A two-dimensional assembly of ultrafine cobalt oxide nanocrystallites anchored on single-layer Ti3C2Tx nanosheets with enhanced lithium storage for Li-ion batteries†
Xuan
Sun
,
Ke
Tan
,
Yang
Liu
,
Jinyang
Zhang
,
Dienguila Kionga
Denis
,
Fakhr uz
Zaman
,
Linrui
Hou
 and
Changzhou
Yuan
and
Changzhou
Yuan
 *
*
School of Material Science & Engineering, University of Jinan, Jinan, 250022, P. R. China. E-mail: mse_yuancz@ujn.edu.cn; ayuancz@163.com
First published on 8th July 2019
Abstract
Recently, Ti-based MXenes were expected to compete with graphene and other carbonaceous materials towards Li-ion batteries (LIBs) due to their two-dimensional (2D) open structure, cost efficiency, superior conductivity and low Li+ diffusion barrier. However, the relatively moderate capacity and aggregation tendency hamper their practical applications in next-generation LIBs. Herein, we explore for the first time a scalable bottom-up approach to fabricate a series of Co3O4@single-layer Ti3C2Tx (s-Ti3C2Tx) hybrids, where numerous homogeneous Co3O4 nanocrystallites (NCs), serving both as a spacer and electroactive phase, are anchored uniformly on the surface of s-Ti3C2Tx nanosheets (NSs) through the Co–O–Ti interfacial bonds. Furthermore, detailed experimental analyses clearly shed light upon the formation mechanism of the hybrid Co3O4@s-Ti3C2Tx NSs. Thanks to the structural and compositional merits, the 2D Co3O4@s-Ti3C2Tx NSs even exhibit a remarkable high-rate capacity of ∼223 mA h g−1 at an ultra-high current density of 10 A g−1, and a long-span cycle life with a high reversible capacity of 550 mA h g−1 at 1 A g−1 after 700 consecutive cycles. Corresponding density functional theory calculation further confirms that the Co–O–Ti interfacial function leads to an even higher pseudocapacitive contribution and faster lithium storage behavior due to the enhanced interfacial electron transfer.
1. Introduction
The emerging market of large-scale energy storage applications, such as electric vehicles and electric grids, encourages a great deal of interest in research on versatile energy storage systems (ESSs).1 Li-ion batteries (LIBs), as a promising platform for ESSs, have drawn significant attention in terms of energy/power density and lifetime merits. Unfortunately, the low-capacity graphite-based anodes (∼372 mA h g−1) seriously limit the applications of commercial LIBs. Many researchers are making enormous efforts to explore eligible anodes for advanced LIBs to meet rigorous future demands.2–4Among the available anode candidates, transition-metal-oxides (TMOs) have been extensively investigated due to their large specific theoretical capacities,5 in which Co3O4 is deemed as an anode for LIBs owing to its large capacity and facile synthesis.6,7 However, the direct utilization of Co3O4, like all other TMOs, still results in several ineluctable disadvantages, including sluggish Li+ kinetics,8 severe volumetric expansion/shrinkage and an unstable solid electrolyte interphase (SEI) layer over successive lithiation/delithiation cycles.9,10 To date, two general efficient strategies have been developed well to address these issues. One is reducing the particle size of Co3O4 from micro- to nano-dimensions, effectively shortening the Li+ diffusion path and facilitating the accommodation of volumetric changes, and the other is the hybridization of Co3O4 with other conductive and flexible matrices, which can improve the conductivity of the Co3O4-based anodes and buffer the internal stress caused by the volume change meanwhile. Although both exhibit positive functions on enhancing the rate and cycle behaviors of LIBs,11 however, the synthesis of well-dispersed nanoscale Co3O4 in appealing matrices still remains a great challenge towards enhancing its lithium storage via smart integration of the two methods mentioned above.
Recently, MXenes, a representative type of two-dimensional (2D) transition metal carbide/nitride, have gained widespread attention in energy-related applications.12–16 Typically, Ti3C2Tx (T for F, OH and O) is one of the most investigated MXenes due to its mature preparation process and low cost. Besides, Ti3C2Tx intrinsically possesses metallic conductivity, continuous 2D charge transfer channels,17,18 a robust structure19 and a low ionic diffusion barrier,20,21 which make it especially serve as a matrix material to support other electroactive materials, such as TMOs, for elegantly engineering robust LIB electrodes. Commonly, multi-layer Ti3C2Tx (m-Ti3C2Tx) is applied for LIBs. However, the huge interlayer space in the m-Ti3C2Tx is just a “dead surface”, which cannot be fully exploited for loading other electroactive phases, or even utilized as electroactive sites for lithium storage. By contrast, single-layer Ti3C2Tx (s-Ti3C2Tx) nanosheets (NSs) emerge with an even larger naked surface. Unfortunately, numerous hydrogen bonds attached on their surface Ti atoms make the s-Ti3C2Tx NSs prone to restacking, resulting in a substantial loss of active area and inaccessibility of electrolyte ions.22–24 Therefore, it is of huge significance to devise several simple yet efficient strategies to construct nanoarchitectured hybrids consisting of s-Ti3C2Tx NSs and nanosized Co3O4.
Inspired by the comprehensive considerations above, in this work, we developed a facile freeze-drying strategy along with the subsequent calcination for large-scale fabrication of 2D hybrid Co3O4@s-Ti3C2Tx NSs, where the s-Ti3C2Tx NSs were uniformly decorated with size-controlled Co3O4 nanocrystallites (NCs). The negatively charged functional groups on the surface of s-Ti3C2Tx NSs anchored and restrained the growth and amalgamation of the Co3O4 NCs; meanwhile the in situ growth of Co3O4 NCs minimized the strong tendency to restacking and/or agglomeration of the s-Ti3C2Tx itself. Owing to the synergetic contributions from their compositional and structural advantages, the as-obtained Co3O4@s-Ti3C2Tx hybrid NSs exhibited a superb rate capability of ∼223 mA h g−1 at 10 A g−1, high reversible capacity and long-term cycling stability (up to ∼550 mA h g−1 after 700 cycles at 1 A g−1 and ∼160 mA h g−1 at 5 A g−1 after 1000 cycles) as a competitive anode material for advanced LIBs.
