M24+ paddlewheel clusters as junction points in single-chain nanoparticles†
Nicolai D.
Knöfel‡
 a,
Hannah
Rothfuss‡
a,
Hannah
Rothfuss‡
 b,
Christopher
Barner-Kowollik
b,
Christopher
Barner-Kowollik
 *bc and
Peter W.
Roesky
*bc and
Peter W.
Roesky
 *a
*a
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstrasse 15, 76131 Karlsruhe, Germany. E-mail: roesky@kit.edu
bMacromolecular Architectures, Institute for Technical Chemistry and Polymer Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstrasse 18, 76131 Karlsruhe, Germany. E-mail: christopher.barner-kowollik@kit.edu
cSchool of Chemistry, Physics and Mechanical Engineering, Queensland University of Technology (QUT), 2 George Street, Brisbane, Queensland 4000, Australia. E-mail: christopher.barnerkowollik@qut.edu.au
First published on 10th November 2018
Abstract
We report the formation of well-defined copper and molybdenum single-chain nanoparticles, exhibiting metal clusters (M24+) as junction points. Upon addition of dimetallic precursor complexes [Cu2(OAc)4] and [Mo2(OAc)4], carboxylic acid functionalized polymer chains collapse into nanoparticles containing a M24+ (M = Cu, Mo) centered paddlewheel folding moiety. This synthetic strategy allows a high crosslinking per metal unit and the incorporation of an adjustable number of dimetallic centers into a single polymer chain. The successful formation of M2-SCNPs is evidenced by size exclusion chromatography and diffusion-ordered NMR spectroscopy. Detailed insight into the dimetallic folding unit is provided by 1H/2H NMR-, IR-, Raman- and UV-Vis spectroscopy as well as comparative analyses of molecular dicopper and dimolybdenum model-complexes. For the first time, M24+ paddlewheel folding motifs, including quadruple bonded dimetallic units, as in the case of Mo24+, are utilized as structure forming elements in the realm of SCNP chemistry.
Introduction
Single-chain nanoparticles (SCNPs) are versatile and multi-functional macromolecular architectures, which have recently attracted particular attention due to their application as highly specialized enzyme-mimetic catalytic systems.1–3 SCNPs consist of single polymer chains, which are folded intramolecularly into nanoparticles by an external trigger.4–6 The collapse of single-chains can be induced by a variety of chemical reactions, yet recent approaches focus on coordinative metal to ligand bond formation. Employing metal-ions as cross-linkers within the chain does not only lead to the formation of compact nanoparticles, yet introduces unique properties to the resulting metal-SCNPs, such as catalytic activity, magnetism or luminescence.7–11 Up to date, a series of metal-crosslinked SCNP systems has been established and to some extend examined in initial catalytic studies, demonstrating promising results, regarding enhanced catalytic activity, selectivity as well as catalyst recyclability.1,12–17 Essential for metal-SCNP formation are suitable metal precursor complexes, exhibiting free coordination sites or leaving groups, which are readily replaceable by the ligand moieties in the polymer chains. Up to now, the literature mainly reports the use of monometallic complexes for SCNP formation, resulting in cross-points, consisting of single metal centers.First results to incorporate dimetallic species into a SCNP system were achieved by the group of Berda. Here, a diiron-cluster was attached to a polymer chain, which – via subsequent light triggered [4 + 4] cycloaddition of anthracene functions in the polymer – was collapsed into a nanoparticle. In this way, a polymeric scaffold is formed around the diiron-cluster, creating a synthetic [FeFe] hydrogenase mimic.18 However, in this approach the cluster is not part of the actual SCNP formation, instead attached to the side chain.
Furthermore, the group of Lemcoff described the synthesis of metal-SCNPs exhibiting a μ-chloro bridged dinuclear binding motif.9,15 The application of polycycloocta-1,5-diene (pCOD) allowed the incorporation of the dimetallic precursors [RhCl(C2H4)2]2 or [IrCl(COE)2]2via ligand exchange reaction. This approach enabled, for the first time, the introduction of dinuclear metal complexes as structure forming elements in SCNPs. A similar μ-chloro bridged dinuclear nickel crosslinking motif was proposed by the group of Hübner, utilizing pyridine-functionalized polymer chains.19 However, in the reported examples the two metal ions within the same folding unit are locally separated from each other. Therefore, a different accessible pathway for the implementation of dimetallic clusters into SCNP systems, enabling a close special distance and interaction between the metal ions, is highly desirable.
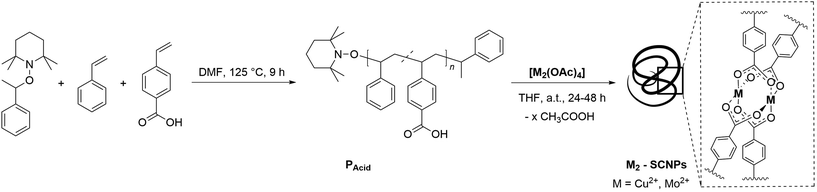
Herein, we report a synthetic strategy to incorporate dimetallic clusters (Cu24+, Mo24+) as junction points into single-chains, thus generating metal-SCNPs. In doing so, not only a high crosslinking within the polymer chain is achieved, yet unique M24+ paddlewheel folding motifs, including multiple bonded metal centers, are introduced into the field of SCNPs (Fig. 1).
