Room-temperature hydrogen generation from water and nanoscale Fe catalyzed by Pd†
Ting
Ni
,
Huan
Zhang
and
Sai
Zhang
 *
*
Shaanxi Key Laboratory of Energy Chemical Process Intensification, School of Chemical Engineering and Technology, Xi'an Jiaotong University, Xi'an, 710049, China. E-mail: zhangsai058@mail.xjtu.edu.cn
First published on 27th November 2018
Abstract
Hydrogen generation under mild conditions is a promising strategy and longstanding challenge for the development of novel clean energy resources. Most current systems involve organic/inorganic hydrides that are catalyzed in basic media and have to release the carbon oxides (CO or CO2). Herein, we describe an alternative pathway for highly efficient production of pure H2 (0.073 mmol gFe−1 min−1) through a redox reaction between water and Fe nanoparticles at room temperature catalyzed by Pd without the activation of light or electricity. Experimental results prove that the small size of Fe nanoparticles and the catalysis of Pd supported on its surface play a key role in the successful generation of hydrogen. With the catalysis of Pd, the reaction activation energy can be obviously reduced, which is beneficial for the enhanced hydrogen generation rate. The hydrogen can also be successfully released from the water and Fe nanoparticle system even at 0 °C reaction temperature, indicating its broad scope of application. Meanwhile, the remnants of the hydrogen generation system are Pd/Fe2O3 nanoparticles, which are useful and recyclable heterogeneous catalysts for various organic reactions.
Introduction
Polymer electrolyte membrane fuel cells (PEMFCs) running on hydrogen, which can efficiently convert energy from chemical fuels into electricity, are attractive alternative power supplies for a range of applications.1–3 Unfortunately, the physical properties of hydrogen make its transportation and handling difficult.4 To overcome this, an in situ stable hydrogen release system is required for the PEMFCs to ensure its safe storage and transportation.5 The use of methanol is particularly interesting in this regard compared with formaldehyde or formic acid, because it is inexpensive and can reform itself with water to release hydrogen with a high volumetric density of 0.037 mmol mL−1.6–13 However, there are still some problems in these hydrogen generation systems. (1) It is unavoidable to produce CO2 and CO during hydrogen generation. The large amount of CO2 in the atmosphere will contribute to global warming,14 while very low content of CO in hydrogen will lead to the toxication of fuel cells. (2) Most of these systems need high temperature to realize the hydrogen generation (even higher than 100 °C).6,15 Therefore, carbon-free hydrogen generation at mild reaction temperature is considered an ideal, environmentally friendly, clean and renewable fuel for an economically and socially sustainable future.16Recently, activated metal, such as Si and Al, can be oxidized by water and release hydrogen during the oxidation process.17–21 It is worth noting that these reactions are found to be near-complete with >95% yield of hydrogen based on the amount of metal. The low-price and safer Fe can be used as a potential material to replace the active Al and expensive Si for these metal–water hydrogen generation systems. In addition to considering the huge price gap between Fe and methanol, the 0.044 mmol mL−1 hydrogen volumetric density of the Fe–water system is still obviously higher than the value of the methanol–water system (0.037 mmol mL−1). However, the practical application of the Fe–water system for hydrogen generation is limited by the high reaction temperature (over 500 °C).18,21 Therefore, achieving hydrogen generation from Fe and water at room temperature is urgently needed.
Herein, we propose a strategy to realize hydrogen generation from the Fe–water system at room temperature through the catalysis of in situ formed Pd nanoparticles on the surface of Fe. The Fe nanoparticles were obtained by the reduction of FeCl2 with NaBH4 solution. After adding Pd2+ ions into the Fe nanoparticles and water system, hydrogen can release continually and steadily with a rate of 0.073 mmol gFe−1 min−1 at room temperature. The Fe nanoparticles and water system can yield 1.5 moles of H2 per mole of Fe after the reaction. Meanwhile, the Fe/water system can not only generate pure H2 molecules as the only gas to avoid the additional cost of gas separation and purification, but also produce Pd/Fe2O3 as efficient Pd heterogeneous catalysts. Therefore, the presence of a hydrogen generation system is a 100% atom economy system and in accordance with the concept of green chemistry in industrial applications.
