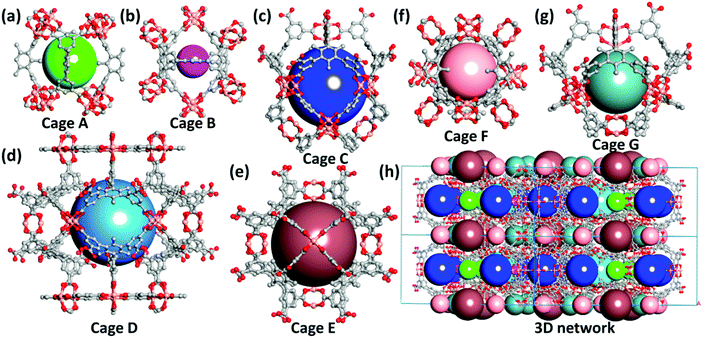
The synthesis of the organic ligand, (1,3,5-trimethylbenzene-2,4,6-triyl) triisophthalate, was facilely accomplished using Suzuki cross-coupling reaction between 1,3,5-trimethyl-2,4,6-tribromobenzene and dimethyl 5-(pinacolboryl) isophthalate followed by hydrolysis and acidification. 1H and 13C NMR confirmed the structure and purity of the ligand. Subsequently, the ligand was subjected to a solvothermal reaction with copper chloride dihydrate in acidified mixed solvents of DMF and water, affording blue block-shaped crystals which we termed ZJNU-100 (Fig. S1†). The PXRD pattern of the bulk phase of ZJNU-100 coincides with the simulated one generated from the single-crystal X-ray diffraction data, verifying that the bulk product is phase-pure (Fig. S2†). TGA analysis showed a weight loss of 37.9% (calcd. 37.7%) from room temperature to 573 K, which is attributed to the departure of solvent molecules and terminal water molecules (Fig. S3†). More importantly, as evidenced by PXRD (Fig. S2†) and 77 K N2 adsorption measurements (Fig. S6†), ZJNU-100 is stable in aqueous HCl/NaOH solutions in the pH range of 3–11 for 24 h at room temperature, which is favourable for practical industrial applications.SCXRD studies show that ZJNU-100 is a porous three-dimensional network crystallizing in the hexagonal space group of P6/mmm, with the unit cell parameters a = b = 63.7383(15) Å, and c = 25.3645(7) Å. Each copper ion is five-coordinated by four oxygen atoms belonging to four distinct carboxylate groups and one oxygen atom coming from the terminal water molecule, thus representing a square-pyramidal geometry. The Cu–Ocarboxylate bond lengths are in the range of 1.854–2.238 Å. As expected, two neighbouring copper ions were linked to each other by four bidentate bridging carboxylate groups to generate a dicopper paddlewheel secondary building unit (SBU) with the Cu–Cu distances lying in the range of 2.593–2.708 Å. These inorganic dicopper paddlewheel units were further interconnected by the organic ligands, eventually resulting in the formation of a complicated three-dimensional network. Noteworthily, some of the organic ligands were fully deprotonated, while the others were partially deprotonated, with a free carboxylate group being immobilized in the pore surface and pointing into the centre of the pore. As such, the uncoordinated carboxylate oxygen atoms are capable of serving as potential binding sites for incoming guest molecules. Besides, the water molecules occupied at the apical position of the bimetallic Cu2(COO)4 node can be readily removed during the activation process to generate Lewis acidic open metal sites. Therefore, ZJNU-100 features two different types of functional active sites for gas adsorption. Interestingly, a close inspection of the crystal structure revealed that there exist seven types of metal–organic polyhedral cages with different shapes and sizes in the overall network. As illustrated in Fig. 1, cage A consists of eight dicopper paddlewheel units and four organic ligands; cage B is composed of six dicopper paddlewheel units and three organic ligands; cage C encompasses nine dicopper paddlewheel units, twelve isophthalate units and four organic ligands; cage D is constructed by linking twelve isophthalate units and twelve organic ligands with eighteen dicopper paddlewheel units; cage E is comprised of six dicopper paddlewheel units and eight organic ligands; cage F is built up from eight dicopper paddlewheel units and four organic ligands; cage G is assembled from six dicopper paddlewheel units, six isophthalate units and four organic ligands. The diameters of the spheres representing the void of the cages A–G were estimated to be about 6.5, 3.0, 12.0, 12.0, 12.0, 6.2 and 8.2 Å based on van der Waals radii, respectively. To the best of our knowledge, ZJNU-100 represents the first example of MOFs incorporating seven different types of cages in the overall structure. After the removal of the solvent molecules and the terminal water molecules, the accessible void fraction was estimated to be 64.4% of the unit cell volume, and the framework density is 0.7802 g cm−3. Topologically, if the dicopper paddlewheel units are regarded as 4-connected nodes and the organic ligands are considered to be 5-connected and 6-connected nodes, respectively, the overall network of ZJNU-100 can be simplified to be a (4,5,6)-connected network with the point (Schläfli) symbol of {42·62·82}3{43·63}4{43·67}2{44·62}5{44·64·82}2{44·66}2{45·610}2{46·69}. Furthermore, if the 5-connected and 6-connected ligands are considered to consist of three and four 3-connected nodes, respectively, the overall network of ZJNU-100 can be simplified to be a (3,4)-connected network with the point symbol of {6·7·8}8{6·72}8{62·82·102}5{62·8}4{63·82·9}4{72·8}10{74·10·12}2{74·102}. The topological structure has not been observed for copper–multicarboxylate frameworks so far.