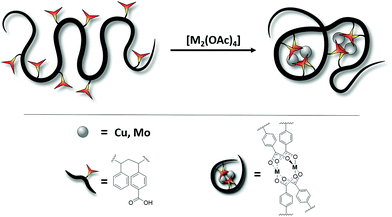 | ||
| Fig. 1 Schematic illustration of M2-SCNP formation via an intramolecular chain collapse upon dimetal cluster coordination (Cu24+, Mo24+). | ||
Experimental
Materials
4-Vinylbenzoic acid (>97%, TCI), 4-ethylbenzoic acid (99%, Alfa Aesar) and copper(II) acetate monohydrate (99.9%, Alfa Aesar) were used as received. Styrene (99%, Merck) was passed through a column of basic alumina (Acros) and stored at −19 °C. [Mo2(OAc)4], [Mo2(OAc)4]-d12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidine12 were synthesized according to literature procedures. Reactions and characterization methods of all molybdenum compounds were performed under exclusion of moisture and oxygen in flame-dried Schlenk-type glassware or in an argon-filled MBraun glovebox.
20 and 2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidine12 were synthesized according to literature procedures. Reactions and characterization methods of all molybdenum compounds were performed under exclusion of moisture and oxygen in flame-dried Schlenk-type glassware or in an argon-filled MBraun glovebox.
Hydrocarbon solvents (THF, n-pentane) were dried using an MBraun solvent purification system (SPS-800). THF was additionally distilled under nitrogen from potassium before storage over 4 Å molecular sieves. Prior to use, dimethyl sulfoxide (DMSO) was distilled under nitrogen from CaH2 and methanol (MeOH) was distilled under nitrogen from magnesium. Dimethyl formamide (DMF) and dichloromethane (DCM) were purchased as analytical grade and used as received.
Instruments
NMR spectra were recorded on a Bruker Avance 400 (400 MHz) spectrometer. 1H and 13C{1H} NMR chemical shifts are reported relative to tetramethylsilane (TMS). 2H NMR measurements were performed in non-deuterated THF. Abbreviations include singlet (s), broad singlet (bs), doublet (d), triplet (t), quartet (q) and unresolved multiplet (m). The diffusion coefficients were measured with Diffusion Ordered NMR Spectroscopy (DOSY) experiments, using a Bruker Smart VT unit to control the temperature which was calibrated to be exactly 298 K.Size-exclusion chromatography (SEC) measurements were performed on a PL-SEC 50 Plus (Polymer Laboratories, Varian), running on THF (HPLC-grade). The applied columns were a PLgel Mixed C guard column (50 × 7.5 mm), followed by three PLgel Mixed C linear columns (300 × 7.5 mm, 5 μm beadsize) and a differential refractive index (RI) detector. The device was operated at 35 °C column temperature with a flow rate of 1 mL min−1. To obtain Mn and Đ values, the integration of the polymer peak was carried out from low elution times to approximately 40 minutes due to overlap with an SEC system peak. No baseline correction was performed. Consequently, Mn and Đ values are estimates.
IR spectra were obtained on a Bruker Tensor 37 FTIR spectrometer, equipped with a room temperature DLaTGS detector and a diamond ATR (attenuated total reflection) unit. Abbreviations used in the compounds’ analysis include very strong (vs), strong (s), medium (m), weak (w) and very weak (vw).
Raman spectra were obtained on a Bruker MultiRam spectrometer. Abbreviations used in the compounds’ analysis include very strong (vs), strong (s), medium (m), weak (w) and very weak (vw).
Elemental analyses were carried out with an Elementar Micro Cube.
Ultraviolet–visible (UV-Vis) spectra were recorded on a VARIAN Cary 50 Scan UV-Visible Spectrophotometer. Spectra were recorded in THF at room temperature and collected between 200 and 800 nm. Samples were baseline corrected with respect to the pure solvent.
Copolymerization of 4-vinyl-benzoic acid and styrene (PAcid)
2,2,6,6-Tetramethyl-1-(1-phenylethoxy)piperidine (9.00 mg, 34.4 μmol, 1.00 equiv.) (synthesized according to literature12), styrene (2.64 g, 25.3 mmol, 735 equiv.) and 4-vinylbenzoic acid (178 mg, 1.21 mmol, 35.0 equiv.) were dissolved in 1.45 mL of degassed DMF. While stirring, the solution was degassed with N2 for 30 min. Subsequently, the reaction mixture was heated at 125 °C for 9 h. Cooling to ambient temperature and opening the flask to air, quenched the reaction. After diluting with DCM, the polymer PAcid was precipitated twice into cold MeOH, resulting in a white powder. Characterization via1H NMR revealed a monomer ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, regarding the higher amount to styrene (0.65 mmol of benzoic acid functionality in 1.00 g PAcid).
14, regarding the higher amount to styrene (0.65 mmol of benzoic acid functionality in 1.00 g PAcid).
SEC characterization (THF, RI) resulted in a number average molecular weight of Mn = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 and a polydispersity of Đ = 1.2.
200 g mol−1 and a polydispersity of Đ = 1.2.
1H NMR (400 MHz, THF-d8): δ [ppm] = 11.2 (bs, COOH), 7.91–7.51 (m, CH-benzoic acid, Ha), 7.25–6.27 (m, CH-aromatic), 2.59–2.83 (m, H-backbone).
IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3082 (vw), 3060 (w), 3026 (s), 3002 (vw), 2923 (s), 2849 (w), 1734 (m), 1690 (m), 1603 (m), 1582 (vw), 1493 (s), 1451 (s), 1423 (w), 1369 (vw), 1315 (vw), 1285 (w), 1179 (w), 1156 (vw), 1086 (vw), 1028 (w), 907 (vw), 856 (vw), 756 (s), 698 (vs), 540 (m).
