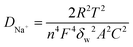Preparation of MoS2/Ti3C2Tx composite as anode material with enhanced sodium/lithium storage performance†
Guangyuan
Du
,
Mengli
Tao
,
Wei
Gao
,
Youquan
Zhang
,
Renming
Zhan
,
Shujuan
Bao
 * and
Maowen
Xu
* and
Maowen
Xu
 *
*
Key Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, Faculty of Materials and Energy, Southwest University, Chongqing 400715, P. R. China. E-mail: baoshj@swu.edu.cn; xumaowen@swu.edu.cn
First published on 12th November 2018
Abstract
MoS2/Ti3C2Tx composite was synthesized by a facile one-step hydrothermal method without further annealing process. When tested as anode material for sodium-ion batteries, it exhibited a high reversible capacity of 331 mA h g−1 at 100 mA g−1 after 70 cycles with only 0.058% decay per cycle. When tested as anode material for lithium-ion batteries, it exhibited a reversible capacity of 614.4 mA h g−1 at 100 mA g−1 after 70 cycles with only 0.05% decay per cycle. Moreover, compared with pristine MoS2 and pure Ti3C2Tx, the composite had better rate performance and faster ion diffusion kinetics, which might be caused by the as-prepared composite material having relatively rough surface, more active sites and more convenient diffusion paths.
Introduction
Developing environmentally friendly, economical, secure and sustainable energy source is the greatest societal and scientific challenge in the 21st century.1–3 To meet these challenges, it is an urgent mission to develop renewable and sustainable energy sources, such as water, solar and tidal power.4,5 However, these types of energy sources have inevitable shortcomings, such as instability and intermittency, and thus cannot be put into application directly. Therefore, energy storage and conversion devices have great significance for smooth integration of renewable energy sources into power grids. Specifically, lithium-ion batteries (LIBs) are playing an important role in powering many electronic devices in our daily lives from laptops to electric vehicles. LIBs have many merits, such as long cycle life, no memory effect, minor self-discharge and environment friendliness. However, the supply of natural lithium sources is limited. In recent years, taking sodium's cost and abundance into consideration, sodium-ion batteries (SIBs) have been regarded as a promising alternative to LIBs. Though many breakthroughs have been achieved, SIBs still have been relatively slowly developed and pales in comparison with that of LIBs.5 Therefore, in the foreseeable future, SIBs will draw more research attention while LIBs will still be the center of the commercial market. As a consequence, electrode materials for LIBs/SIBs with merits such as high reversible capacity, long lifespan and good electronic conductivity are promising.6In terms of anode materials, graphene has excellent electrochemical performance due to its two-dimensional structure, which facilitates the insertion/extraction of ions between layers.7–9 As a new type of two-dimensional material, MXene has received great attention since it was first reported in 2011 by Naguib et al.10 MXene is obtained by selectively etching the atom layers (such as Al or Si) from MAX phase with hydrofluoric acid. M represents early transition metal elements, X represents carbon and/or nitrogen and Tx represents the surface hydrophilic groups in the formula Mn+1XnTx (n = 1, 2, 3) of MXene.10 The applications of MXene are wide, including energy storage11 and catalysis.12 MXene possesses a surface with abundant functional groups, and it has merits such as high electronic conductivity and low ion diffusion resistance in LIBs/SIBs.13–15 Nevertheless, pure MXene suffers from low capacity when used as LIBs/SIBs anode material, which is an obstacle for its application. Fortunately, it has surface hydrophilic groups (such as −OH, −F, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and a layered structure; hence, appropriate strategies can be devised to synthesize MXene composites with improved capacity. Many studies with the purpose of improving the reversible capacity of MXene composites have been reported,16,17e.g., preparation of SnS/Ti3C2Tx composites.18 Therefore, combining sulfide with Ti3C2Tx is a worthy idea to improve the capacity of MXene.
O) and a layered structure; hence, appropriate strategies can be devised to synthesize MXene composites with improved capacity. Many studies with the purpose of improving the reversible capacity of MXene composites have been reported,16,17e.g., preparation of SnS/Ti3C2Tx composites.18 Therefore, combining sulfide with Ti3C2Tx is a worthy idea to improve the capacity of MXene.