To characterize the permanent porosity and pore textural properties of ZJNU-100, we performed the N2 adsorption and desorption experiments at 77 K. Prior to gas adsorption, the as-synthesized ZJNU-100 was subjected to guest exchange with dry acetone, and the acetone-exchanged ZJNU-100 was evacuated under dynamic vacuum at room temperature for 24 h and then at 353 K until the degas rate of 0.27 Pa min−1 (2 μmHg min−1) was reached. As depicted in Fig. 2, the N2 adsorption isotherm is fully reversible and features a type-I profile, reflecting that ZJNU-100 is a porous material with permanent microporosity. Based on the N2 adsorption isotherm and the saturated N2 uptake of 487.2 cm3 (STP) g−1, the Brunauer–Emmett–Teller (Langmuir) specific surface areas and pore volume are estimated to be 1933 (2113) m2 g−1 and 0.754 cm3 g−1, respectively (Fig. S5†). The BET surface area of ZJNU-100 is significantly higher than that of UTSA-20 (1156 m2 g−1)10 based on the BHB ligand, NPC-5 (1140 m2 g−1)9a and SDU-1 (779 m2 g−1)9a constructed from the H6L ligand, but lower than that of some other copper-triisophthalate frameworks such as Cu-TDPAT (1938 m2 g−1),17 SDU-6 (2826 m2 g−1),18 PCN-61 (3000 m2 g−1),19 NU-125 (3120 m2 g−1),20 Cu-TPBTM (3160 m2 g−1),21 and NOTT-112 (3800 m2 g−1)22 (Table S2†). The inset in Fig. 2 shows the DFT PSD of ZJNU-100, falling in the range of 5.36–15.91 Å with the maximum value of 5.90, 8.04 and 11.79 Å.
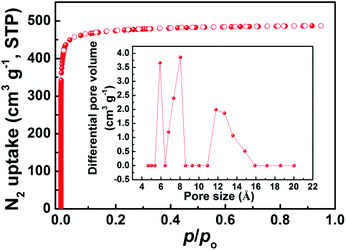 | ||
| Fig. 2 77 K N2 adsorption and desorption isotherm of ZJNU-100. The solid and open symbols refer to adsorption and desorption, respectively. The inset shows the DFT PSD of ZJNU-100. | ||
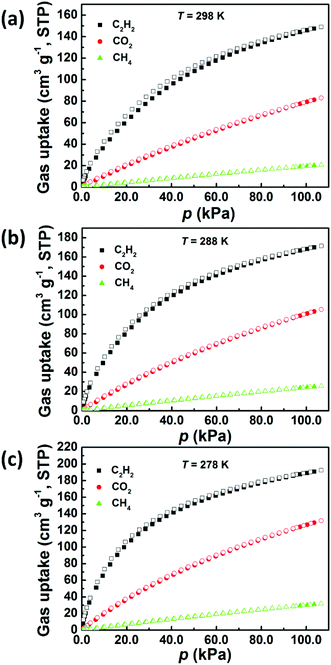
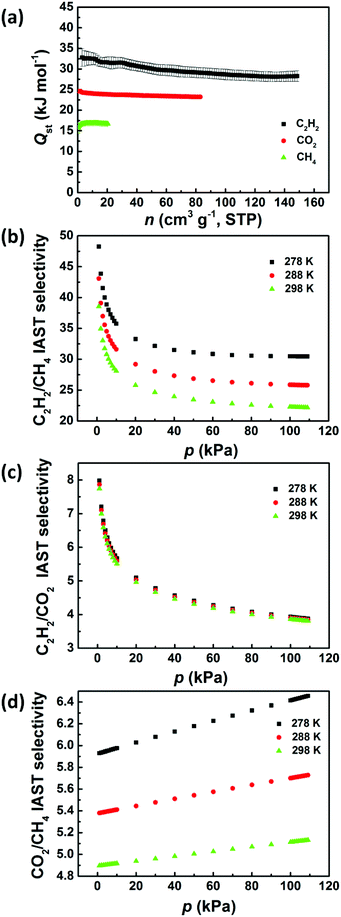
Next, we investigated the potential of ZJNU-100 for C2H2/CH4, C2H2/CO2, and CO2/CH4 separations. Accordingly, simple-component sorption isotherms for C2H2, CO2, and CH4 were collected at three different temperatures of 278 K, 288 K, and 298 K and pressures up to 106.6 kPa (800 mmHg). As shown in Fig. 3, no obvious hysteresis was observed for all isotherms at all the temperatures and pressures