[cm−1] = 3082 (vw), 3060 (w), 3026 (s), 3002 (vw), 2923 (s), 2849 (w), 1734 (m), 1690 (m), 1603 (m), 1582 (vw), 1493 (s), 1451 (s), 1423 (w), 1369 (vw), 1315 (vw), 1285 (w), 1179 (w), 1156 (vw), 1086 (vw), 1028 (w), 907 (vw), 856 (vw), 756 (s), 698 (vs), 540 (m).
Raman (solid state): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3055 (s), 3002 (vw), 2978 (vw), 2903 (m), 2851 (w), 1604 (s), 1584 (m), 1451 (w), 1329 (vw), 1183 (m), 1156 (w), 1032 (m), 1002 (vs), 798 (w), 662 (m), 224 (w).
[cm−1] = 3055 (s), 3002 (vw), 2978 (vw), 2903 (m), 2851 (w), 1604 (s), 1584 (m), 1451 (w), 1329 (vw), 1183 (m), 1156 (w), 1032 (m), 1002 (vs), 798 (w), 662 (m), 224 (w).
Synthesis of Cu2-SCNPs
4.60 mg of copper(II) acetate hydrate (11.5 μmol, 0.25 equiv.) was dissolved in 25 mL of THF (c = 4.6 × 10−4 mol L−1). 70.0 mg of copolymer PAcid (45.5 μmol benzoic acid functionality, 1.00 equiv.) was dissolved in 100 mL of THF. While stirring the metal salt solution, the polymer solution was added dropwise with a dropping rate of ∼5 mL h−1. After complete addition (20 h), the blue solution was stirred for additional 4 h. The solution was concentrated under reduced pressure and subsequently precipitated into cold MeOH. The solid was filtered off and dried under vacuum, obtaining Cu2-SCNPs.SEC characterization (THF, RI) resulted in a number average molecular weight of Mn = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 and a polydispersity of Đ = 1.2.
500 g mol−1 and a polydispersity of Đ = 1.2.
1H NMR (400 MHz, THF-d8): δ [ppm] = 7.95–7.51 (m, CH-benzoic acid), 7.59–5.82 (m, CH-aromatic), 2.36–0.62 (m, H-backbone) – The multiplet resonance at δ = 7.95–7.51 ppm (CH-benzoic acid) is significantly broadened, compared to PAcid, due to the coordination of paramagnetic Cu(II) ions. At δ = 11.2 ppm a singlet resonance is detected for R-COOH, indicating free carboxylic acid moieties, most likely due to an axial coordination of the acidic functionalities to the Cu24+ moieties.
IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3083 (vw), 3060 (w), 3026 (s), 3001 (vw), 2923 (s), 2849 (w), 1733 (w), 1691 (w), 1619 (m), 1603 (m), 1583 (vw), 1565 (vw), 1493 (s), 1452 (s), 1407 (s), 1374 (vw), 1181 (w), 1155 (vw), 1070 (vw), 1028 (w), 907 (w), 857 (vw), 757 (s), 698 (vs), 540 (m).
[cm−1] = 3083 (vw), 3060 (w), 3026 (s), 3001 (vw), 2923 (s), 2849 (w), 1733 (w), 1691 (w), 1619 (m), 1603 (m), 1583 (vw), 1565 (vw), 1493 (s), 1452 (s), 1407 (s), 1374 (vw), 1181 (w), 1155 (vw), 1070 (vw), 1028 (w), 907 (w), 857 (vw), 757 (s), 698 (vs), 540 (m).
Synthesis of Mo2-SCNPs
7.00 mg of molybdenum(II) acetate (16.3 μmol, 0.25 equiv.) was dissolved in 50 mL of THF (c = 3.3 × 10−4 mol L−1). 100 mg of copolymer PAcid (65.0 μmol benzoic acid functionality, 1.00 equiv.) was dissolved in 120 mL of THF. While stirring the metal salt solution, the polymer solution was added dropwise with a dropping rate of ∼3 mL h−1. After complete addition (40 h), the yellow solution was stirred for additional 8 h. The solution was concentrated under reduced pressure and subsequently precipitated into cold MeOH. The solid was filtered off and dried under vacuum, yielding Mo2-SCNPs.1H NMR (400 MHz, CDCl3): δ [ppm] = 8.16–7.54 (m, CH-benzoic acid), 7.37–6.13 (m, CH-aromatic), 2.55–0.89 (m, H-backbone). Additional NMR measurements in THF-d8 revealed no free carboxylic acid proton resonances.
Raman (solid state): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3055 (s), 3000 (vw), 2977 (vw), 2910 (m), 2851 (w), 1606 (s), 1583 (m), 1513 (m), 1451 (w), 1411 (s), 1401 (s), 1332 (vw), 1184 (m), 1156 (w), 1032 (m), 1002 (vs), 795 (w), 622 (m), 408 (w), 225 (w).
[cm−1] = 3055 (s), 3000 (vw), 2977 (vw), 2910 (m), 2851 (w), 1606 (s), 1583 (m), 1513 (m), 1451 (w), 1411 (s), 1401 (s), 1332 (vw), 1184 (m), 1156 (w), 1032 (m), 1002 (vs), 795 (w), 622 (m), 408 (w), 225 (w).
IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] = 3082 (vw), 3060 (w), 3026 (s), 3001 (vw), 2922 (s), 2849 (w), 1602 (m), 1556 (w), 1493 (s), 1451 (s), 1401 (m), 1181 (w), 1154 (vw), 1068 (vw), 1028 (w), 907 (w), 845 (vw), 756 (s), 698 (vs), 540 (m).
[cm−1] = 3082 (vw), 3060 (w), 3026 (s), 3001 (vw), 2922 (s), 2849 (w), 1602 (m), 1556 (w), 1493 (s), 1451 (s), 1401 (m), 1181 (w), 1154 (vw), 1068 (vw), 1028 (w), 907 (w), 845 (vw), 756 (s), 698 (vs), 540 (m).
UV-Vis: λmax. [nm] = 433.
For the synthesis and analysis of the Cu2- and Mo2-model complexes, as well as all original data, refer to the ESI.†
Results and discussion
Polymer synthesis and SCNP formation
To synthesize cluster folded SCNPs, the dimeric tetra-carboxylates [[M2(O2CR)4] (M = Cu, Mo)] were chosen as suitable metal precursors. These complexes form characteristic paddlewheel structures, in which the carboxylates act as bidentate bridging ligands of the dimetallic center.21–23 The bridging ligands cause a close proximity of the metal ions, thus allowing quadruple bonded clusters in the case of molybdenum.24 Of specific interest are the readily accessible tetraacetates [Cu2(OAc)4] and [Mo2(OAc)4], as both complexes are known to readily substitute their acetate ligands by addition of stronger carboxylic acids at room temperature,25–27 ideal reaction conditions for SCNP formation. This synthetic strategy allows a high crosslinking of up to four linkages per dimetallic unit. Furthermore, [Cu2(OAc)4] has already been successfully employed as a crosslinking agent in polymeric systems, modulating the mechanical properties of metallopolymers.28Thus, the precursor polymer chains require benzoic acid moieties to allow, via simple carboxylate exchange reactions, the incorporation of dimetallic M24+ units. Provided that at least two acid linkers in the polymer coordinate to the dimetallic unit, a chain collapse is anticipated. Benzoic acid moieties were incorporated into the polymer chains by statistical copolymerization of styrene and 4-vinylbenzoic acid via nitroxide-mediated polymerization (NMP),29,30 resulting in PAcid (Scheme 1). The copolymer composition was determined by 1H NMR spectroscopy (ESI, Fig. S4†), distinguishing the aromatic proton resonances of both monomers. Hence, a monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, with regard of the higher amount to styrene, was identified, resulting in approximately 7 mol% of carboxylic acid units (approx. 20 units per chain) for PAcid, in good accordance to the feed ratio. Size exclusion chromatography (SEC) analysis [THF, RI] of PAcid indicated a Mn of 32
14, with regard of the higher amount to styrene, was identified, resulting in approximately 7 mol% of carboxylic acid units (approx. 20 units per chain) for PAcid, in good accordance to the feed ratio. Size exclusion chromatography (SEC) analysis [THF, RI] of PAcid indicated a Mn of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 and a narrow distribution of Đ ≈ 1.2.
200 g mol−1 and a narrow distribution of Đ ≈ 1.2.
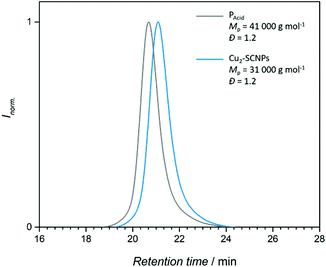
For the subsequent SCNP formation, PAcid was dissolved in THF and added to a solution of the metal precursors [Cu2(OAc)4]·2H2O or [Mo2(OAc)4]. Working under high dilution is necessary to prevent network formation of the polymer chains by intermolecular reaction. After isolation of the polymeric structures, the collapse into nanoparticles and the structural motif of the cross-links, were evidenced. The folding of copolymer PAcid into a nanoparticle upon [Cu24+] coordination was initially confirmed by SEC analysis [THF, RI]. In comparison to PAcid, the SEC trace for the Cu2-SCNPs is shifted towards longer retention times (Fig. 2), indicating a smaller hydrodynamic radius, and thus the exclusive formation of SCNP nanostructures (Đ ≈ 1.2). However, SEC analysis of the Mo2-SCNPs was not feasible, as Mo24+ compounds are known to be highly reactive towards moisture and air, hence prohibiting a meaningful analysis.
The decrease of the particles’ mean hydrodynamic radius was additionally confirmed by diffusion ordered NMR spectroscopy (DOSY).31 The diffusion coefficient of molecules in solution is dependent on their hydrodynamic radius, hence particles of different sizes can be distinguished. The mean hydrodynamic radius of PAcid resulted in Rh = 3.9 nm, whereas a radius of Rh = 2.3 nm for the Cu2-SCNPs and Rh = 2.6 nm for the Mo2-SCNPs was determined (ESI, Fig. S14–S16†). Thus, a reduction of the hydrodynamic radius by approximately 35–40% evidenced a successful SCNP formation for both dimetallic-crosslinked structures.