2. Experimental section
2.1 Material preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min per cycle) to ensure that the pH reaches ∼7.0. A stable s-Ti3C2Tx colloidal solution was collected by centrifugation (10
000 rpm, 5 min per cycle) to ensure that the pH reaches ∼7.0. A stable s-Ti3C2Tx colloidal solution was collected by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min per cycle) after sonication (1 h) of the sedimentation. Although the texture of s-Ti3C2Tx could be preserved largely in the initial 5 days in aqueous solution at room temperature (RT), the colloidal solution was stored in a refrigeration environment (at ∼4 °C) to improve the stability and lifetime of s-Ti3C2Tx. To obtain freeze-dried Ti3C2Tx (f-Ti3C2Tx), the resultant colloidal solution (2.5 mg mL−1) was rapidly frozen using liquid nitrogen, and further freeze-dried at −90 °C.
000 rpm, 5 min per cycle) after sonication (1 h) of the sedimentation. Although the texture of s-Ti3C2Tx could be preserved largely in the initial 5 days in aqueous solution at room temperature (RT), the colloidal solution was stored in a refrigeration environment (at ∼4 °C) to improve the stability and lifetime of s-Ti3C2Tx. To obtain freeze-dried Ti3C2Tx (f-Ti3C2Tx), the resultant colloidal solution (2.5 mg mL−1) was rapidly frozen using liquid nitrogen, and further freeze-dried at −90 °C.
2.2 Material characterization
The zeta potentials of the s-Ti3C2Tx colloidal solution and the Ti3C2Tx/Co salt mixture were measured using a Zetasizer Nano-ZS90 (Malvern Instruments). The thickness of the Ti3C2Tx NSs was measured using an atomic force microscope (AFM, Bruker Dimension Icon). The crystalline phases of all samples were characterized by powder X-ray diffraction (XRD, Cu Kα radiation, Rigaku Ultima IV) at a step scan of 10° min−1 ranging from 5 to 80°. The morphologies of the samples were investigated by field-emission scanning electron microscopy (FESEM, JEOL-6300F, 15 kV), transmission electron microscopy (TEM), scanning TEM (STEM), and high-resolution TEM (HRTEM) (JEOL JEM 2100 system) with energy dispersive X-ray spectroscopy (EDS). The chemical composition and surface elemental electronic states were characterized by EDS and X-ray photoelectron spectroscopy (XPS, Thermo, an Escalab 250xi spectrometer) with an Al Kα monochromatic X-ray source (1486.6 eV). Raman spectroscopy was performed using a Raman spectroscope (Lab RAM HR, excited by a 514.5 nm laser).2.3 Electrochemical evaluation
Coin-type cells (CR2032) were fabricated in an argon-filled glove box (MBRAUN, Germany) with oxygen and moisture contents being under 0.5 ppm to evaluate the electrochemical performance of the electrodes. The working electrodes were obtained by mixing the active materials with acetylene black and carboxymethyl cellulose (CMC, average Mw: ∼250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) (80
000) (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in weight) in DI water. Then the homogeneous slurry was coated on copper foil and dried in a vacuum oven at 110 °C. The typical loading mass of the electroactive materials was ∼1.5 mg cm−2. In a half-cell configuration, metallic lithium foil was used as the counter/reference electrode and a microporous membrane (Celgard 2302) as the separator. The electrolyte was a solution of 1.0 M LiPF6 in ethylene carbonate and diethylene carbonate with a volumetric ratio of 1
10 in weight) in DI water. Then the homogeneous slurry was coated on copper foil and dried in a vacuum oven at 110 °C. The typical loading mass of the electroactive materials was ∼1.5 mg cm−2. In a half-cell configuration, metallic lithium foil was used as the counter/reference electrode and a microporous membrane (Celgard 2302) as the separator. The electrolyte was a solution of 1.0 M LiPF6 in ethylene carbonate and diethylene carbonate with a volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Galvanostatic charge–discharge cycling tests were carried out using the LAND CT2001A battery testing system (Wuhan, China) with a voltage window range of 0.01–3.00 V (vs. Li/Li+). Cyclic voltammetry (CV) tests were also performed in a voltage range between 0.01 and 3.00 V (vs. Li/Li+). The electrical impedance spectroscopy (EIS) measurements were performed between 0.01 and 100 kHz with a potential amplitude of 5.0 mV. Both CV and EIS were conducted on an electrochemical workstation (IviumStat.h, The Netherlands). All electrochemical measurements were conducted at RT.
1. Galvanostatic charge–discharge cycling tests were carried out using the LAND CT2001A battery testing system (Wuhan, China) with a voltage window range of 0.01–3.00 V (vs. Li/Li+). Cyclic voltammetry (CV) tests were also performed in a voltage range between 0.01 and 3.00 V (vs. Li/Li+). The electrical impedance spectroscopy (EIS) measurements were performed between 0.01 and 100 kHz with a potential amplitude of 5.0 mV. Both CV and EIS were conducted on an electrochemical workstation (IviumStat.h, The Netherlands). All electrochemical measurements were conducted at RT.