With the aim of developing secondary batteries for energy storage, electrode materials with long life span and low cost are favorable. MoS2 is a type of layered transition metal dichalcogenide. It is an ideal electrode material for LIBs/SIBs because it has much higher capacity than traditional commercial materials. Particularly, Mo atoms have many different oxidation states, and MoS2 has favorable charge storage capability.19
However, in the process of ion insertion/extraction, pristine MoS2 suffers drastic capacity fading caused by large volume change owing to its large surface energy, leading to the stacking and aggregation of MoS2 layers. Furthermore, with only a few accessible active sites and low electronic conductivity, MoS2 has limited rate performance in LIBs/SIBs. In contrast, Ti3C2Tx has excellent electronic conductivity but lower capacity. Hence, combining Ti3C2Tx with MoS2 may combine the merits of these two materials by synergistic effect.16,20–22
In this work, MoS2/Ti3C2Tx composite was synthesized by a facile one-step hydrothermal method. MoS2 nanosheets anchored on Ti3C2Tx layers uniformly to create a hierarchical structure, which enhanced the capacity of Ti3C2Tx and the cycling stability of the MoS2 nanosheets due to the synergistic effect. Furthermore, MoS2/Ti3C2Tx composite, with the enlarged interlayer space of Ti3C2Tx and a rough surface, was conducive to ion diffusion and expanded the electrode–electrolyte contact surface area. Therefore, when used as anode material for SIBs and LIBs, impressive cycling stability, excellent rate performance and faster ion diffusion kinetics were achieved owing to the helpful synergistic effect.
Results and discussion
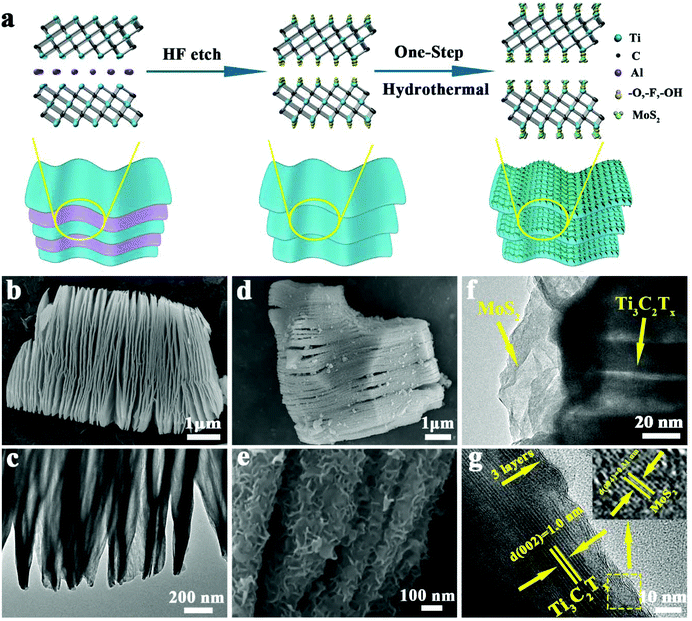
The MoS2/Ti3C2Tx composite was synthesized by a facile one-step hydrothermal method without further annealing process. A schematic of the synthesis is shown in Fig. 1a. First, during the selective etching process, Al atom layers were removed from the MAX (Ti3AlC2) phase by HF solution and a loosely accordion-like MXene (Ti3C2Tx) phase was obtained. Then, the as-prepared Ti3C2Tx powder, Na2MoO4·2H2O and CH3CSNH2 were dispersed into deionized water, due to which the functional groups (−F, −OH,![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) on the surface of Ti3C2Tx could firmly attach to the Mo6+ derived from Na2MoO4·2H2O. Subsequently, CH3CSNH2 acted as a reductant and sulfur source; then, MoS2 nanosheets were in situ deposited on the Ti3C2Tx substrate. This method has the following two merits: it required no further annealing process, and MoS2 nanosheets could firmly and uniformly be decorated onto the Ti3C2Tx substrate.
O) on the surface of Ti3C2Tx could firmly attach to the Mo6+ derived from Na2MoO4·2H2O. Subsequently, CH3CSNH2 acted as a reductant and sulfur source; then, MoS2 nanosheets were in situ deposited on the Ti3C2Tx substrate. This method has the following two merits: it required no further annealing process, and MoS2 nanosheets could firmly and uniformly be decorated onto the Ti3C2Tx substrate.
Fig. 1b and Fig. S1† are the FESEM images of pure Ti3C2Tx after the etching process. The structure appears accordion-like, where each layer of Ti3C2Tx is similar to graphene's two-dimensional structure and forms a hierarchical structure. The TEM image (Fig. 1c) further confirms the hierarchical structure of pure Ti3C2Tx.
The FESEM images of the MoS2/Ti3C2Tx composite are shown in Fig. 1d and e. They demonstrate that MoS2/Ti3C2Tx composite has a structure with MoS2 nanosheets uniformly distributed on the Ti3C2Tx substrate. The energy dispersive X-ray mapping of the MoS2/Ti3C2Tx composite is shown in Fig. S2,† which illustrates the uniform distribution of Ti (green), Mo (red) and S (yellow) on the surface and interlayers of Ti3C2Tx substrates, and further confirms the presence of MoS2. Moreover, it can be clearly seen in Fig. 1e that a large number of MoS2 nanosheets were deposited in Ti3C2Tx interlayers, providing many sites for ion intercalation and shortening the paths for ion diffusion. The FESEM image of pristine MoS2 is shown in Fig. S3,† in which the MoS2 nanosheets stacked closely with each other and few active sites were accessible. Further observation of MoS2/Ti3C2Tx composite by TEM is shown in Fig. 1f, which confirmed that MoS2 nanosheets were attached on the Ti3C2Tx layers. HRTEM images, as shown in Fig. 1g and inset, reveal spacings of the lattice fringe as 0.31 nm and 1.0 nm, corresponding to the (004) plane of MoS2 and the (002) plane of Ti3C2Tx. Nevertheless, it is evident that MoS2 nanosheets have a hierarchical structure with a few (∼3–5) monolayers. The coupling of few-monolayered MoS2 nanosheets and highly conductive substrates such as graphene not only accelerates the electron transfer but also improves capacity and cycling stability.19 Therefore, it is a worthy idea to combine MoS2 nanosheets with a highly conductive substrate such as Ti3C2Tx in order to achieve electrode materials with higher capacity, longer lifespan, lower electronic diffusion resistance and faster ion diffusion kinetics.
