Investigations of the generality of quaternary ammonium salts as alkylating agents in direct C–H alkylation reactions: solid alternatives for gaseous olefins†
Abstract
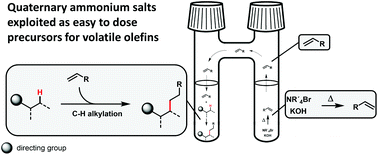
C–H alkylation reactions using short chain olefins as alkylating agents could be operationally simplified on the lab scale by using quaternary ammonium salts as precursors for these gaseous reagents: Hofmann elimination delivers in situ the desired alkenes with the advantage that the alkene concentration in the liquid phase is high. In case a catalytic system did not tolerate the conditions for Hofmann elimination, a very simple spatial separation of both reactions, Hofmann elimination and direct alkylation, was achieved to circumvent possible side reactions or catalyst deactivation. Additionally, the truly catalytically active species of a rhodium(I) mediated alkylation reaction could be identified by using this approach.
- This article is part of the themed collections: Synthetic methodology in OBC and Direct C-H Functionalization in Method Development and Late Stage Functionalization


 Please wait while we load your content...
Please wait while we load your content...