investigated, indicating that the adsorption and desorption processes are completely reversible. Most remarkably, the gas adsorption capacity of ZJNU-100 follows the hierarchy: C2H2 > CO2 > CH4. Concretely, ZJNU-100 is capable of adsorbing up to 149.1 cm3 (STP) g−1 of C2H2 at 298 K and 106.6 kPa. As expected, the C2H2 uptake increases with the decreasing temperature due to exothermic physical adsorption, reaching 171.5 cm3 (STP) g−1 at 288 K, and 192.4 cm3 (STP) g−1 at 278 K. Comparatively, the amount of C2H2 adsorbed in ZJNU-100 exceeds those of the vast majority of MOF materials reported thus far under the same measurement conditions such as PCM-48 (25.54 cm3 (STP) g−1),23 ZJNU-62 (33.9 cm3 (STP) g−1),24 APPT-Cd-ClO4− (39.3 cm3 (STP) g−1),25 ZJNU-63 (39.4 cm3 (STP) g−1),26 ZJNU-61a (48.0 cm3 (STP) g−1),27 FJI-C4 (72.5 cm3 (STP) g−1),11f MFM-130a (85.9 cm3 (STP) g−1),28 FJI-Y9-ht (119.2 cm3 (STP) g−1),29 UTSA-34b (120.8 cm3 (STP) g−1), ZJU-199 (128 cm3 (STP) g−1),30 and UPC-21 (139.5 cm3 (STP) g−1).31 As for CO2 adsorption, the uptake amount of CO2 at 106.6 kPa is 83.1 cm3 (STP) g−1 at 298 K, 105.4 cm3 (STP) g−1 at 288 K, and 131.7 cm3 (STP) g−1 at 278 K. The CO2 uptake of ZJNU-100 is also comparable to and better than those of some reported copper–hexacarboxylate frameworks such as HNUST-5 (56 cm3 (STP)),32 Cu-NTTA (65.5 cm3 (STP) g−1),33 GDMU-2 (74 cm3 (STP) at 273 K),34 and UTSA-20 (82.4 cm3 (STP) g−1)10 (Table S2†). In contrast, ZJNU-100 only adsorbs a very limited amount of CH4, with the CH4 uptake of 20.8 cm3 (STP) g−1 at 298 K, 25.7 cm3 (STP) g−1 at 288 K, and 31.8 cm3 (STP) g−1 at 278 K at 106.6 kPa, respectively. To evaluate the binding energy between gas molecules and the framework, we calculated the coverage-dependent isosteric heat (Qst) of gas adsorption from multiple temperature-dependent isotherms according to the Clausius–Clapeyron equation. During the entire adsorption process, the order of Qst values is consistent with the uptake amounts (Fig. 4a). Specially, at near zero coverage, the Qst values for C2H2, CO2, and CH4 adsorption are 32.8 ± 2.2, 24.6 ± 0.4 and 15.7 ± 1.2 kJ mol−1 respectively.