2.4 Computational method
All the spin-polarized density functional theory (DFT) calculations were performed with the Perdew–Burke–Ernzerhof (PBE)25 function within the generalized gradient approximation (GGA) using the Vienna ab initio simulation package (VASP).26 The projector augmented wave (PAW) method was used to describe the electron–ion interaction.27 The van der Waals (vdW) interaction was described using the opt-B86b approach.28 The Hubbard-U (GGA+U, U = 3 eV)29 correction was applied for a better description of the localized d-electrons of the Co element. A hydroxylation structure consisting of Ti3C2 and the (222) surface of Co3O4 was used to model the Co3O4/Ti3C2 periodic interface. A relatively small supercell, i.e., 1 × 1 of Co3O4 (222) on 4 × 4 of Ti3C2, is large enough to maintain the lattice mismatch between Ti3C2 and Co3O4 (222) within 2.5%. What's more, among all the low index facets, the (222) surface of Co3O4 has the lowest surface energy.30 A plane wave basis set with a cut-off energy of 500 eV was used. The Brillouin zone was sampled by using a 3 × 3 × 1 Monkhorst–Pack k-point mesh. All structures were optimized based on the electronic and ionic degrees of freedom using thresholds for the total energy of 10−5 eV and a force of 0.02 eV Å−1. A vacuum region of about 15 Å was adopted to avoid unnecessary interactions.3. Results and discussion
3.1. Physicochemical and structural characterization
The overall synthetic procedure for the assembly of uniform Co3O4 NCs anchored on 2D s-Ti3C2Tx NSs is schematically illustrated in Scheme 1. Firstly, m-Ti3C2Tx is etched from the bulk Ti3AlC2 with the HF solution. After being delaminated by TMAOH under sonication via an ion exchange between protons and TMA+ in the interlayer of m-Ti3C2Tx, swelling and exfoliation take place, and the s-Ti3C2Tx colloidal solution is obtained with an average zeta potential of −3.34 μm cm V s−1, owing to the negatively charged functional groups on the surface of s-Ti3C2Tx. With the subsequent addition of cobalt ions into the above solution, the Co2+ ions were spontaneously attached on the s-Ti3C2Tx NSs through the electrostatic effect, rendering the mixture with a decreased zeta potential of −0.539 μm cm V s−1. The hybrid solution was rapidly frozen by using liquid nitrogen. After being dried via a freeze-drying process, the sample is then annealed in N2, which induces the in situ decomposition of the cobalt salt into the Co3O4 NCs on the s-Ti3C2Tx surface, and the 2D Co3O4@s-Ti3C2Tx hybrid NSs are produced accordingly.Fig. 1 shows the wide-angle X-ray diffraction (XRD) patterns of Co3O4@s-Ti3C2Tx with various Co3O4 loadings (i.e., H-2, H-4 and H-8), along with those for the pure Co3O4 and f-Ti3C2Tx. All these discernable peaks for H-2, H-4 and H-8 are indexed and assigned to the reflections of the cubic Co3O4 phase (JCPDS file No. 76-1802) with a space group of Fd3m. Besides, typical diffraction signals from Co3O4 become more and more intense as the Co3O4 content increases in the hybrids. It is worth noting that the (002) peak from Ti3C2Tx in the XRD pattern of Co3O4@s-Ti3C2Tx hybrids obviously disappears. This unique phenomenon may be ascribed to the fact that the in situ growth of Co3O4 NCs, as a smart spacer, efficiently restrains the restacking of the s-Ti3C2Tx NSs in the 2D hybrids.31 In addition, the defect introduction in s-Ti3C2Tx components due to the formation of Co3O4 NCs would weaken the (002) peaks from the Ti3C2Tx.1 It is noteworthy that no signals for TiOx phases can be detected in the reflections for the hybrids, which confirms that the oxidation of s-Ti3C2Tx can be prevented by low-temperature annealing under a N2 atmosphere.
Raman spectroscopy was conducted to further figure out the surface structure of the products, as comparably shown in Fig. 2. The characteristic peak at 205 cm−1 results from the out-of-plane A1g vibration of the Ti atoms in the f-Ti3C2Tx, and those peaks at 370 and 571 cm−1 correspond to the C vibrations.32,33 As noted, the peaks detected at around 193, 481, 523, and 670 cm−1 can be attributed to the F2g, Eg, F12g, and A1g modes of the Co3O4, respectively.34 Strikingly, the H-4 specimen combines the prominent Raman features of the Co3O4 and f-Ti3C2Tx, while the peak intensities of the Co3O4 in H-4 become even weaker, compared to that for the pure Co3O4, for which the Co3O4 NCs separated well by s-Ti3C2Tx NSs should be well responsible.35 Notably, in the H-4 specimen, the characteristic peaks for the Co3O4 exhibit an obvious shift to the lower wavenumber while those for the f-Ti3C2Tx shift to the higher wavenumber, indicating the strong chemical interactions between the s-Ti3C2Tx and Co3O4.
X-ray photoelectron spectroscopy (XPS) was further performed to explore the chemical composition and valence information of the obtained products. Compared with m-Ti3C2Tx, the increase in the intensity of O 1s is obviously observed for the f-Ti3C2Tx and H-4, as visualized in the survey spectra (Fig. 3a), which is related to more oxygen functional groups (such as –OH and –O) terminating the larger naked surface of the s-Ti3C2Tx and Co3O4 NCs in the H-4 sample, respectively. The high-resolution XPS spectra and the corresponding fitted peaks of Ti 2p for the three samples are shown in Fig. 3b, and the corresponding quantitative analysis is shown in Fig. 3c. In contrast to m-Ti3C2Tx, the peaks attributed to Ti3+ and Ti–O from the f-Ti3C2Tx and H-4 samples become even stronger, which increase from 13.3 at% (m-Ti3C2Tx) to 23.9 at% (f-Ti3C2Tx)/28.7 at% (H-4) for Ti3+, and from 9.2 at% (m-Ti3C2Tx) to 35 at% (f-Ti3C2Tx)/71.3 at% (H-4) for Ti–O, respectively. In contrast, the peaks corresponding to Ti and Ti2+ become significantly weakened in the f-Ti3C2Tx, and even disappear in H-4, revealing the increase in the valence of Ti atoms in the hybrid. In particular, the shift in the binding energy for the H-4 sample confirms the existence of chemical bonding between the s-Ti3C2Tx and Co3O4 NCs, which will result in the spontaneous charge transfer from the adsorbates to the substrate via the Co–O–Ti bonds at the interface between the two components.36,37 To straightforwardly elucidate the impact of –OH terminations and Co–O–Ti bonds on the valence state of Ti atoms, atomic charge calculation was conducted using the Bader charge analysis.38 The analysis for the relaxed configuration about the two-layer bare Ti3C2 and the hydroxylation structure of the hybrid is conducted. As for the bare Ti3C2 (Fig. 3d), the Bader charge of Ti atoms ranges from 1.03 to 1.57, in which the Ti3 surface from the interlayer possesses the lowest valence state due to the vdW interaction between the two layers. While for the hydroxylated Ti5 surface, the Bader charge increases from 1.13 to 1.50 since the O draws electrons from the atoms of Ti5, which is consistent with the report by Wang's group.39 As shown in Fig. 3e, the Co atoms on the Co3O4 (222) surface are anchored on the surface of s-Ti3C2Tx, forming Co–O–Ti bonds at the interface between the two components. As a result, with the above calculation results in mind, it is easy to conclude that the charges of Co atoms would transfer to Ti4 through O ions via the interfacial bonds, and that the surface Ti4 in the hybrid has the highest valence state of 1.59, which provides further evidence for the strong interaction between the s-Ti3C2Tx NSs and Co3O4 NCs. Accordingly, Ti elements in the hybrids display a higher binding energy in average, which is in good agreement with the XPS spectra (Fig. 3b).