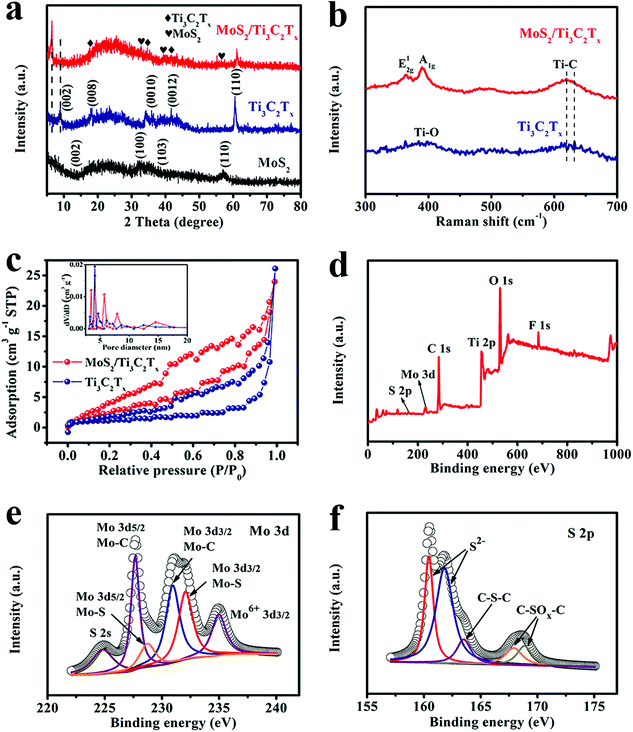
The XRD patterns of MoS2/Ti3C2Tx composite, pure Ti3C2Tx and pristine MoS2 are shown in Fig. 2a. The XRD pattern of pure Ti3C2Tx clearly exhibits the planes of (002), (008), (0010), (0012) and (110), which are in agreement with the previously reported data.23 In the XRD pattern of MoS2/Ti3C2Tx composite, the MoS2 diffraction peaks are clearly visible. Diffraction peaks at 2θ = 33.8°, 39.4° and 57.1° correspond to (100), (103) and (110) planes of MoS2 (JCPDS No. 37-1492), which are consistent with a previous report.24 It should be mentioned that the peak intensity of (002) signifies a structure of stacked layers.25 However, the (002) peak (2θ = 14.378°) is not observed in this work, illustrating that the obtained MoS2 is a very thin layered structure, which is consistent with the HRTEM image (Fig. S4†). The characteristic diffusion peaks of MoS2 and Ti3C2Tx were well retained in the MoS2/Ti3C2Tx composite, indicating successful synthesis of the MoS2/Ti3C2Tx composite. On comparing the XRD patterns of Ti3C2Tx and MoS2/Ti3C2Tx composite materials, we can see that the diffusion peaks of MoS2/Ti3C2Tx have lower intensity; this illustrates that Ti3C2Tx flakes have been effectively modified by MoS2 nanosheets. Moreover, the XRD pattern of the MoS2/Ti3C2Tx composite illustrates that the (002) peak of Ti3C2Tx shifts to a lower angle, indicating that the Ti3C2Tx interplanar spacing increased, which may facilitate the diffusion of ions. It is noticeable that there is a TiO2 diffraction peak at 61°, which may have evolved due to exposing the composite to air.
The Raman spectra of pure Ti3C2Tx and MoS2/Ti3C2Tx composite are shown in Fig. 2b. For pure Ti3C2Tx, the Raman modes located at 410 cm−1 and 638 cm−1 represent the vibrations from Ti–O and Ti–C bonds, respectively. It is clear that there are two typical peaks in the Raman spectrum of the MoS2/Ti3C2Tx composite: the E12g peak at 364.7 cm−1, which originate from the vibration of Mo–S in-plane mode and the A1g peak at around 389.6 cm−1 from out-of-plane vibrations, which are typical first-order Raman active modes E12g and A1g due to in-plane vibrational modes within the S–Mo–S layer.25 Notably, there is a shift in the peak of Ti–C to lower energy, implying the appearance of strong interactions at the interfaces between the MoS2 nanosheets and Ti3C2Tx layers.