Results and discussion
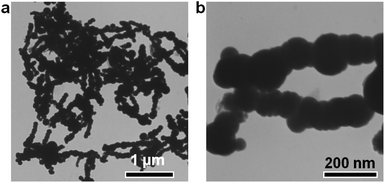
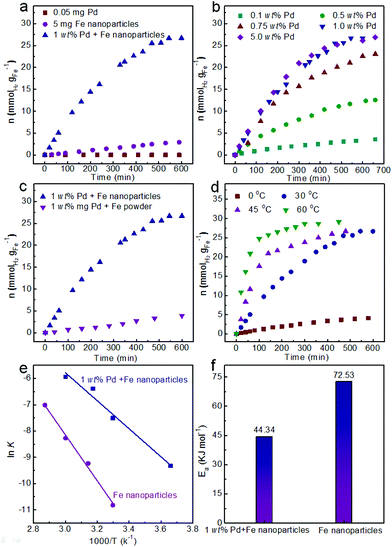
The Fe nanoparticles were synthesized by the reduction of FeCl2 with NaBH4 solution at room temperature. Transmission electron microscopy (TEM) was used to observe the morphology and size of the obtained Fe nanoparticles. From the TEM images, the Fe nanoparticles exhibited a spherical structure with a smooth surface and an average diameter of 105 ± 16 nm (Fig. 1a and b). The diffraction peaks at 2θ = 44, 65 and 82° can be indexed to the (110), (200) and (211) reflections of the Fe (PDF # 65-4899) from the XRD analysis (Fig. S1a†). After washing three times with deionized water under argon protection, the obtained Fe nanoparticles were re-dispersed in another 4 mL water. Then, the generated hydrogen could be tested by GC analysis.A series of optimal experiments were performed to explore the influence of various factors on the hydrogen generation rate. With 5 mg Fe nanoparticles in water (4 mL), hydrogen could slowly release with the rate of only 0.005 mmol gFe−1 min−1 at room temperature (Fig. 2a). After 600 min reaction, only 2.9 mmol gFe−1 of hydrogen was generated from the Fe/water system. In contrast, the hydrogen generation rate could be greatly enhanced to 0.073 mmol gFe−1 min−1 after adding 1 wt% Pd2+ ions (Fig. 2a), which was 14.6 times higher than the rate in the absence of Pd2+ ions under the same reaction conditions. And the final hydrogen yield could reach 26.7 mmol gFe−1 after 600 min reaction, as shown in Fig. 2a. Meanwhile, no hydrogen generated from the Pd2+ solution under the same reaction conditions, indicating the catalysis of Pd2+ ions in the Fe/water hydrogen generation system.
To further confirm the catalysis of Pd2+ ions, the hydrogen generation rates were tested in various amounts of initial Pd2+ ions, as shown in Fig. 2b. Obviously, the rate of hydrogen generation could be enhanced with the increasing amount of initial Pd2+ ions from 0.1 wt% to 1.0 wt%. Specifically, the rates of hydrogen generation were 0.0053 mmol gFe−1 min−1, 0.025 mmol gFe−1 min−1, 0.051 mmol gFe−1 min−1 and 0.073 mmol gFe−1 min−1 corresponding to 0.1 wt%, 0.5 wt%, 0.75 wt% and 1.0 wt% of initial Pd2+ ions, respectively (Fig. 2b). The enhanced rate of hydrogen generation could be attributed to the increasing initial Pd2+ ion catalysts. Meanwhile, after 600 min reaction, the yields of hydrogen also increased from 3.4 mmol gFe−1 to 12.3 mmol gFe−1, then to 22.6 mmol gFe−1 and finally to 26.7 mmol gFe−1, respectively. However, the hydrogen generation rate slightly enhanced from 0.073 mmol gFe−1 min−1 to 0.074 mmol gFe−1 min−1, when the initial Pd2+ ions further increased from 1 wt% to 5 wt% (Fig. 2b). Therefore, 1 wt% of initial Pd2+ ions was selected as the optimized reaction condition for hydrogen generation.