The C 1s region of the m-Ti3C2Tx, f-Ti3C2Tx and H-4 samples (Fig. S1a and Table S1, ESI†) displays four components corresponding to the C–Ti bonds from Ti3C2, C–C bonds from the testing type (284.6 eV),40,41 and C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from oxygen terminations, respectively. The component assigned to C–Ti shows an obvious decline in f-Ti3C2Tx and H-4, which can possibly be attributed to the defect introduction in the Ti–C layers due to the exfoliation and annealing processes.36 The O 1s XPS spectra (Fig. S1b and Table S1, ESI†) can be fitted with four kinds of peaks, corresponding to the Co–O and/or Ti–O bonds (M–O), oxygen termination bonds (–Ox, –(OH)x), adsorbed H2O (H2Oads) and organic compounds (OR) due to atmospheric contaminations, which overlap with, and obscure, many other peaks.36,37 Notably, the peak intensity of the M–O bonds obviously increases in H-4, indicating the formation of Co–O bonds. Quantitative analysis (Fig. S1c, ESI†) implies that the specific content of Co3O4 in the H-4 sample is ∼58.95 wt% based on its stoichiometry, which is in good agreement with the theoretical data.
O bonds from oxygen terminations, respectively. The component assigned to C–Ti shows an obvious decline in f-Ti3C2Tx and H-4, which can possibly be attributed to the defect introduction in the Ti–C layers due to the exfoliation and annealing processes.36 The O 1s XPS spectra (Fig. S1b and Table S1, ESI†) can be fitted with four kinds of peaks, corresponding to the Co–O and/or Ti–O bonds (M–O), oxygen termination bonds (–Ox, –(OH)x), adsorbed H2O (H2Oads) and organic compounds (OR) due to atmospheric contaminations, which overlap with, and obscure, many other peaks.36,37 Notably, the peak intensity of the M–O bonds obviously increases in H-4, indicating the formation of Co–O bonds. Quantitative analysis (Fig. S1c, ESI†) implies that the specific content of Co3O4 in the H-4 sample is ∼58.95 wt% based on its stoichiometry, which is in good agreement with the theoretical data.
Fig. 4a shows the field-emission scanning electron microscopy (FESEM) image of the s-Ti3C2Tx. Micro-sized NSs are apparently observed, which is wholly distinct from the accordion-like m-Ti3C2Tx (Fig. S2, ESI†). The typical Tyndall effect, also known as the Willis–Tyndall scattering phenomenon (Fig. 4b), visually evidences the good dispersion of the s-Ti3C2Tx in aqueous solution. The atomic force microscopy (AFM) measurement was further performed to detect the topography of the resulting s-Ti3C2Tx NSs. As shown in Fig. 4c, the thickness of the s-Ti3C2Tx NSs with a lateral dimension of approximately 500 nm is approximately 1.5 nm, somewhat larger than that of unilamellar Ti3C2Tx NSs (∼0.98 nm).42,43 The increase in the thickness of the s-Ti3C2Tx observed here should mainly result from the presence of surface adsorbates, such as water molecules, similar to the observations on other 2D materials as well.44Fig. 4d shows the FESEM image of the f-Ti3C2Tx specimen. Loose and microscale NSs are still evident. The TEM images (Fig. 4e and f) illustrate the thin and electron-beam transparent but slightly restacked morphology of f-Ti3C2Tx, which proves the vdW interaction between the NSs. In addition, the flakes of f-Ti3C2Tx, as marked in Fig. 4f, are composed of only about 2–3 layered NSs, which can be verified from the HRTEM image (Fig. 4g), which authenticates the restacking few-layer structure of the f-Ti3C2Tx itself.