Interestingly, in the 1H NMR spectrum (THF-d8) of the Cu2-SCNPs a resonance at δ = 11.2 ppm, belonging to the acidic protons (R-COOH) of PAcid, is still detected (approximately 30% compared to PAcid, Fig. S6†). Remaining carboxylic acid moieties in the polymer chains derive most likely from additional axial coordination of the acidic functionalities to the Cu2 moiety (for details, refer to section ‘model complexes’). In contrast to the Cu2-SCNPs, no resonance for acidic protons is detected in the 1H NMR spectrum of the Mo2-SCNPs (ESI, Fig. S9†). Given the stoichiometry applied (0.25 mol% of metal precursor), a substitution of all four acetate ligands by the benzoic acid moieties of PAcid is presumed, considering the error range of NMR spectroscopy. To quantify the actual number of substituted acetate groups during the folding process, deuterated [Mo2(OAc)4]-d12 was applied as a metal precursor. 2H NMR spectroscopy of the corresponding Mo2-SCNPs revealed no remaining deuterium resonances, indicating a complete substitution of all deuterated acetate ligands (ESI, Fig. S12 and S13†). However, analogous experiments with deuterated [Cu2(OAc)4]-d12 were not feasible. NMR analytics were hindered by the paramagnetic Cu24+ moieties, which is in agreement with the corresponding dicopper tetra carboxylate model complexes (see next section).
Model complexes
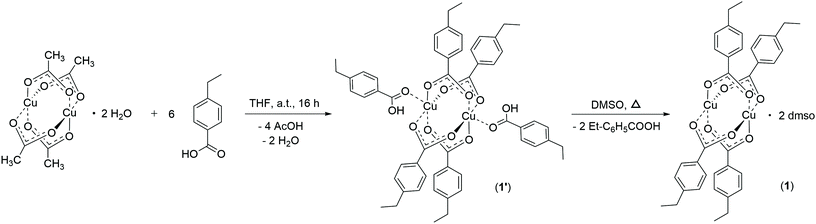
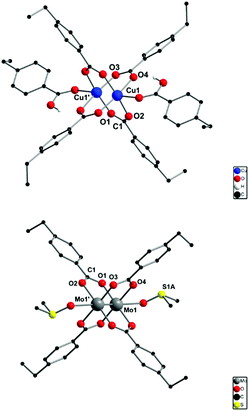
After evidencing the particles’ size reduction, a detailed look into the dimetallic linkages was crucial for understanding the composition and structure of the folding moieties. Therefore, corresponding molecular dimetallic model complexes of both copper and molybdenum were synthesized. To simulate the benzoic acid moieties within the polymer chain, 4-ethylbenzoic acid was chosen as the most suitable ligand (Scheme 2).Under similar reaction conditions as for the M2-SCNP synthesis, 4-ethylbenzoic acid reacted with [Cu2(OAc)4]·2H2O in THF, resulting in the compound [Cu2(p-O2CPhEt)4(p-HO2CPhEt)2] (1′), in which six ligand molecules coordinate in different coordination modes to one dicopper unit (solid state structure, Fig. 3, top).32 Four ligands arrange as carboxylates in a typical bidentate bridged paddlewheel structure. In addition, two carboxylic acid molecules coordinate axially to both sides of the dicopper unit via their carbonyl functionality to saturate the coordination sphere. Most likely, this structural arrangement explains the remaining carboxylic acid functionalities observed in the 1H NMR spectrum of the Cu2-SCNPs. However, subsequent crystallization from DMSO leads to the substitution of both axially binding 4-ethyl benzoic acid moieties and the formation of [Cu2(p-O2CPh-Et)4(dmso)2] (1) (ESI, Fig. S1†). A Cu–Cu distance of 2.602 Å is detected for complex 1 in the solid state, in agreement to structurally similar dicopper complexes,33 indicating no direct bond between the two copper atoms.
The analogous reaction with [Mo2(OAc)4], resulted in the homoleptic compound [Mo2(p-O2CPh-Et)4] (2), in which additional THF molecules saturate the axial coordination sites of the dimolybdenum Mo24+ unit. The molecular structure of 2 (crystallized from a DMSO solution) in the solid state (Fig. 3, bottom) confirms the expected, highly symmetric, tetracarboxylato-bridged paddlewheel structure with two axially coordinating solvent molecules. The Mo-Mo quadruple bond length is 2.115 Å, which is a typical value in a bidentate bridged coordination geometry (2.06–2.13 Å).34
The synthesis of both model complexes proceeded at ambient temperature, yielding exclusively homoleptic paddlewheel structures (neglecting axial solvent coordination) in high yields. Therefore, a similar reactivity is expected for the formation of the Cu2-SCNPs and Mo2-SCNPs. The complexes 1, 1′ and 2 are assumed to depict the actual folding motif of the SCNP linkages, exhibiting a tetracarboxylate dimetallic folding unit. However, a complete substitution of all four acetate ligands of the precursor complexes by the benzoic acid moieties of PAcid might be hampered, due to steric hindrance in the collapsed nanoparticles after a certain folding degree. Hence, to a small extend, heteroleptic structures, containing unsubstituted acetate ligands, are likely to be present in the SCNP structures.