The nanoscale Fe nanoparticles also play multiple roles in hydrogen generation from the Fe/water system. Because of their high surface area, Fe nanoparticles are naturally expected to generate hydrogen more rapidly than bulk Fe. To expose their advantages, the commercial Fe powder with a diameter of 10 μm was also employed as the raw material for hydrogen generation. As shown in Fig. 2c, the hydrogen generation rate was only 0.0067 mmol gFe−1 min−1, which was 10.87 times lower than the rate of the Fe nanoparticles under the same reaction conditions. Obviously, the hydrogen generation rate was obviously improved with the Fe nanoparticles from the Fe/water system.
Meanwhile, the hydrogen generation rate was also affected by the reaction temperature. As shown in Fig. 2d, hydrogen generation rates were obviously enhanced with the increase in reaction temperature from 0 °C to 60 °C. It is noteworthy that the system could yield 0.011 mmol gFe−1 min−1 of hydrogen without the activation of light or electricity even at 0 °C reaction temperature, therefore it has wide applications in several areas and long-term prospects. When the reaction temperature increased to 30 °C, the rate of hydrogen generation was 0.073 mmol gFe−1 min−1. The hydrogen generation rates could further enhance to 0.19 mmol gFe−1 min−1 and 0.34 mmol gFe−1 min−1 at 45 °C and 60 °C, respectively. The final yield of hydrogen was about 26.7 mmol gFe−1 with 180 min, 480 min and 600 min corresponding to the reaction temperature of 60 °C, 45 °C and 30 °C, respectively.
The activation energy (Ea) indicates the degree of difficulty of the reaction from the kinetic analysis. It can be calculated from following eqn (1):
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = −Ea/RT + ln k = −Ea/RT + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A. A. | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and 1/T.
k and 1/T.
Based on the hydrogen generation at 0–60 °C and the zero-order reaction characteristic in the initial period (Fig. 2d and Fig. S2a†), k could be obtained from the slope of the fitting line and is summarized in Fig. S2b.† The Pd catalyzed Fe/water system followed a good linearity between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and 1/T, as shown in Fig. 2e. The corresponding slope of the plot yielded Ea. The calculated Ea was 44.34 kJ mol−1 for the Pd catalyzed Fe/water system (Fig. 2f). Meanwhile, the Ea for the Fe/water system in the absence of Pd catalysts was also calculated with a value of 72.53 kJ mol−1 (Fig. 2e, f and Fig. S2c and d†), which was 1.64 times higher than the value of the catalyzed system. The obviously reduced Ea also indicated the catalysis of Pd catalysts for the Fe/water system. Therefore, the enhanced hydrogen generation rate can also be understood from the value of Ea.
k and 1/T, as shown in Fig. 2e. The corresponding slope of the plot yielded Ea. The calculated Ea was 44.34 kJ mol−1 for the Pd catalyzed Fe/water system (Fig. 2f). Meanwhile, the Ea for the Fe/water system in the absence of Pd catalysts was also calculated with a value of 72.53 kJ mol−1 (Fig. 2e, f and Fig. S2c and d†), which was 1.64 times higher than the value of the catalyzed system. The obviously reduced Ea also indicated the catalysis of Pd catalysts for the Fe/water system. Therefore, the enhanced hydrogen generation rate can also be understood from the value of Ea.
As we all know, the Pd2+ ions can be easily reduced by Fe0 as shown in the following reaction eqn (2):
| Pd2+ + Fe0 = Pd0 + Fe2+. | (2) |
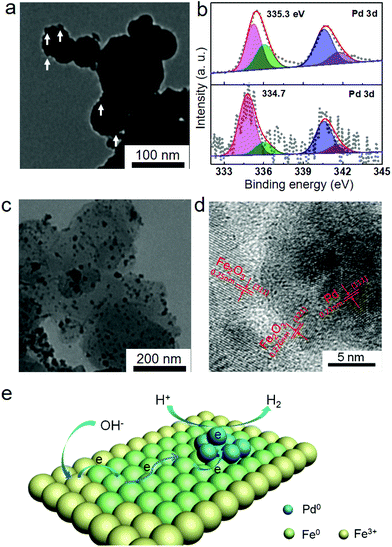
Therefore, the Pd2+ ions can be reduced to Pd nanoparticles by Fe nanoparticles. The concentration of Pd2+ ions in the Fe/water system after 1 h reaction could not be tested by ICP-OES analysis, which also indicated the complete reduction of Pd2+ ions to Pd nanoparticles by Fe nanoparticles. In order to further prove this assumption, the Fe nanoparticles after 1 h reaction were separated from the reaction system and then analyzed by TEM and XPS. As shown in Fig. 3a, the rough surface also indicated that small Pd nanoparticles were formed on the surface of Fe nanoparticles. Meanwhile, the binding energy of Pd(3d5/2) was 334.7 eV (Fig. 3b), indicating the metallic phase Pd on the obtained Fe nanoparticles. Therefore, the reduced Pd2+ ions were supported on the surface of Fe nanoparticles and formed the Pd/Fe nanoparticles.