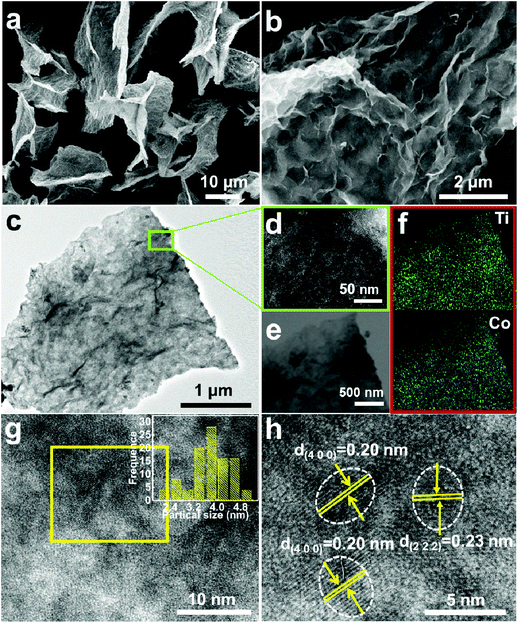
Fig. 5a and b show the FESEM images with various magnifications for the H-4 sample. Delightfully, the H-4 sample exhibits a kinetically favorable 2D structure with a crumpled surface (Fig. 5a and b). Obviously, it is wholly different from the frozen product obtained using the refrigerator pre-freezing method (Fig. S3, ESI†), which shows a simple agglomerated structure. These distinct observations fully corroborate that the rapid pre-freezing process is critical in the efficient formation of 2D Co3O4@s-Ti3C2Tx hybrids. As is well known, the liquid nitrogen pre-freezing process shows a higher freezing rate than the common refrigerator pre-freezing avenue, and the slow freezing rate would allow water molecules to exclude foreign particles and eventually lead them to aggregation.45 Therefore, if the dispersion was frozen at a slower freezing rate, such as in a refrigerator, the hybrids may have enough time to form strong aggregates. Delightfully, the lateral size of H-4 can reach over 50 μm, which is much larger than that of f-Ti3C2Tx flakes (Fig. 4c). This result indicates that the negatively charged s-Ti3C2Tx NSs are assembled with each other due to the induction of positively charged metal ions, and accordingly the 2D assembly of the Co3O4@s-Ti3C2Tx NSs is obtained. Notably, the extended 2D structure of hybrids is vitally important to provide sufficient and effective electron conduction channels for Co3O4 NCs during cycling. Interestingly, after a careful inspection into the TEM images (Fig. 5c and d) with various magnifications, no discernable particles could be found on the surface of the s-Ti3C2Tx NSs. However, the scanning TEM (STEM) image (Fig. 5e) and the corresponding EDS elemental (Ti and Co) mappings (Fig. 5f) evidently authenticate the uniform distribution of the element Co over the s-Ti3C2Tx substrate, and the atomic Co/Ti ratio is found to be ∼3.9 in the H-4 sample (Fig. S4b, ESI†). Further HRTEM observations (Fig. 5g and h) suggest that the high-quality Co3O4 NCs with an ultra-small size less than 5 nm (the inset of Fig. 5g) are uniformly dispersed on the flexible s-Ti3C2Tx matrix without obvious agglomeration. With the strong Co–O–Ti bonds, numerous separated Co3O4 NCs are strongly anchored on the surface of s-Ti3C2Tx, which in turn self-limits the growth of Co3O4 NCs. The lattice distance of 0.20 and 0.23 nm, respectively, corresponds to the (400) and (222) crystalline plane distance of Co3O4, as well demonstrated in Fig. 5e. In sharp contrast, only the aggregates with large particles are obtained in the absence of the s-Ti3C2Tx matrix (Fig. S5, ESI†).
For comparison, the micro-morphologies of the H-2 and H-8 samples are also characterized, and shown in Fig. 6. As presented in Fig. 6a, similar to that of the H-4 sample, the H-2 sample exhibits a 2D open NS structure with an atomic Co/Ti ratio of ∼2.3 (Fig. S4a, ESI†), which is lower than H-4. With regard to the H-8 sample (Fig. 6b), the Co3O4 NCs seriously aggregate and overlap on the surface of the s-Ti3C2Tx NSs, which can be attributed to the limited surface of the s-Ti3C2Tx and the high loading (atomic Co/Ti ratio = ∼7.8, Fig. S4c, ESI†) of the Co3O4 in the hybrid. Furthermore, the TEM (Fig. 6c and d) and HRTEM (Fig. 6e and f) images clearly reveal that the size of the Co3O4 NCs becomes even larger and denser as the concentration of the cobalt salt gradually increases. Specifically, for the H-2 sample, the Co3O4 NCs are ultrafine with an average size less than 3 nm (the inset in Fig. 6c), isolated from each other (Fig. 6c and e), and uniformly dispersed on the s-Ti3C2Tx matrix (Fig. 6g). While for the case of H-8 (Fig. 6d and f), the average size of the Co3O4 NCs is even up to ∼9 nm (the inset in Fig. 6d), but were still well dispersed throughout the s-Ti3C2Tx NSs, as supported by the STEM and corresponding EDS elemental (Ti and Co) mapping images (Fig. 6h). In contrast, for the H-12 sample (Fig. S6, ESI†), namely the specimen with the highest loading of Co3O4, the Co3O4 NCs agglomerated severely and separated with the Co3O4@s-Ti3C2Tx hybrids due to the excessive introduction of Co3O4, which produces huge volume expansion in the charging and discharging processes.