Characterization of the folding unit
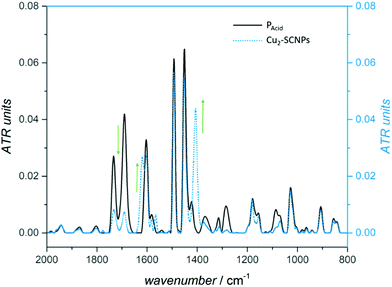
At first, vibrational spectroscopy analysis was employed to determine the structural arrangement within the SCNP folding units, comparing the M2-complexed nanoparticles with the dimetallic model complexes (1 and 2). In a bridged bidentate coordination of a carboxylate unit (RCO2−), the two C–O bonds are equivalent and the corresponding frequencies of the symmetric and anti-symmetric stretching modes are detected as strong bands in the region of 1400–1600 cm−1, mainly depending on the ligand design and the metal ions.20,33,35Applying IR spectroscopy, a characteristic band for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of the benzoic acid moieties (R-COOH) at
O stretching mode of the benzoic acid moieties (R-COOH) at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1690 cm−1 is detected for copolymer PAcid (Fig. 4, black). In the IR spectrum of the Cu2-SCNPs, the band at 1690 cm−1 (C
= 1690 cm−1 is detected for copolymer PAcid (Fig. 4, black). In the IR spectrum of the Cu2-SCNPs, the band at 1690 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) decreased significantly, yet did not disappear completely. This is in good agreement with the NMR data of the Cu2-SCNPs in which approximately 30% of free carboxylic acid moieties are still observed. Additionally, two characteristic bands appear at
O) decreased significantly, yet did not disappear completely. This is in good agreement with the NMR data of the Cu2-SCNPs in which approximately 30% of free carboxylic acid moieties are still observed. Additionally, two characteristic bands appear at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) a = 1619 and
a = 1619 and ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) s = 1374 cm−1 for the asymmetric and symmetric C–O stretching modes (Fig. 4, blue).36 In conclusion, IR spectroscopy clearly indicates a bridged carboxylate coordination of the polymers’ acid moieties to the dicopper units. The IR spectrum of the Cu2-model complex 1 further supports this assumption, exhibiting similar bands at
s = 1374 cm−1 for the asymmetric and symmetric C–O stretching modes (Fig. 4, blue).36 In conclusion, IR spectroscopy clearly indicates a bridged carboxylate coordination of the polymers’ acid moieties to the dicopper units. The IR spectrum of the Cu2-model complex 1 further supports this assumption, exhibiting similar bands at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) a = 1619 and
a = 1619 and ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) s = 1398 cm−1 (ESI, Fig. S20†).
s = 1398 cm−1 (ESI, Fig. S20†).
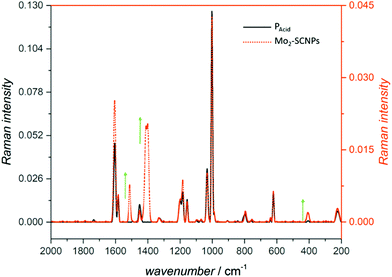
IR spectroscopy of the Mo2-SCNPs indicates, in accordance to 1H NMR spectroscopy, a coordination of nearly all carboxylic acid moieties, as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of free carboxylic acids has almost vanished. In addition, an intense band at
O stretching mode of free carboxylic acids has almost vanished. In addition, an intense band at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) s = 1401 cm−1 appears, associated with the symmetric C–O stretching mode of a bridged bidentate carboxylate (ESI, Fig. S19†). However, due to overlaying bands, a band for the asymmetric stretching mode cannot be observed. Therefore, Raman spectroscopy was applied as a complementary vibrational spectroscopic method for the Mo2-SCNPs. In the spectrum of the Mo2-SCNPs, two bands at
s = 1401 cm−1 appears, associated with the symmetric C–O stretching mode of a bridged bidentate carboxylate (ESI, Fig. S19†). However, due to overlaying bands, a band for the asymmetric stretching mode cannot be observed. Therefore, Raman spectroscopy was applied as a complementary vibrational spectroscopic method for the Mo2-SCNPs. In the spectrum of the Mo2-SCNPs, two bands at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) a = 1513 cm−1 and
a = 1513 cm−1 and ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) s = 1411/1401 cm−1 (asymmetric and symmetric C–O stretching modes) are visible, indicating a bridged carboxylate coordination (Fig. 5, orange). The relatively small distance between these two bands (approx. 100 cm−1) is typical for a dimolybdenum species.33 Additionally, the characteristic band at
s = 1411/1401 cm−1 (asymmetric and symmetric C–O stretching modes) are visible, indicating a bridged carboxylate coordination (Fig. 5, orange). The relatively small distance between these two bands (approx. 100 cm−1) is typical for a dimolybdenum species.33 Additionally, the characteristic band at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 408 cm−1 is associated with the quadruple bonded Mo–Mo stretching mode,33,37 proving an intact Mo–Mo quadruple bond within the Mo2-SCNP structure. Similar results are received for the Mo2-model compound 2 (
= 408 cm−1 is associated with the quadruple bonded Mo–Mo stretching mode,33,37 proving an intact Mo–Mo quadruple bond within the Mo2-SCNP structure. Similar results are received for the Mo2-model compound 2 (![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) a = 1515 cm−1;
a = 1515 cm−1; ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) s = 1415/1398 cm−1;
s = 1415/1398 cm−1; ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) Mo–Mo = 403 cm−1; Fig. S24†), yet the axial coordination of different solvent molecules (THF, DMSO) needs to be considered.
Mo–Mo = 403 cm−1; Fig. S24†), yet the axial coordination of different solvent molecules (THF, DMSO) needs to be considered.
The M2-SCNPs and their respective model complexes were further analyzed by UV-Vis spectroscopy. Upon metal coordination, the resulting M2-SCNPs exhibit either a blue (Cu2) or a dark yellow/orange (Mo2) color. UV-Vis measurements of the Cu2-SCNPs in THF showed a broad absorption between 550–800 nm (ESI, Fig. S25†), characteristic for Cu(II) carboxylates and typically considered as the result of d–d transitions,36 yet recent studies also consider different contributions.38 UV-Vis measurements of the Cu2-model complex (1) and the starting material [Cu2(OAc)4]·2H2O showed similar trace shapes and absorption maxima (ESI, Fig. S25 and S27†), validating the assumption of a successful implementation of Cu(II) ions into the polymeric structure.