As shown in Fig. 2c is the comparison of N2 adsorption/desorption curves. The specific surface areas of Ti3C2Tx and MoS2/Ti3C2Tx composite are 4.0 m2 g−1 and 12.91 m2 g−1, respectively. This enhancement can be described as the intercalation of MoS2 nanosheets into Ti3C2Tx interlayers and anchoring on the surface. Pore size distribution (inset of Fig. 2c) data demonstrated that the pores of MoS2/Ti3C2Tx composite were mainly mesoporous. The increased specific surface area and pore size distribution of MoS2/Ti3C2Tx composite were beneficial to expand the electrode–electrolyte contact area and contributed more active sites to improve the electrochemical performance.
To detect the valences and chemical components of the MoS2/Ti3C2Tx composite, XPS analysis was performed. From the survey spectrum of MoS2/Ti3C2Tx composite in Fig. 2d, the peaks of elements sulfur, molybdenum, carbon, titanium, oxygen and fluoride can be clearly seen. The Mo 3d region is displayed in Fig. 2e, in which the two peaks centered at 228.78 eV and 232.08 eV correspond to Mo 3d5/2 (Mo–S bond) and Mo 3d3/2 (Mo–S bond), respectively.26 The peak at 224.98 eV corresponds to S 2s of MoS2. The other two peaks at 227.68 eV and 230.98 eV are ascribed to Mo 3d5/2 (Mo–C bond) and Mo 3d3/2 (Mo–C bond), respectively. Mo 3d5/2 and Mo 3d3/2 are the characteristic Mo4+ oxidation states of MoS2, while the peak at 235.1 eV corresponds to MoO3 and MoO42−, indicating that the MoS2/Ti3C2Tx composite was partly oxidized due to preparation and exposure to air. Fig. 2f shows the S 2p spectrum. The two main peaks at 160.5 eV and 161.8 eV are ascribed to the characteristic S2− species in MoS2, while the peak at 163.6 eV corresponds to C–S–C bonding, which illustrates the firm binding between MoS2 nanosheets and Ti3C2Tx substrate. The other two peaks at 167.9 eV and 168.9 eV coincide with C–Sox–C bonding, which may occur due to the exposure of the composite to air.27 All the XPS analysis results confirm that the MoS2/Ti3C2Tx composite was successfully synthesized and the MoS2 nanosheets had firm interfacial contact with the Ti3C2Tx layers, which could maintain the stable structure and ensure steady cycling performance.
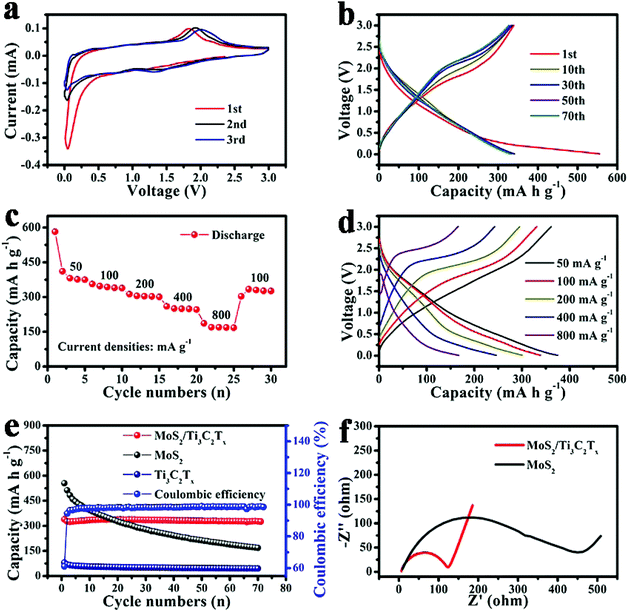
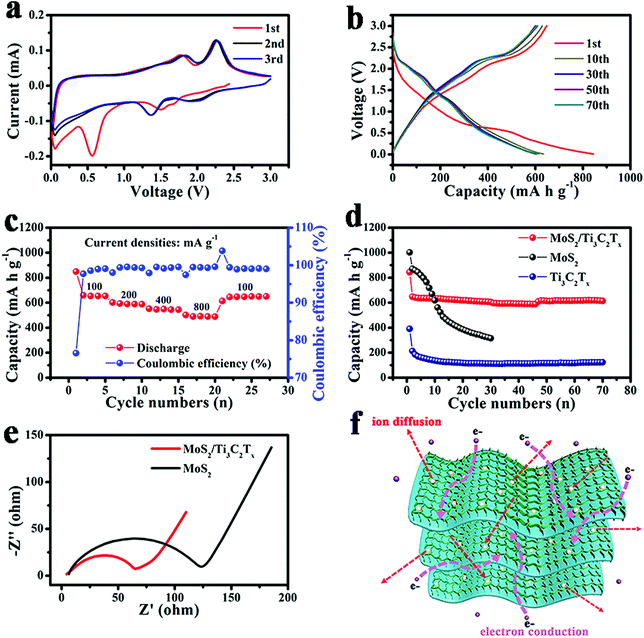
In order to determine the advantages of the MoS2/Ti3C2Tx composite, it was used as the anode material for sodium-ion batteries. Fig. 3a shows the CV curves of the first three cycles. In the first cathodic process, an irreversible peak at around 0.06 V could be ascribed to the decomposition of the electrolyte and formation of the solid electrolyte interface (SEI). In the second and third cathodic process, a distinct peak at around 1.32 V can be associated with Na+ insertion into the MoS2 lattice. Moreover, the peaks overlap very well, which demonstrates a highly reversible reaction between MoS2 and Na+. In contrast, there is one oxidation peak at around 1.83 V in the first cycle, which could be ascribed to the reversible reaction of Na2S to polysulfide,28 and the oxidation peak shifting to a higher voltage in the following two cycles should be ascribed to the polarization of the electrode.
Fig. 3b shows the galvanostatic charge and discharge voltage profiles of the MoS2/Ti3C2Tx composite at 100 mA g−1. The charge and discharge capacities of the MoS2/Ti3C2Tx composite in the first cycle were 339 mA h g−1 and 543 mA h g−1, respectively, with a coulombic efficiency of only 62.4%. Formation of SEI is the cause of the initial irreversible capacity. In the subsequent cycles, there is one charge plateau and one discharge plateau, which corresponds to the cyclic voltammetry analysis.
Fig. 3c and d show the rate performance and the corresponding charge and discharge curves of the MoS2/Ti3C2Tx composite, which delivered high discharge capacities of 411 mA h g−1, 356.2 mA h g−1, 312.6 mA h g−1, 259.6 mA h g−1 and 186.2 mA h g−1 at 50 mA g−1, 100 mA g−1, 200 mA g−1, 400 mA g−1 and 800 mA g−1, respectively. It is worth mentioning that the electrode can still deliver a reversible capacity of 333.6 mA h g−1 when the current density returned to 100 mA g−1 almost without capacity decay. The rate performance and the corresponding charge–discharge curves of pristine MoS2 and pure Ti3C2Tx are shown in Fig. S5.† It is clearly demonstrated that the pristine MoS2 has a higher reversible discharge capacity but suffers fast capacity decay during cycling, while pure Ti3C2Tx is stable but its capacity is too low for its application. The excellent rate performance illustrates that the MoS2/Ti3C2Tx composite electrode could maintain a stable structure even under a high current density. Furthermore, the performance could also be explained as the result of synergistic effect. On the one hand, the Ti3C2Tx substrate prevented the stacking of MoS2 nanosheets during the cycling and contributed more active sites for the reversible actions. On the other hand, the MoS2 nanosheets anchored in the Ti3C2Tx interlayers expanded the space of the interlayers to improve the electrode–electrolyte contact area and shorten the ion diffusion paths. As a consequence, an excellent rate performance was obtained.
The cycling performances of the MoS2/Ti3C2Tx composite, pristine MoS2 and pure Ti3C2Tx are shown in Fig. 3e. The MoS2/Ti3C2Tx composite electrode still had a high reversible capacity of 331 mA h g−1 even after 70 cycles at 100 mA g−1, with a low capacity decay rate of only ∼0.058% per cycle. Pristine MoS2 and pure Ti3C2Tx delivered reversible capacities of only 170.7 mA h g−1 and 45.7 mA h g−1, respectively. Additionally, coulombic efficiency of the MoS2/Ti3C2Tx composite electrode remained at around 100% during cycling, proving that the MoS2/Ti3C2Tx composite possessed stable structural integrity. Moreover, it is evident that the MoS2/Ti3C2Tx composite electrode had a more stable cycling performance than pristine MoS2 and a much higher charge/discharge capacity than pure Ti3C2Tx. Therefore, the MoS2/Ti3C2Tx composite is an electrode material with great promise for sodium-ion batteries.
Fig. 3f shows the comparison of the electrochemical impedance spectra (EIS) of MoS2/Ti3C2Tx composite and pristine MoS2 used as anode materials for sodium-ion batteries and Fig. S6† displays the dependence of reciprocal square root of angular frequency on the real impedance in the low frequencies of MoS2/Ti3C2Tx composite and pristine MoS2. According to the equation:
| Z′ = Rs + Rct + δwω−1/2 |
under the same conditions, the diffusivity of Na+ (DNa+) is inversely proportional to δw2. δw2 of the MoS2/Ti3C2Tx composite was smaller than that of pristine MoS2; therefore, the diffusivity of Na+ in the MoS2/Ti3C2Tx composite is faster than that in pristine MoS2. The faster diffusion kinetics of the MoS2/Ti3C2Tx composite may be due to the unique structure of MoS2 nanosheets uniformly decorated on Ti3C2Tx layers. The Ti3C2Tx substrate with expanded interlayer space favored the electrode–electrolyte contact, while the ultrathin MoS2 nanosheets shortened the ion diffusion path; as a consequence, fast reaction kinetics is obtained.