3.2. Electrochemical evaluation
Detailed electrochemical evaluations were conducted at RT to investigate the suitability of Co3O4@s-Ti3C2Tx hybrids as an anode material for LIBs. Fig. 7a shows the initial three CV profiles (0.01 mV s−1) of the H-4 electrode within the potential range from 0.01 to 3.0 V (vs. Li/Li+). The representative appearance of CV profiles generally corresponds to the co-contributions from the cobalt oxide and Ti3C2Tx itself.5,10,46–52 Specifically, the distinct reduction peak at around 0.50 V in the first cathodic scan corresponds to the reduction of Co3O4 to metallic cobalt (see eqn (1)) and formation of a solid electrolyte interphase (SEI) film due to the electrolyte decomposition. The small tail located at 0.07 V is ascribed to the reaction of Li+ ions with Ti3C2Tx (see eqn (2)). There are two distinct anodic peaks in the oxidation process. The one, appearing between 1.07 and 1.80 V, is assigned to the reactions of Co and Li2O, and the delithiation process of the s-Ti3C2Tx, and the other, situated near 2.11 V, is attributed to the transformation of Co into Co3O4, indicating the multi-step electrochemical reaction between Li2O and Co. The cyclic voltammetry (CV) curves of the 3rd and 5th cycles are almost the same and well overlapped, confirming the desirable cycling stability and electrochemical reversibility of the H-4 anode. For the f-Ti3C2Tx (Fig S7a, ESI†), only a couple small peaks located at 0.07 V and 0.12 V, corresponding to the lithiation and delithiation reaction between Li+ and the surface active sites of the s-Ti3C2Tx,53,54 are detected along with the embodied capacitive contribution. Furthermore, the low current responses in the CV curves of the f-Ti3C2Tx verify its modest Li-storage behavior. In contrast, the phase-pure Co3O4 sample (Fig S7b, ESI†) exhibits a series of strong and sharp peaks, corresponding to the typical redox process. Obviously, the CV profiles of the hybrid materials combine all the characteristic anodic/cathodic peaks belonging to the Ti3C2Tx and active Co3O4, indicating the combination of Co3O4 and Ti3C2Tx phases. The CV profiles of the H-2 (Fig. S7c, ESI†) and H-8 samples (Fig. S7d, ESI†) comparatively seem to be closely related to the Co3O4/s-Ti3C2Tx ratios in the hybrids. Typically, the H-8 sample shows similar CV curves to the Co3O4, owing to the high loading of electroactive Co3O4 NCs upon the surface of s-Ti3C2Tx NSs.| Co3O4 + 8Li+ + 8e− ↔ 3Co + 4Li2O | (1) |
| Ti3C2Tx + xLi+ + xe− ↔ LixTi3C2Tx | (2) |
Fig. 7b displays the typical galvanostatic charge–discharge curves of the H-4 anode at a current density of 100 mA g−1. The H-4 anode exhibits a discharge capacity of ∼1168 mA h g−1 and a charge capacity of ∼747 mA h g−1 indicating an initial coulombic efficiency (CE) of ∼64%. As observed in Fig. 7b the discharge plateaus appearing at ∼0.9 V in the first cycle move up to ∼1.4 V in the subsequent cycles and the charge plateaus are always located at ∼1.3 and ∼2.0 V in all cycles, which is consistent with the starting position of redox peaks in the CV results. In comparison with the hybrid anode, the f-Ti3C2Tx and bare Co3O4 (Fig. S8 ESI†) show only the first discharge/charge capacities of ∼167/∼71.0 mA h g−1 and ∼1267/∼924 mA h g−1 corresponding to the initial CE values of ∼43% and ∼73% respectively. The larger irreversibility of f-Ti3C2Tx should arise from numerous functional groups such as –OH and –F on its surface. Compared to the pure Co3O4 in micron dimension, the lower initial CE of the hybrids can be reasonably attributed to two main reasons. One is the low CE of the Ti3C2Tx NSs and the other is the enhanced electrode/electrolyte surfaces/interfaces resulting from ultrafine Co3O4 NCs, which leads to the serious consumption of Li+ due to the formation of a SEI film.
The rate capabilities within a wide current range from 0.1 to 10 A g−1 for the H-2, H-4, H-8 and f-Ti3C2Tx samples are also compared and collected in Fig. 7c. It should be noted that the f-Ti3C2Tx electrode delivers decreasing discharge specific capacities (DSCs) from ∼70 to ∼21 mA h g−1 with the current density up to 10 from 0.1 A g−1. Through a preliminary inspection it is easy to find that the DSCs increase with the increasing content of Co3O4 gradually at the initial current rates due to the high loading of the large-capacity Co3O4. Strikingly, the H-8 electrode achieves the highest capacity of ∼772 mA h g−1 at 100 mA g−1 but it declines quickly down to ∼235 mA h g−1 equaling to that of the H-4 electrode at a high rate of 10 A g−1. This should be related to the failure of efficient electronic conductive channels due to the coverage of Co3O4 with a larger size on the surface of the s-Ti3C2Tx NSs in the H-8 electrode. Because of the low weight ratio of Co3O4 to s-Ti3C2Tx the H-2 electrode exhibits minimum DSCs (i.e. ∼772 mA h g−1 at 100 mA g−1 and ∼87 mA h g−1 at 10 A g−1) in all hybrids. Encouragingly the specific capacities are estimated as ∼672, ∼629, ∼559, ∼486, ∼408, ∼297 and ∼223 mA h g−1 for the H-4 anode at current rates of 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 A g−1, respectively, which are much larger than those of the f-Ti3C2Tx and H-2. Furthermore with the current density of 0.1 A g−1, the capacity of the H-4 anode could recover to ∼682.2 mA h g−1 indicating its superb rate capability. As summarized in Fig. 7d benefiting from the fast charge storage and electron transfer properties, the H-4 and H-8 electrodes exhibit appealing capacity retention with the current rate increasing, which is superior to many other cobalt oxide-based/MXene-based anodes reported previously especially at the high rates.23,46–52 Therefore the s-Ti3C2Tx NSs can be highly expected as an ideal matrix for fabricating 2D hybrid anodes with superb electrochemical behavior for LIBs.
