Palladium-catalyzed tandem one-pot synthesis of π-expanded imidazoles through a sequential Heck and oxidative amination reaction†
Abstract
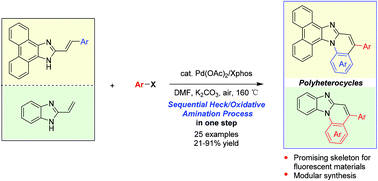
An efficient palladium-catalyzed route for tandem synthesis of imidazole-fused polyheterocycles from 2-vinyl imidazoles and aryl halides is described. The sequentially accomplished process comprises intermolecular Heck arylation of 2-vinyl imidazoles followed by an intramolecular aerobic oxidative C–H amination reaction promoted by the same Pd catalyst. This Pd-catalyzed tandem approach offers a straightforward protocol for the assembly of benzimidazo/phenanthroimidazo[1,2-a]quinolines in moderate to high yields, serving as a useful tool for the discovery of fluorescent materials.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...