After the hydrogen generation reaction, the solid products in the Fe/water system were also analyzed by XRD, TEM and XPS. As shown in Fig. S1b,† the peaks observed at 2θ = 30.2, 35.6, 43.2, 53.7, 57.2 and 62.9° were attributed to the (220), (311), (400), (422), (511) and (440) reflections of Fe2O3 (PDF # 39-1346), which indicated the transformation from initial Fe nanoparticles to Fe2O3 nanoparticles during the hydrogen generation process. The transformation could be further obtained from the TEM images of solid products at various reaction times. As shown in Fig. S3,† the Fe nanoparticles could be continually transferred to Fe2O3 with supported small Pd nanoparticles. Meanwhile, two different components could be observed from the TEM image of the final solid product (Fig. 3c). The small dark nanoparticles could be attributed to the Pd nanoparticles, while the large bright ones were Fe2O3 nanoparticles. After careful analysis of the HRTEM image (Fig. 3d), the measured planar spacing of 0.21 nm for the small nanoparticles was in good agreement with the (111) crystalline phase of Pd nanoparticles. The planar spacings of 0.25 nm and 0.226 nm for the large nanoparticles were in good agreement with the (311) and (321) crystalline phases of Fe2O3 nanoparticles, respectively. The metallic phase of Pd nanoparticles could be further confirmed from the 335.3 eV binding energy of Pd(3d5/2) by XPS analysis (Fig. 3b).
From the above analysis, the Fe nanoparticles could be transformed into Fe2O3 nanoparticles along with hydrogen generation, which indicates the valence state change of the Fe element from zero to plus three. Based on the final yield of hydrogen, one mole Fe could generate 1.5 moles H2. Therefore, three electrons per Fe atom were contributed to H+ and then formed hydrogen molecules. In the Fe/water system, the H element of generated hydrogen necessarily came from the H+ of ionization water. After receiving electrons from Fe nanoparticles, two reduced H+ ions could combine with each other releasing a hydrogen molecule immediately. During the hydrogen generation process, no other gas (such as O2, CO or CO2) was released from the Fe/water system and only hydrogen was tested by GC analysis, as shown in Fig. S4.† Meanwhile, the residual OH− ions would combine with an Fe3+ ion to form Fe(OH)3, which could be further transformed to Fe2O3 along with the dehydration reaction. The complete cyclic reaction equations are shown as follows (3)–(7):
| Fe0 = Fe3+ + 3e− | (3) |
| H2O = H+ + OH− | (4) |
| 2H+ + 2e− = H2↑ | (5) |
| Fe3+ + 3OH− = Fe(OH)3 | (6) |
| Fe(OH)3 = Fe2O3 + H2O. | (7) |
Generally, it is hard for Fe0 atoms in bulk or powder to lose three electrons to H+ and the reaction can be realized at high reaction temperature.18,21 Benefitting from the great surface energy of nanoscale Fe nanoparticles, it can slowly release hydrogen in the water without catalysts (as shown in Fig. 2a). Obviously, the electron transformation process can be enhanced after Pd nanoparticles are supported on the surface of Fe nanoparticles, resulting in the improvement of the hydrogen generation rate. Although Pd nanoparticles have been widely used as good catalysts for hydrogen generation, it is still hard to understand the increased hydrogen generation rate.9,10,22,23 A bimetallic structure, with one metal element exerting an influence on another metal element to provide some unique physical and chemical properties, is well documented from previous reports.9,24,25 Therefore, it is possible that the enhanced catalytic activity of Pd nanoparticles is derived from the retention of the desirable Pd surface feature, with electronic modification of Pd using Fe nanoparticles.