Fig. 7e shows the cycling performance of the H-2, H-4, H-8, pure Co3O4 and f-Ti3C2Tx samples over 700 consecutive cycles which were all conducted at a high current density of 1 A g−1 after 4-cycle activation at 100 mA g−1. The pure Co3O4 and H-8 anodes present much higher capacities than other anodes in the initial several cycles but suffer a dramatic decline as the charge/discharge cycles continue. This significant capacity degradation may result from the destruction of the structure caused by the strain during the electrochemical cycling. As noted the capacities of ∼420 and ∼550 mA h g−1 can be achieved by the H-2 and H-4 electrodes after 700 cycles respectively which are much higher than that of f-Ti3C2Tx (∼44.9 mA h g−1). It is worth mentioning that the cycling plots of the H-2 and H-4 anodes are almost as stable as the f-Ti3C2Tx sample and the CE value of the H-4 anode was close to 100% after the first few activation cycles until the 700th cycle, indicating their ultrafast lithium storage and excellent long-term cycling stability. Remarkably the H-4 anode holds excellent capability to balance the high reversible capacity and stability exhibiting its superb lithium storage properties for practical application. What's more, even after evaluating under 5 A g−1, the H-4 anode can still deliver a discharge capacity as large as ∼160 mA h g−1 after the 1000th cycle (Fig. 7f). The obviously improved rate performance and the cycling stability of H-4 could be ascribed to its desirable kinetic properties as evidenced by the EIS study (Fig. S9, ESI†). For each spectrum there is a depressed semicircle at a high frequency and a linear Warburg factor at a low frequency (Fig. S9a, ESI†). In the circuit model, Rs corresponds to the intercept with the x-axis which can reflect the conductivity of electrodes under the same test conditions. The size of the semicircle is attributed to the charge transfer resistance (Rct) at the interface between the reaction sites and electrodes. The fitted results (Table S2, ESI†) indicate that the f-Ti3C2Tx electrode shows the lowest Rs (1.16 Ω) and Rct (99.3 Ω) due to the excellent conductivity. Note that the much lower Rct (130.0 Ω) and Rs (1.30 Ω) of the H-4 anode than the pure Co3O4 (Rct of 238.2 Ω and Rs of 1.60 Ω) is beneficial for the enhancement of the charge transfer kinetics. Moreover the Rct value is only 175.4 Ω even after 700 cycles at a current density of 1 A g−1 which can be well responsible for the large reversible capacity, superb rate behavior and better stability of the H-4 anode. On the other hand the lithium diffusion kinetics can be investigated by plotting the real part of the complex impedance (Z′) vs. angular frequency (ω−1/2) (Fig. S9b, ESI†). The slope of the fitted line represents the Warburg coefficient (δω). The H-4 electrode shows a lower Warburg factor (δ = 80.6) than the Co3O4 electrode (δ = 114.4). It corresponds to a faster lithium diffusion rate in H-4 with respect to Co3O4 since the lithium diffusion coefficient (DLi+) is inversely proportional to δ2 with a relationship of DLi+ = R2T2/2A2n4F4C2δ2, where C (concentration of lithium ions), A (area of the electrode), F (Faraday constant), T (absolute temperature) and R (universal gas constant) are all constant under the same conditions and n (number of electrons per molecule in the reaction) has little effect on the overall value.1 The higher DLi+ implies that the layered structure of the H-4 electrode ensures fast transport of Li+ during cycling.
To further understand the charge-storage properties of the optimized H-4 anode, the corresponding kinetics analyses based on CV curves were carried out. Fig. 8a shows the CV curves at various sweep rates ranging from 0.4 to 2.4 mV s−1. The contributions of the surface-induced pseudo-capacitive process (k1v) and the diffusion-controlled insertion process (k2v0.5) of the H-4 electrode can be distinguished quantitatively by using the following formula: i (V) = k1v + k2v0.5 = avb (ref. 55 and 56), where i (V) and v are the response current at a fixed potential V and the specific scan rate respectively. k1 and k2 are constants and a and b are adjustable values. Generally the case of b = 0.5 indicates an ideal diffusion controlled intercalation process while the b value approaching 1.0 implies the surface-induced pseudo-capacitance controlled process. The b value can be obtained by the slope of the fitted line of log(i) and log(v). As illustrated in Fig. 8b, the b values for typical anodic and cathodic peaks are 0.67 and 0.74 respectively. The result verifies that the dual model charge storage mechanism is involved for the H-4 electrode. As calculated from the CV profile (Fig. 8c), the pseudo-capacitive contribution ratio is estimated as ∼68.1% at a scanning rate of 1.8 mV s−1, indicative of a favored surface/subsurface-induced charge storage process of the hybrid.57 Notably upon increasing the sweep rate the H-4 anode displays enlarged capacitive contributions of ∼44.3%, ∼56.7% and ∼79.4% at the scan rates of 0.4, 1.0 and 2.4 mV s−1 respectively as plotted in Fig. 8d. As a result, the high-ratio surface induced pseudo-capacitive process would highly promote the rate performance of the H-4 electrode.
All in all the enhanced electrochemical behaviors of the H-4 anode, especially high-rate behavior and long-span cycling stability, can be rationally ascribed to the following aspects. First the intrinsic Co–O–Ti interaction at the s-Ti3C2Tx/Co3O4 interfaces effectively limits the growth of the Co3O4 NCs and further prevents these electroactive NCs from shedding from the s-Ti3C2Tx surface during cycling, which in turn avoids the aggregation of ultrathin s-Ti3C2Tx NSs. Meanwhile the unique Co–O–Ti interfacial bonds allow convenient interfacial electron transfer between the Co3O4 and s-Ti3C2Tx. Second the 2D opened s-Ti3C2Tx as a matrix of hybrid materials not only promotes the electronic conductivity of the full electrode but also allows accessibility of the electroactive sites by Li+ ions in the electrolytes. Finally the Co3O4 NCs with a tiny size of ∼5 nm provide an extremely shortened diffusion pathway over charge storage, and the internal strain of the hybrid electrode over a continuous lithiation/de-lithiation process can be efficiently buffered with the synergistic contribution of the flexible s-Ti3C2Tx NSs. These results highlight the promising application of the Co3O4@s-Ti3C2Tx NS hybrids as high-performance anodes for next-generation LIBs.