The hierarchy of uptake capacity and isosteric adsorption heat for C2H2, CO2, and CH4 in ZJNU-100 suggests that it has promising potential for the separation of C2H2–CH4, C2H2–CO2, and CO2–CH4 binary gas mixtures. In order to assess the gas-mixture separation performance, we utilized the ideal adsorbed solution theory (IAST) model to predict the adsorption selectivity.35 Prior to calculations, the pure-component experimental adsorption isotherms were fitted with the Langmuir–Freundlich equation (Fig. S7†). The fitting parameters obtained are provided in Table S3 in the ESI.†Fig. 4b–d show the IAST adsorption selectivities for the equimolar C2H2–CH4, C2H2–CO2, and CO2–CH4 gas mixtures at three different temperatures of 298 K, 288 K, and 278 K. It can be observed that the adsorption selectivity is closely correlated with the temperature and pressure. When the total gas pressure increases, the C2H2/CH4 and C2H2/CO2 adsorption selectivities decrease, while the CO2/CH4 adsorption selectivity slightly increases. With the decreasing temperature, the C2H2/CH4 and CO2/CH4 adsorption selectivities increase, while the C2H2/CO2 adsorption selectivity almost remains unchanged. In particular, the C2H2/CH4, C2H2/CO2, and CO2/CH4 adsorption selectivities at atmospheric pressure reach 22.2, 3.81, and 5.13 at 298 K, 25.8, 3.85, and 5.73 at 288 K, and 30.5, 3.88, and 6.46 at 278 K. Given the impressive adsorption selectivity, high uptake capacity, and good hydrostability, ZJNU-100 could be a promising solid adsorbent applied in the separation and purification of C2H2 and natural gas.
4. Conclusions
In summary, we employed the ligand conformation preorganization design strategy to construct a copper–hexacarboxylate framework. The obtained MOF not only shows a novel topological structure, high porosity and good hydrostability, but also displays selective C2H2/CH4, C2H2/CO2, and CO2/CH4 adsorption capabilities. The presented results will pave the way to the strategic design and synthesis of MOF materials with novel topologies and functional properties by sterically controlling the conformation of the organic building blocks.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 21771162, 21761003), the Natural Science Foundation of Zhejiang Province, China (LR16B010001), and the Qianjiang Talents Project in Zhejiang Province (ZC304015017).Notes and references
- (a) D.-X. Xue, Q. Wang and J. Bai, Coord. Chem. Rev., 2019, 378, 2–16 CrossRef CAS; (b) R.-B. Lin, S. Xiang, H. Xing, W. Zhou and B. Chen, Coord. Chem. Rev., 2019, 378, 87–103 CrossRef CAS; (c) Y. He, F. Chen, B. Li, G. Qian, W. Zhou and B. Chen, Coord. Chem. Rev., 2018, 373, 167–198 CrossRef CAS; (d) R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS; (e) R.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS; (f) K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC; (g) S. C. King, R.-B. Lin, H. Wang, H. D. Arman and B. Chen, Mater. Chem. Front., 2017, 1, 1514–1519 RSC; (h) Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657–5678 RSC; (i) Z. R. Herm, E. D. Bloch and J. R. Long, Chem. Mater., 2014, 26, 323–338 CrossRef CAS; (j) Y. Yan, S. Yang, A. J. Blake and M. Schröder, Acc. Chem. Res., 2014, 47, 296–307 CrossRef CAS; (k) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS; (l) H. Wu, Q. Gong, D. H. Olson and J. Li, Chem. Rev., 2012, 112, 836–868 CrossRef CAS PubMed; (m) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS; (n) S. Ma and H.-C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC.
- (a) W. Jiang, J. Yang, Y.-Y. Liu, S.-Y. Song and J.-F. Ma, Inorg. Chem., 2017, 56, 3036–3043 CrossRef CAS; (b) M. Zhao, S. Ou and C.-D. Wu, Acc. Chem. Res., 2014, 47, 1199–1207 CrossRef CAS PubMed; (c) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed; (d) A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS; (e) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- (a) W.-Q. Zhang, Q.-Y. Li, J.-Y. Cheng, K. Cheng, X. Yang, Y. Li, X. Zhao and X.-J. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 31352–31356 CrossRef CAS PubMed; (b) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC; (c) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed; (d) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. V. Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- Y. He, B. Li, M. O'Keeffe and B. Chen, Chem. Soc. Rev., 2014, 43, 5618–5656 RSC.