The same electrochemical measurements were adopted in lithium-ion batteries to prove the advantages of the MoS2/Ti3C2Tx composite.
Fig. 4a shows the first three CV curves of the MoS2/Ti3C2Tx composite with a scan rate of 0.1 mV s−1. In the first cycle, it is clear that there is an irreversible reduction peak at around 0.6 V that disappeared in the subsequent cycles, which could be ascribed to the lattice transformation from triangular prism into octahedral structure. The reason for the irreversibility is that the nearly amorphous MoS2 only reformed after the first charge process.24,29 The reduction peaks at around 1.4 V in the second and third cycles correspond to the reaction MoS2 + 4Li + 4e− → Mo + 2Li2S. Moreover, a broad reduction peak is located at around 2.0–2.5 V. In the anodic scanning, the distinguishable peaks at around 1.8 V and 2.3 V can be ascribed to lithium extraction and oxidation of Mo to MoS2.28 It is noticeable that the CV curves of the second and the third cycles are looped very well, which illustrates that the action between the MoS2/Ti3C2Tx composite and Li+ is highly reversible.
Fig. 4b shows the galvanostatic charge and discharge voltage profiles at 100 mA g−1. The charge and discharge capacities of the first cycle are 647.2 mA h g−1 and 843.7 mA h g−1, respectively, and the coulombic efficiency is 76.7%. The drastic initial irreversible capacity may be obtained because of the decomposition of electrolyte and formation of SEI. It is clear that the discharge profiles have two plateaus at around 2.0–2.5 V and 1.4 V. Moreover, the charge profiles have two plateaus at around 1.8 V and 2.3 V, which corresponds to the cyclic voltammetry analysis.
The rate performance and corresponding charge/discharge curves are shown in Fig. 4c and Fig. S7,† respectively. The discharge capacities at 100 mA g−1, 200 mA g−1, 400 mA g−1 and 800 mA g−1 are 653 mA h g−1, 587 mA h g−1, 544 mA h g−1 and 488 mA h g−1, respectively, with coulombic efficiency being almost 100%. Impressively, the MoS2/Ti3C2Tx composite electrode maintained reversible capacity of 645 mA h g−1, with almost no capacity decay, when the current density returned to 100 mA g−1. It was speculated that the MoS2/Ti3C2Tx composite electrode had a stable structure even under high current density. The rate performances of pristine MoS2 and pure Ti3C2Tx and the corresponding charge–discharge curves are shown in Fig. S8.† The drastic decay of reversible discharge capacity of pristine MoS2 when the current density was turned to 100 mA g−1 illustrates that the structure of pristine MoS2 is not stable. Pure Ti3C2Tx had a low capacity of 170.4 mA h g−1, making it difficult for use in practical applications. Therefore, the MoS2/Ti3C2Tx composite has the best rate performance of all the three electrode materials.
The cycling performance is exhibited in Fig. 4d. The MoS2/Ti3C2Tx composite could maintain a reversible capacity of 614.4 mA h g−1 at 100 mA g−1 after 70 cycles with only a 0.05% decay per cycle. In contrast, pristine MoS2 suffered a drastic capacity decay of 63.8% after only 30 cycles, while pure Ti3C2Tx delivered a very low capacity of 123.2 mA h g−1. The MoS2/Ti3C2 composite had the best cycling performance of all the three materials, which was attributed to its unique structure and synergistic effect. On the one hand, the MoS2 nanosheets with ultrathin thickness shortened the diffusion paths of Li+ and promoted the intercalation and accommodation of Li+. On the other hand, the Ti3C2Tx substrate prevented the stacking of MoS2 nanosheets and maintained the stability of the structure during the cycling process. Furthermore, the Ti3C2Tx substrate had lower resistance for electron conduction, which also promoted the cycling stability of the MoS2/Ti3C2Tx composite.
The comparison of electrochemical impedance spectra (EIS) of MoS2/Ti3C2Tx composite and pristine MoS2 as anodes for lithium-ion batteries is shown in Fig. 4e. The semicircles at high and medium frequencies can be described as the resistance of charge-transfer and SEI film, while the sloping line at low-frequency corresponds to the diffusion of lithium ions. Similar to the MoS2/Ti3C2Tx composite being used as the anode material in sodium-ion batteries, when used in lithium-ion batteries, the composite electrode exhibited a smaller semicircle and a steeper line than the pristine MoS2 electrode, which implied that the MoS2/Ti3C2Tx composite electrode had smaller electrochemical impedance and faster Li+ diffusion kinetics than pristine MoS2. Overall, the superior electrochemical performance of MoS2/Ti3C2Tx could be ascribed to the unique structure and synergistic effect, as shown in Fig. 4f, in which two-dimensional MoS2 nanosheets are uniformly decorated on the Ti3C2Tx layers. First, the MoS2 nanosheets shortened the ionic diffusion paths and contribute more active sites for ion intercalation. Second, the improved specific surface area facilitated electrode–electrolyte contact. Finally, Ti3C2Tx matrix promoted electronic conduction and prevented the stacking of MoS2 nanosheets, which also enhanced the electrochemical performance.
Experimental
Preparation of the MoS2/Ti3C2Tx composite
Ti3C2Tx powder was obtained as described in our previous study.18The MoS2/Ti3C2Tx composite was synthesized by a facile one-step hydrothermal method. In detail, 100 mg Ti3C2Tx powder was dispersed in 36 mL DI water with 192 mg Na2MoO4·2H2O and 66 mg CH3CSNH2 under mechanical stirring at room temperature for 1 h. Then, the suspension was transferred into a Teflon-lined stainless-steel autoclave and kept at 180 °C for 12 h. After cooling, the product was washed with ethyl alcohol and DI water several times and collected by filtration, and then dried in a vacuum oven at 60 °C for 12 h. For comparison, pristine MoS2 was synthesized by the same method without Ti3C2Tx.
Characterization of materials
The morphologies of the MoS2/Ti3C2Tx composite were observed by field emission electron microscopy (FESEM, JSM-7800F, Japan) and transmission electron microscopy (TEM, JEM-2100, Japan). X-ray diffraction (XRD MAXima-X XRD-7000) patterns were recorded with Cu Kα radiation (λ = 1.5406 Å). Raman spectra were recorded by Invia Refl (Renishaw, UK) with a laser wavelength of 532 nm. X-ray photoelectron spectroscopy was conducted on an electron spectrometer (ESCALAB 250Xi). The specific surface area and distribution of pore size were determined by Quadrasorb evo 2QDS-MP-30 (Quantachrome Instruments, USA). The elemental analysis was performed on an energy dispersive X-ray spectrometer attached to a SEM instrument.Electrochemical measurements
MoS2/Ti3C2Tx composite, pure Ti3C2Tx and pristine MoS2 were tested as anode materials in SIBs and LIBs by assembling CR2032 type coin cells in an argon filled glovebox with sodium and lithium metals as counter electrodes. Typically, the anode electrodes were prepared by a slurry coating procedure. In detail, the as-prepared material was mixed with polyvinylidene fluoride (PVDF) and acetylene black (AB) with a mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidinone (NMP) to get a slurry. Following this, the slurry was uniformly coated on the copper foil current collector, and then dried under vacuum at 120 °C for 12 h. The area of each electrode is 1.13 cm2.
1 in N-methyl-2-pyrrolidinone (NMP) to get a slurry. Following this, the slurry was uniformly coated on the copper foil current collector, and then dried under vacuum at 120 °C for 12 h. The area of each electrode is 1.13 cm2.
Celgard 2400 was used as a separator and 1 M NaClO4 dissolved in a mixed solution of diethyl carbonate (DEC) and ethylene carbonate (EC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was used as the electrolyte. Galvanostatic charge and discharge cycling was tested between 0.01 V and 3.0 V vs. Na/Na+ and Li/Li+ on the Land battery test system. The rate performance was tested with a variety of current densities. Cyclic voltammetry (CV) was conducted between 0.01 V and 3.0 V with a scanning rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS) was conducted on an electrochemical workstation (CHI 660C). All electrochemical measurements were conducted under normal environment.
1, v/v) was used as the electrolyte. Galvanostatic charge and discharge cycling was tested between 0.01 V and 3.0 V vs. Na/Na+ and Li/Li+ on the Land battery test system. The rate performance was tested with a variety of current densities. Cyclic voltammetry (CV) was conducted between 0.01 V and 3.0 V with a scanning rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS) was conducted on an electrochemical workstation (CHI 660C). All electrochemical measurements were conducted under normal environment.
Conclusions
In summary, the MoS2/Ti3C2Tx composite was successfully synthesized by a facile one-step hydrothermal method. The MoS2 nanosheets were tightly and uniformly anchored on the Ti3C2Tx layers. Benefiting from the rough surface, firm contact between MoS2 nanosheets and Ti3C2Tx layers, improved number of active sites, and high electrical conductivity of Ti3C2Tx substrate, the MoS2/Ti3C2Tx composite as anode material for sodium/lithium ion batteries delivered high reversible discharge capacity, better rate performance and lower electrochemical impedance than those of pristine MoS2 and pure Ti3C2Tx. Therefore, we think that the MoS2/Ti3C2Tx composite is an anode material for SIBs and LIBs with great promise.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by grants from the National Natural Science Foundation of China (No. 21773188), Fundamental Research Funds for the Central Universities (XDJK2017A002, XDJK2017B048) and Program for Innovation Team Building at Institutions of Higher Education in Chongqing (CXTDX201601011).References
- L. Y. Wen, M. Zhou, C. L. Wang, Y. Mi and Y. Lei, Adv. Energy Mater., 2016, 6, 1600468 CrossRef.
- V. R. Stamenkovic, D. Strmcnik, P. P. Lopes and N. M. Markovic, Nat. Mater., 2017, 16, 57–69 CrossRef CAS PubMed.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- X. Wang, H.-M. Kim, Y. Xiao and Y.-K. Sun, J. Mater. Chem. A, 2016, 4, 14915–14931 RSC.
- Y. Zhao, X. F. Li, B. Yan, D. B. Xiong, D. J. Li, S. Lawes and X. L. Sun, Adv. Energy Mater., 2016, 6, 1502175 CrossRef.
- Y. Q. Fu, Q. L. Wei, G. X. Zhang and S. H. Sun, Adv. Energy Mater., 2018, 8, 1870057 CrossRef.
- K.-X. Sheng, Y.-X. Xu, C. Li and G.-Q. Shi, New Carbon Mater., 2011, 26, 9–15 CrossRef CAS.
- L. Zhang and G. Q. Shi, J. Phys. Chem. C, 2011, 115, 17206–17212 CrossRef CAS.
- H. Shan, X. F. Li, Y. H. Cui, D. B. Xiong, B. Yan, D. J. Li, A. Lushington and X. L. Sun, Electrochim. Acta, 2016, 205, 188–197 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed.
- X. J. Li, G. Y. Fan and C. M. Zeng, Int. J. Hydrogen Energy, 2014, 39, 14927–14934 CrossRef CAS.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. Dall'Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- Q. Tang, Z. Zhou and P. W. Shen, J. Am. Chem. Soc., 2012, 134, 16909–16916 CrossRef CAS PubMed.
- C. C. Leong, H. Pan and S. K. Ho, Phys. Chem. Chem. Phys., 2016, 18, 7527–7534 RSC.
- C. J. Shen, L. B. Wang, A. G. Zhou, H. Zhang, Z. H. Chen, Q. K. Hu and G. Qin, J. Electrochem. Soc., 2017, 164, A2654–A2659 CrossRef CAS.
- M. L. Tao, Y. Q. Zhang, R. M. Zhan, B. S. Guo, Q. J. Xu and M. W. Xu, Mater. Lett., 2018, 230, 173–176 CrossRef CAS.
- Y. Q. Zhang, B. S. Guo, L. Y. Hu, Q. J. Xu, Y. Li, D. Y. Liu and M. W. Xu, J. Alloys Compd., 2018, 732, 448–453 CrossRef CAS.
- D. W. Su, S. X. Dou and G. X. Wang, Adv. Energy Mater., 2015, 5, 1401205 CrossRef.
- Y. T. Wu, P. Nie, J. M. Jiang, B. Ding, H. Dou and X. G. Zhang, ChemElectroChem, 2017, 4, 1560–1565 CrossRef CAS.
- K. Ma, H. Jiang, Y. J. Hu and C. Z. Li, Adv. Funct. Mater., 2018, 1804306 CrossRef.
- M. Zheng, R. S. Guo, Z. C. Liu, B. Y. Wang, L. C. Meng, F. Y. Li, T. T. Li and Y. N. Luo, J. Alloys Compd., 2018, 735, 1262–1270 CrossRef CAS.
- F. Wang, C. H. Yang, C. Y. Duan, D. Xiao, Y. Tang and J. F. Zhu, J. Electrochem. Soc., 2015, 162, B16–B21 CrossRef CAS.
- C. Li, J. Q. Li, Z. J. Wang, S. Zhang, G. J. Wei, J. Zhang, H. Wang and C. H. An, Inorg. Chem. Front., 2017, 4, 309–314 RSC.
- M. W. Xu, F. L. Yi, Y. B. Niu, J. L. Xie, J. K. Hou, S. G. Liu, W. H. Hu, Y. T. Li and C. M. Li, J. Mater. Chem. A, 2015, 3, 9932–9937 RSC.
- T. Zheng, G. D. Li, J. H. Dong, Q. Q. Sun and X. G. Meng, Inorg. Chem. Front., 2018, 5, 1587–1593 RSC.
- Y. Q. Zhang, R. M. Zhan, Q. J. Xu, H. Liu, M. L. Tao, Y. S. Luo, S. J. Bao, C. M. Li and M. W. Xu, Chem. Eng. J., 2019, 357(1), 220–225 CrossRef CAS.
- W. J. Tang, X. L. Wang, Y. Zhong, D. Xie, X. Q. Zhang, X. H. Xia, J. B. Wu, C. D. Gu and J. P. Tu, Chem. – Eur. J., 2018, 24, 11220–11226 CrossRef CAS PubMed.
- S. J. Ding, D. Y. Zhang, J. S. Chen and X. W. Lou, Nanoscale, 2012, 4, 95–98 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qi01081a |
| This journal is © the Partner Organisations 2019 |