Analogous UV-Vis measurements of the Mo2-SCNPs show a pronounced absorption band between 350–500 nm, with an absorption maximum at approximately 430 nm (Fig. 6). This absorption originates from the electronic transition between the δ orbitals of the Mo2 unit and π* orbitals of the aromatic ligands. It represents a fully-allowed metal to ligand charge transfer (MLCT), which can be tuned over a wide wavelength range, strongly dependent on the π acceptor-ligands (e.g. O2CR).27,39–41 In comparison, the starting material [Mo2(OAc)4] does not show any absorption in this area, as no π acceptor ligands are present (ESI, Fig. S28†). Thus, the characteristic absorption of the Mo2-SCNPs at λ = 430 nm proves the substitution of acetate by the π acceptor ligand moieties of the polymer chains, allowing a δ–π* transition. UV-Vis measurement of the Mo2-model complex (2) shows an almost identical absorption between 350–500 nm (Fig. 6), further evidencing the proposed structural motif within the Mo2-SCNPs and the presence of a quadruple bonded dimolybdenum unit.
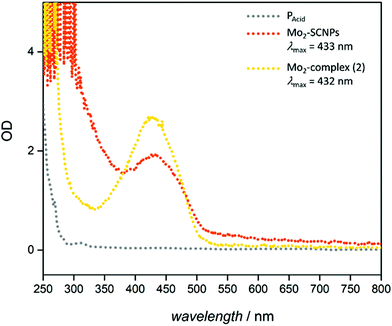 | ||
| Fig. 6 UV-Vis spectra of PAcid, Mo2-SCNPs and the Mo2-model complex 2. A local maximum absorbance at 430–435 nm is observed for the Mo2 containing compounds. | ||
The combined results of vibrational (IR, Raman) and UV-Vis spectroscopy clearly point towards a successful incorporation of the dimetallic clusters and the formation of a paddlewheel folding motif, as proposed by the model complexes 1 and 2. Thus, a bridged carboxylate dimetallic moiety is postulated as the actual junction point in the SCNPs.
Conclusions
Herein, we present the synthesis and in-depth characterization of polymeric single-chain nanoparticles, exhibiting M24+ (M = Cu, Mo) dimetallic units as novel crosslinking junctions (M2-SCNPs). Reacting the dimeric precursor complexes [Cu2(OAc)4] and [Mo2(OAc)4] with carboxylic acid functionalized polymer chains, multiple cross-links within the chains are formed, leading to intramolecular collapse. The successful formation of nanoparticles was evidenced by SEC and DOSY, whereas the dimetallic folding unit was analyzed by 1H/2H NMR, IR-, Raman- and UV-Vis spectroscopy. In addition, the synthesis of dicopper and dimolybdenum model complexes (1, 1′ and 2) provided detailed insight into the paddlewheel structured folding motif. The described M2-SCNPs employ metal clusters as structure forming elements, introducing a unique M24+ paddlewheel folding motif to SCNP chemistry. Importantly, Mo2-SCNPs display the first nanostructured polymeric system exhibiting quadruple bonded metal centers, thus creating novel soft matter materials with promising reactivity and characteristics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. B.-K. and P. W. R. acknowledge support from the SFB 1176 (project A2) funded by the German Research Council (DFG). H. R.'s and N. K.'s PhD studies are additionally supported by the Fonds der Chemischen Industrie (FCI). C. B.-K. acknowledges key support from the Australian Research Council (ARC) in the context of a Laureate Fellowship as well as by the Queensland University of Technology (QUT). C. B.-K. additionally acknowledges continued support by the Helmholtz association via the STN and BIFTM programs.References
- H. Rothfuss, N. D. Knöfel, P. W. Roesky and C. Barner-Kowollik, J. Am. Chem. Soc., 2018, 140, 5875 CrossRef CAS.
- Y. Liu, P. Turunen, B. F. M. de Waal, K. G. Blank, A. E. Rowan, A. R. A. Palmans and E. W. Meijer, Mol. Syst. Des. Eng., 2018, 3, 609 RSC.
- J. P. Cole, A. M. Hanlon, K. J. Rodriguez and E. B. Berda, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 191 CrossRef CAS.
- S. Mavila, O. Eivgi, I. Berkovich and N. G. Lemcoff, Chem. Rev., 2016, 116, 878 CrossRef CAS.
- J. A. Pomposo, J. Rubio-Cervilla, A. J. Moreno, F. Lo Verso, P. Bacova, A. Arbe and J. Colmenero, Macromolecules, 2017, 50, 1732 CrossRef CAS.
- A. M. Hanlon, C. K. Lyon and E. B. Berda, Macromolecules, 2016, 49, 2 CrossRef CAS.
- A. Latorre-Sánchez and J. A. Pomposo, Polym. Int., 2016, 65, 855 CrossRef.
- Y. Bai, X. Feng, H. Xing, Y. Xu, B. K. Kim, N. Baig, T. Zhou, A. A. Gewirth, Y. Lu, E. Oldfield and S. C. Zimmerman, J. Am. Chem. Soc., 2016, 138, 11077 CrossRef CAS.