- F. Chen, D. Bai, Y. Wang, M. He, X. Gao and Y. He, Dalton Trans., 2018, 47, 716–725 RSC.
- Y. Wang, M. He, Z. Tian, H. Zhong, L. Zhu, Y. Zhang, X. Zhang, D.-L. Chen and Y. He, Dalton Trans., 2018, 47, 2444–2452 RSC.
- D. Zhao, D. Yuan, D. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2009, 131, 9186–9188 CrossRef CAS PubMed.
- (a) L. Li, S. Tang, X. Lv, M. Jiang, C. Wang and X. Zhao, New J. Chem., 2013, 37, 3662–3670 RSC; (b) X. Zhao, X. Wang, S. Wang, J. Dou, P. Cui, Z. Chen, D. Sun, X. Wang and D. Sun, Cryst. Growth Des., 2012, 12, 2736–2739 CrossRef CAS.
- Z. Guo, H. Wu, G. Srinivas, Y. Zhou, S. Xiang, Z. Chen, Y. Yang, W. Zhou, M. O'Keeffe and B. Chen, Angew. Chem., Int. Ed., 2011, 50, 3178–3181 CrossRef CAS.
- (a) Y.-L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K.-J. Chen, K. A. Forrest, B. Space, P. Cheng, M. J. Zaworotko and Z. Zhang, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS; (b) X. Duan, Y. Zhou, R. Lv, B. Yu, H. Chen, Z. Jia, Y. Cui, Y. Yang and G. Qian, J. Solid State Chem., 2018, 260, 31–33 CrossRef CAS; (c) Z.-J. Guo, J. Yu, Y.-Z. Zhang, J. Zhang, Y. Chen, Y. Wu, L.-H. Xie and J.-R. Li, Inorg. Chem., 2017, 56, 2188–2197 CrossRef CAS; (d) J.-W. Zhang, M.-C. Hu, S.-N. Li, Y.-C. Jiang and Q.-G. Zhai, Dalton Trans., 2017, 46, 836–844 RSC; (e) W. Fan, Y. Wang, Q. Zhang, A. Kirchon, Z. Xiao, L. Zhang, F. Dai, R. Wang and D. Sun, Chem. – Eur. J., 2017, 24, 2137–2143 CrossRef; (f) L. Li, X. Wang, J. Liang, Y. Huang, H. Li, Z. Lin and R. Cao, ACS Appl. Mater. Interfaces, 2016, 8, 9777–9781 CrossRef CAS PubMed; (g) J. Jiao, L. Dou, H. Liu, F. Chen, D. Bai, Y. Feng, S. Xiong, D.-L. Chen and Y. He, Dalton Trans., 2016, 45, 13373–13382 RSC; (h) J. Duan, W. Jin and R. Krishna, Inorg. Chem., 2015, 54, 4279–4284 CrossRef CAS; (i) Y. He, Z. Zhang, S. Xiang, H. Wu, F. R. Fronczek, W. Zhou, R. Krishna, M. O'Keeffe and B. Chen, Chem. – Eur. J., 2012, 18, 1901–1904 CrossRef CAS; (j) Y. He, Z. Zhang, S. Xiang, F. R. Fronczek, R. Krishna and B. Chen, Chem. – Eur. J., 2012, 18, 613–619 CrossRef CAS; (k) Z. Chen, S. Xiang, H. D. Arman, P. Li, S. Tidrow, D. Zhao and B. Chen, Eur. J. Inorg. Chem., 2010, 3745–3749 CrossRef CAS.
- (a) Y. Wang, M. He, X. Gao, P. Long, Y. Zhang, H. Zhong, X. Wang and Y. He, Dalton Trans., 2018, 47, 12702–12710 RSC; (b) F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O'Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS.
- R.-B. Lin, L. Li, H. Wu, H. Arman, B. Li, R.-G. Lin, W. Zhou and B. Chen, J. Am. Chem. Soc., 2017, 139, 8022–8028 CrossRef CAS.
- C. Song, Y. Ling, Y. Feng, W. Zhou, T. Yildirim and Y. He, Chem. Commun., 2015, 51, 8508–8511 RSC.
- (a) A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS; (b) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
- B. Li, Z. Zhang, Y. Li, K. Yao, Y. Zhu, Z. Deng, F. Yang, X. Zhou, G. Li, H. Wu, N. Nijem, Y. J. Chabal, Z. Lai, Y. Han, Z. Shi, S. Feng and J. Li, Angew. Chem., Int. Ed., 2012, 51, 1412–1415 CrossRef CAS PubMed.
- X. Zhao, D. Sun, S. Yuan, S. Feng, R. Cao, D. Yuan, S. Wang, J. Dou and D. Sun, Inorg. Chem., 2012, 51, 10350–10355 CrossRef CAS PubMed.
- D. Yuan, D. Zhao, D. Sun and H.-C. Zhou, Angew. Chem., Int. Ed., 2010, 49, 5357–5361 CrossRef CAS PubMed.
- C. E. Wilmer, O. K. Farha, T. Yildirim, I. Eryazici, V. Krungleviciute, A. A. Sarjeant, R. Q. Snurr and J. T. Hupp, Energy Environ. Sci., 2013, 6, 1158–1163 RSC.
- B. Zheng, J. Bai, J. Duan, L. Wojtas and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 133, 748–751 CrossRef CAS.
- Y. Yan, X. Lin, S. Yang, A. J. Blake, A. Dailly, N. R. Champness, P. Hubberstey and M. Schröder, Chem. Commun., 2009, 1025–1027 RSC.
- J. E. R. III, K. M. Walsh, B. Li, P. Kunal, B. Chen and S. M. Humphrey, Chem. Commun., 2018, 54, 9937–9940 RSC.
- D. Bai, F. Chen, D. Jiang and Y. He, Inorg. Chem. Front., 2017, 4, 1501–1508 RSC.
- G.-X. Jin, X. Niu, J. Wang, J.-P. Ma, T.-L. Hu and Y.-B. Dong, Chem. Mater., 2018, 30, 7433–7437 CrossRef CAS.
- D. Bai, Y. Wang, M. He, X. Gao and Y. He, Inorg. Chem. Front., 2018, 5, 2227–2237 RSC.
- Y. Ling, J. Jiao, M. Zhang, H. Liu, D. Bai, Y. Feng and Y. He, CrystEngComm, 2016, 18, 6254–6261 RSC.
- Y. Yan, M. Juríček, F. O.-X. Coudert, N. A. Vermeulen, S. Grunder, A. Dailly, W. Lewis, A. J. Blake, J. F. Stoddart and M. Schröder, J. Am. Chem. Soc., 2016, 138, 3371–3381 CrossRef CAS PubMed.
- F.-J. Zhao, Y.-X. Tan, W. Wang, Z. Ju and D. Yuan, Inorg. Chem., 2018, 57, 13312–13317 CrossRef CAS PubMed.
- L. Zhang, C. Zou, M. Zhao, K. Jiang, R. Lin, Y. He, C.-D. Wu, Y. Cui, B. Chen and G. Qian, Cryst. Growth Des., 2016, 16, 7194–7197 CrossRef CAS.
- M. Zhang, X. Xin, Z. Xiao, R. Wang, L. Zhang and D. Sun, J. Mater. Chem. A, 2017, 5, 1168–1175 RSC.
- B. Zheng, H. Wang, Z. Wang, N. Ozaki, C. Hang, X. Luo, L. Huang, W. Zeng, M. Yang and J. Duan, Chem. Commun., 2016, 52, 12988–12991 RSC.
- X. Guo, Z. Zhou, C. Chen, J. Bai, C. He and C. Duan, ACS Appl. Mater. Interfaces, 2016, 8, 31746–31756 CrossRef CAS PubMed.
- J. Liu, G. Liu, C. Gu, W. Liu, J. Xu, B. Li and W. Wang, J. Mater. Chem. A, 2016, 4, 11630–11634 RSC.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Electronic graphs (Fig. S1); PXRD patterns (Fig. S2); TGA curves (Fig. S3); FTIR spectra (Fig. S4); BET and Langmuir surface area plots (Fig. S5); comparison of 77 K N2 isotherms and pore size distributions of ZJNU-100 before and after treatment with water (Fig. S6); gas adsorption isotherm fitting (Fig. S7); NMR spectra (Fig. S8); structure refinement parameters (Table S1); summaries of gas adsorption properties and pore textural parameters of some reported MOFs based on triisophthalate ligands (Table S2); fitting parameters (Table S3). CCDC 1877489. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8qi01225c |
| This journal is © the Partner Organisations 2019 |