Herein, we have considered charge redistribution as the main factor for the enhanced catalytic activity. From XPS analysis, the binding energy of Pd(3d5/2) for the Pd/Fe nanoparticles (334.7 eV) was obviously lower than that for the Pd/Fe2O3 nanoparticles (335.3 eV), as shown in Fig. 3b. Therefore, the electronic density of Pd on Fe nanoparticles is obviously enhanced from the binding energy of Pd(3d5/2). The enhanced electronic density of Pd proved that the electrons transfer from Fe nanoparticles to the Pd nanoparticles. Meanwhile, the transferred electrons on Pd nanoparticles can reduce H+ in the solution and generate hydrogen molecules on the surface. After the electrons were consumed, some electrons from the Fe nanoparticles can be transferred on the Pd nanoparticles again. Therefore, the possible catalytic mechanism is shown in Fig. 3e. Firstly, the hydrogen generation reaction works on the surface of Pd nanoparticles. H+ ions can be reduced by the large amount of redundant electrons on the surface of Pd nanoparticles. Two reduced H+ ions can come into contact with each other and a hydrogen molecule can be generated. Then, the consumed electrons on Pd can be supplied by the neighbouring Fe0 atoms. After that, the Fe0 atoms neighbouring with Pd nanoparticles should be oxidized to Fe3+ atoms, which can be rapidly reduced to Fe0 atoms again after accepting the electrons from the outside Fe0 atoms. The redox process can continuously occur until consumption of all the electrons in Fe nanoparticles. Finally, Fe(OH)3 will form with the co-precipitation of Fe3+ ions and residual OH− ions, and the Fe2O3 nanoparticles are formed after decomposition of molecular water.
The hydrogen generation by the rich electrons on the surface of Pd at room temperature can be further confirmed by investigation of the reaction in the presence of electron scavenger KBrO3. As shown in Fig. 4, various concentrations of KBrO3 were added into the hydrogenation generation system after 50 min reaction. Before adding the electron scavenger, the Fe/water system exhibited almost the same hydrogen generation rate. However, the hydrogen generation rates would be obviously affected after the addition of KBrO3. When 0.03 mmol KBrO3 was added, the hydrogen generation rate decreased to 0.033 mmolH2 gFe−1 min−1 along with an obvious reduced yield of hydrogen. Meanwhile, the hydrogen generation would be completely blocked with the addition of 0.1 and 0.2 mmol KBrO3. Although the hydrogen generation rate was not reduced after the addition of 0.01 mmol KBrO3, the final yield of hydrogen was also significantly reduced compared with the system in the absence of KBrO3. The consumption of electrons by KBrO3 would compete with the reduction of H+ to generate hydrogen, leading to the reduced hydrogen generation rate. In addition, the decreased yield of hydrogen also proved the consumption of electrons by KBrO3.
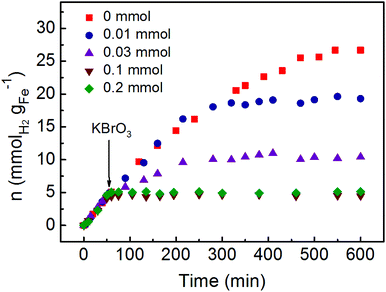 | ||
| Fig. 4 The hydrogen generation from the Fe/water system with various amounts of electron scavenger KBrO3. | ||
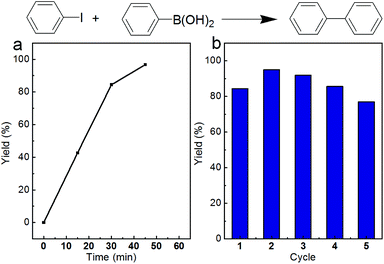
The final Pd/Fe2O3 nanoparticles are efficient heterogeneous catalysts for various organic reactions. Herein, the Suzuki C–C coupling reaction was selected as a model reaction to expose the catalytic activity for the Pd/Fe2O3 nanoparticles. The time course of biphenyl yield catalyzed by Pd/Fe2O3 at 60 °C is shown in Fig. 5a. The 96.7% yield of biphenyl was obtained after 45 min reaction. Catalytic stability of Pd/Fe2O3 nanoparticles is another important feature for heterogeneous catalysts. After being used for the Suzuki coupling reaction of iodobenzene and phenylboronic acid, the Pd/Fe2O3 nanoparticles could be recovered from the reaction solution via an external magnetic field and reused for the same reaction, due to the excellent magnetic properties. As shown in Fig. 5b, the Pd/Fe2O3 nanoparticles also exhibited excellent catalytic stability with almost no loss in catalytic activity after 5 cycles.