- S. Mavila, C. E. Diesendruck, S. Linde, L. Amir, R. Shikler and N. G. Lemcoff, Angew. Chem., Int. Ed., 2013, 52, 5767 CrossRef CAS.
- J. Rubio-Cervilla, E. González and J. Pomposo, Nanomaterials, 2017, 7, 341 CrossRef PubMed.
- Y. Liu, S. Pujals, P. J. M. Stals, T. Paulöhrl, S. I. Presolski, E. W. Meijer, L. Albertazzi and A. R. A. Palmans, J. Am. Chem. Soc., 2018, 140, 3423 CrossRef CAS.
- N. D. Knöfel, H. Rothfuss, J. Willenbacher, C. Barner-Kowollik and P. W. Roesky, Angew. Chem., Int. Ed., 2017, 56, 4950 CrossRef PubMed.
- A. Sanchez-Sanchez, A. Arbe, J. Colmenero and J. A. Pomposo, ACS Macro Lett., 2014, 3, 439 CrossRef CAS.
- T. Terashima, T. Mes, T. F. A. De Greef, M. A. J. Gillissen, P. Besenius, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 4742 CrossRef CAS.
- S. Mavila, I. Rozenberg and N. G. Lemcoff, Chem. Sci., 2014, 5, 4196 RSC.
- E. Huerta, P. J. M. Stals, E. W. Meijer and A. R. A. Palmans, Angew. Chem., Int. Ed., 2013, 52, 2906 CrossRef CAS.
- S. Thanneeru, J. K. Nganga, A. S. Amin, B. Liu, L. Jin, A. M. Angeles-Boza and J. He, ChemCatChem, 2017, 9, 1157 CrossRef CAS.
- C. A. Tooley, S. Pazicni and E. B. Berda, Polym. Chem., 2015, 6, 7646 RSC.
- K. Freytag, S. Säfken, K. Wolter, J. C. Namyslo and E. G. Hübner, Polym. Chem., 2017, 8, 7546 RSC.
- T. A. Stephenson, E. Bannister and G. Wilkinson, J. Chem. Soc., 1964, 2538 RSC.
- P. Sharrock and M. Melník, Can. J. Chem., 1985, 63, 52 CrossRef CAS.
- P. K. Ross, M. D. Allendorf and E. I. Solomon, J. Am. Chem. Soc., 1989, 111, 4009 CrossRef CAS.
- D. Lawton and R. Mason, J. Am. Chem. Soc., 1965, 87, 921 CrossRef CAS.
- F. A. Cotton, C. A. Murillo and R. A. Walton, Multiple Bonds Between Metal Atoms, Springer Science and Business Media, Inc., New York, 3rd edn, 2005 Search PubMed.
- J. Hicks, S. P. Ring and N. J. Patmore, Dalton Trans., 2012, 41, 6641 RSC.
- F. A. Cotton, L. R. Falvello, A. H. Reid and J. H. Tocher, J. Organomet. Chem., 1987, 319, 87 CrossRef CAS.
- N. D. Knöfel, C. Schweigert, T. J. Feuerstein, C. Schoo, N. Reinfandt, A.-N. Unterreiner and P. W. Roesky, Inorg. Chem., 2018, 57, 9364 CrossRef.
- V. Yuval, B. Suwon and M. N. Silberstein, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 1117 CrossRef.
- C. J. Hawker, G. G. Barclay and J. Dao, J. Am. Chem. Soc., 1996, 118, 11467 CrossRef CAS.
- G. Moad and E. Rizzardo, Macromolecules, 1995, 28, 8722 CrossRef CAS.
- E. Blasco, B. T. Tuten, H. Frisch, A. Lederer and C. Barner-Kowollik, Polym. Chem., 2017, 8, 5845 RSC.
- A. C. Sunil, B. C. B. Bezuidenhoudt and J. M. Janse van Rensburg, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, m939 CrossRef CAS.
- J. Catterick and P. Thornton, in Advances in Inorganic Chemistry and Radiochemistry, ed. H. J. Emeléus and A. G. Sharpe, Academic Press, 1977, vol. 20, p. 291 Search PubMed.
- F. A. Cotton, L. M. Daniels, E. A. Hillard and C. A. Murillo, Inorg. Chem., 2002, 41, 2466 CrossRef CAS.
- F. A. Cotton and D. G. Lay, Inorg. Chem., 1981, 20, 935 CrossRef CAS.
- M. Mikuriya, R. Indrawati, R. Hashido, S. Matsubara, C. Nakamura, D. Yoshioka, K. Yokota, M. Fukuzaki and M. Handa, Magnetochemistry, 2018, 4, 22 CrossRef.
- A. J. Hempleman, R. J. H. Clark and C. D. Flint, Inorg. Chem., 1986, 25, 2915 CrossRef CAS.
- M. Kyuzou, W. Mori and J. Tanaka, Inorg. Chim. Acta, 2010, 363, 930 CrossRef CAS.
- M. H. Chisholm, T. L. Gustafson and C. Turro, Acc. Chem. Res., 2013, 46, 529 CrossRef CAS.
- M. H. Chisholm, S. E. Brown-Xu and T. F. Spilker, Acc. Chem. Res., 2015, 48, 877 CrossRef CAS PubMed.
- B. G. Alberding, M. H. Chisholm, C. B. Durr, J. C. Gallucci, Y. Ghosh and T. F. Spilker, Dalton Trans., 2014, 43, 11397 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1873610 and 1873611. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8py01486h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |