Synthesis of 4-styrylcoumarins via FeCl3-promoted cascade reactions of propargylamines with β-keto esters†
Abstract
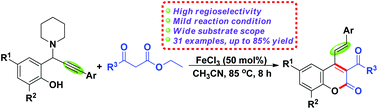
A versatile and highly regioselective FeCl3-promoted tandem cyclization reaction of in situ generated alkynyl o-quinone methides (o-AQMs) with β-keto esters has been developed on the basis of the mode involving an intermolecular 1,4-conjugate addition/alkyne–allene isomerization/intramolecular transesterification/isomerization cascade. Using this method, a variety of diversely substituted 4-styryl-2H-chromen-2-ones were prepared with good efficiency and exclusive site-selectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...