Conclusions
In summary, a new system for the safe, green and unlimited hydrogen generation is successfully realized by the redox reaction between water and cheap Fe nanoparticles catalyzed by Pd. There is stable release of hydrogen at the rate of 0.073 mmol gFe−1 min−1 at room temperature. Kinetics analysis indicates that the presence of Pd nanoparticles on the surface of Fe nanoparticles can effectively decrease the reaction activation energy (Ea) of the Fe/water system. The spontaneous electron transformation from Fe nanoparticles into supported Pd nanoparticles is the key factor for the hydrogen generation reaction. The H+ reduction and hydrogen generation occur on the surface of Pd nanoparticles with rich electrons. Meanwhile, finally Pd/Fe2O3 nanoparticles are found to yield a useful heterogeneous catalyst for the Suzuki C–C coupling reaction. Notably, hydrogen can be of high-purity according to the Fe/water system. Such an idea can open new opportunities for portable devices for outdoor use or in gas stations for hydrogen supply in industrial parks and in PEMC fuel cells.Experimental
Hydrogen generation reaction
25 mg FeSO4·7H2O was dissolved in 5 mL deionized water, and then another 5 mL of freshly prepared NaBH4 solution (1 mg mL−1) was added slowly with vigorous stirring. Fe nanoparticles were obtained by magnetic separation and washed with water three times under argon gas protection. After that the Fe nanoparticles were re-dispersed in another 5 mL water in a 30 mL flask by bubbling argon through the solution for 10 minutes. Finally, the Na2PdCl4 solution was added into the solution and the amount of hydrogen was obtained by GC.Characterization
The phase evolution of the as-synthesized nanostructures was monitored by powder X-ray diffraction (XRD). The XRD patterns with diffraction intensity versus 2θ were recorded on a Shimadzu X-ray diffractometer (Model 6000) using Cu Kα radiation. Transmission electron microscopy (TEM) studies were conducted on a Hitachi HT-7700 transmission electron microscope with an accelerating voltage of 120 kV. High-resolution TEM was performed on a JEM-2100F (JEOL, Japan) operating at 200 kV. X-ray photoelectron spectra (XPS) were acquired using a Thermo Electron Model K-Alpha with Al Kα as the excitation source. The contents of Pd and Au were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis (Agilent 7500ce).Suzuki C–C coupling reaction
Firstly, 0.5 mmol iodobenzene, 0.6 mmol phenylboronic acid and 1.5 mmol K2CO3 were added to a mixture of 3 mL of water and 2 mL of DMF. The reaction mixture was stirred at 60 °C for a desired time. The products were extracted with 12 mL ethyl acetate. Dodecane was used as the internal standard. After purification on a microcolumn filled with silica gel, the products were analyzed by GC-MS and GC.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the China Postdoctoral Science Foundation Grant 2017M620453, 2018T111067.Notes and references
- P. Jena, J. Phys. Chem. Lett., 2011, 2, 206–211 CrossRef CAS.
- L. Schlapbach and A. Zuttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- Y. J. Wang, N. Zhao, B. Fang, H. Li, X. T. Bi and H. Wang, Chem. Rev., 2015, 115, 3433–3467 CrossRef CAS PubMed.
- U. Eberle, M. Felderhoff and F. Schüth, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS PubMed.
- A. Rossin and M. Peruzzini, Chem. Rev., 2016, 116, 8848–8872 CrossRef CAS PubMed.
- L. Lili, Z. Wu, G. Rui, Y. Siyu, Z. Xiao, X. Wenqian, Z. Shijian, J. Zheng, Y. Qiaolin, L. Yongwang, S. Chuan, W. Xiaodong and M. Ding, Nature, 2017, 544, 80–85 CrossRef PubMed.
- M. Nielsen, E. Alberico, W. Baumann, H. J. Drexler, H. Junge, S. Gladiali and M. Beller, Nature, 2013, 495, 85–89 CrossRef CAS PubMed.
- A. Monney, E. Barsch, P. Sponholz, H. Junge, R. Ludwiga and M. Beller, Chem. Commun., 2014, 50, 707–709 RSC.
- K. Tedsree, T. Li, S. Jones, C. W. A. Chan, K. M. K. Yu, P. A. J. Bagot, E. A. Marquis, G. D. W. Smith and S. C. E. Tsang, Nat. Nanotechnol., 2011, 6, 302–307 CrossRef CAS PubMed.
- N. Wang, Q. Sun, R. Bai, X. Li, G. Guo and J. Yu, J. Am. Chem. Soc., 2016, 138, 7484–7487 CrossRef CAS PubMed.
- C. Broicher, S. R. Foit, M. Rose, P. J. C. Hausoul and R. Palkovits, ACS Catal., 2017, 7, 8413–8419 CrossRef CAS.
- Z. Li and Q. Xu, Acc. Chem. Res., 2017, 50, 1449–1458 CrossRef CAS PubMed.
- K. i. Fujita, R. Kawahara, T. Aikawa and R. Yamaguchi, Angew. Chem., Int. Ed., 2015, 54, 9057–9060 CrossRef CAS PubMed.
- A. Goeppert, M. Czaun, J. P. Jones, G. K. S. Prakash and G. A. Olah, Chem. Soc. Rev., 2014, 43, 7995–8048 RSC.
- D. KLiguras, D. IKondarides and X. EVerykios, Appl. Catal., B, 2003, 43, 345–354 CrossRef.
- Y. Yuan, Z. Yu, D. Chen and Z. Zou, Chem. Soc. Rev., 2017, 46, 603–631 RSC.
- H. Z. Wang, D. Y. C. Leung, M. K. H. Leung and M. Ni, Renewable Sustainable Energy Rev., 2009, 13, 845–853 CrossRef CAS.
- P. Charvin, S. Abanades, G. Flamant and F. Lemort, Energy, 2007, 32, 1124–1133 CrossRef CAS.
- C. E. Bunker, M. J. Smith, K. A. S. Fernando, B. A. Harruff, W. K. Lewis, J. R. Gord, E. A. Guliants and D. K. Phelps, ACS Appl. Mater. Interfaces, 2010, 2, 11–14 CrossRef CAS PubMed.
- F. Erogbogbo, T. Lin, P. M. Tucciarone, K. M. LaJoie, L. Lai, G. D. Patki, P. N. Prasad and M. T. Swihart, Nano Lett., 2013, 13, 451–456 CrossRef CAS PubMed.
- T. Kodama and N. Gokon, Chem. Rev., 2007, 107, 4048–4077 CrossRef CAS PubMed.
- M. Ye, J. Gong, Y. Lai, C. Lin and Z. Lin, J. Am. Chem. Soc., 2012, 134, 15720–15723 CrossRef CAS PubMed.
- K. Mori, M. Dojo and H. Yamashita, ACS Catal., 2013, 3, 1114–1119 CrossRef CAS.
- H. Zhang, M. Jin, J. Wang, W. Li, P. H. C. Camargo, M. J. Kim, D. Yang, Z. Xie and Y. Xia, J. Am. Chem. Soc., 2011, 133, 6078–6089 CrossRef CAS PubMed.
- J. Sun, A. M. Karim, H. Zhang, L. Kovarik, X. S. Li, A. J. Hensley, J. S. McEwen and Y. Wang, J. Catal., 2013, 306, 47–57 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More detailed information of kinetic data at various reaction temperatures, the XRD analysis of Pd/Fe2O3 and the GC analysis of obtained hydrogen. See DOI: 10.1039/c8qi01061g |
| This journal is © the Partner Organisations 2019 |