4. Conclusions
In summary we developed for the first time an extremely facile method for the large-scale fabrication of 2D Co3O4@s-Ti3C2Tx hybrid NSs where ultrafine Co3O4 NCs are dispersed and anchored evenly upon the s-Ti3C2Tx surface through the Co–O–Ti interfacial bonds. The formation process of the Co3O4@s-Ti3C2Tx hybrids has been rationally clarified here. Benefiting from the appealing structural and compositional merits, the resultant H-4 anode exhibited remarkable Li-storage behavior even superior to many other MXene-based/cobalt oxide-based anodes in terms of rate capability and cycling stability. Impressively the H-4 electrode delivered a superb rate capability of ∼223 mA h g−1 at a rate of 10 A g−1 and maintained a reversible capacity of ∼550 mA h g−1 after 700 cycles at 1 A g−1. These results convincingly highlighted the promising application of the Co3O4@s-Ti3C2Tx NSs as advanced anodes for LIBs. More significantly the methodologies we devised here hold enormous promise in generalizing to other 2D metal oxides/s-Ti3C2Tx hybrids for versatile energy-related applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51572005, 51772127), Major Program of Shandong Province Natural Science Foundation (No. ZR2018ZB0317), Taishan Scholars (No. ts201712050), Natural Science Doctoral Foundation of Shandong Province (ZR2019BEM038), Natural Science Doctoral Foundation of the University of Jinan (XBS1830), and Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong.References
- S. Niu, Z. Wang, M. Yu, M. Yu, L. Xiu, S. Wang, X. Wu and J. Qiu, ACS Nano, 2018, 12, 3928–3937 CrossRef CAS.
- Y. Wu, P. Nie, L. Wu, H. Dou and X. G. Zhang, Chem. Eng. J., 2018, 334, 932–938 CrossRef CAS.
- L. Liang, W. Chen, X. Sun, S. Xuan, L. Hou, J. Sun and C. Yuan, Adv. Mater. Interfaces, 2017, 4, 1700382 CrossRef.
- J. Meng, Z. Liu, C. Niu, L. Xu, X. Wang, Q. Li, X. Wei, W. Yang, L. Huang and L. Mai, Mater. Horiz., 2018, 5, 78–85 RSC.
- X. Xu, S. Chen, C. Xiao, K. Xi, C. Guo, S. Guo, S. Ding, D. Yu and R. V. Kumar, ACS Appl. Mater. Interfaces, 2016, 8, 6004–6010 CrossRef CAS PubMed.
- H. Long, M. Zhang, Q. Wang, L. Xing, S. Wang and X. Xue, J. Alloys Compd., 2017, 701, 200–207 CrossRef CAS.
- L. Sun, Q. Deng, Y. Li, H. Mi, S. Wang, L. Deng, X. Ren and P. Zhang, Electrochim. Acta, 2017, 241, 252–260 CrossRef CAS.
- G. Liu and J. Shao, J. Mater. Chem. A, 2017, 5, 9801–9806 RSC.
- K. Xie, P. Wu, Y. Zhou, Y. Ye, H. Wang, Y. Tang, Y. Zhou and T. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 10602–10607 CrossRef CAS.
- W. Kang, Y. Zhang, L. Fan, L. Zhang, F. Dai, R. Wang and D. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 10602–10609 CrossRef CAS.
- M. Xu, Q. Xia, J. Yue, X. Zhu, Q. Guo, J. Zhu and H. Xia, Adv. Funct. Mater., 2018, 29, 1807377 CrossRef.
- M. Xu, S. Lei, J. Qi, Q. Dou, L. Liu, Y. Lu, Q. Huang, S. Shi and X. Yan, ACS Nano, 2018, 12, 3733–3740 CrossRef CAS.
- B. Ding, J. Wang, Y. Wang, Z. Chang, G. Pang, H. Dou and X. Zhang, Nanoscale, 2016, 8, 11136–11142 RSC.
- W. Bao, L. Liu, C. Wang, S. Choi, D. Wang and G. Wang, Adv. Energy Mater., 2018, 8, 1702485 CrossRef.
- A. Lipatov, H. Lu, M. Alhabeb, B. Anasori, A. Gruverman, Y. Gogotsi and A. Sinitskii, Sci. Adv., 2018, 4, eaat0491 CrossRef.
- X. Sun, Y. Liu, J. Y. Zhang, L. R. Hou, J. F. Sun and C. Z. Yuan, Electrochim. Acta, 2019, 295, 237–245 CrossRef CAS.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- D. Xiong, X. Li, Z. Bai and S. Lu, Small, 2018, 14, e1703419 CrossRef.
- H. Wang, Y. Wu, X. Yuan, G. Zeng, J. Zhou, X. Wang and J. W. Chew, Adv. Mater., 2018, 30, e1704561 CrossRef.
- Q. Tang, Z. Zhou and P. Shen, J. Am. Chem. Soc., 2012, 134, 16909–16916 CrossRef CAS.
- S. Sun, C. Liao, A. M. Hafez, H. Zhu and S. Wu, Chem. Eng. J., 2018, 338, 27–45 CrossRef CAS.
- X. T. Gao, Y. Xie, X. D. Zhu, K. N. Sun, X. M. Xie, Y. T. Liu, J. Y. Yu and B. Ding, Small, 2018, 14, e1802443 CrossRef.
- M. Q. Zhao, M. Torelli, C. E. Ren, M. Ghidiu, Z. Ling, B. Anasori, M. W. Barsoum and Y. Gogotsi, Nano Energy, 2016, 30, 603–613 CrossRef CAS.
- C. J. Zhang, S. Pinilla, N. McEvoy, C. P. Cullen, B. Anasori, E. Long, S.-H. Park, A. Seral-Ascaso, A. Shmeliov, D. Krishnan, C. Morant, X. Liu, G. S. Duesberg, Y. Gogotsi and V. Nicolosi, Chem. Mater., 2017, 29, 4848–4856 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- J. Klimeš, D. R. Bowler and A. Michaelides, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 195131 CrossRef.
- T. Sharifi, Y. Xie, X. Zhang, H. R. Barzegar, J. Lei, G. Coulter, S. Sun, C. Tiwary, A. Zettl, B. Yakobson and P. M. Ajayan, Carbon, 2019, 141, 266–273 CrossRef CAS.
- S. L. Dudarev, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- X. Wu, Z. Wang, M. Yu, L. Xiu and J. Qiu, Adv. Mater., 2017, 29, 1607017 CrossRef.
- C. Chen, X. Xie, B. Anasori, A. Sarycheva, T. Makaryan, M. Zhao, P. Urbankowski, L. Miao and J. Jiang, Angew. Chem., Int. Ed., 2018, 57, 1846–1850 CrossRef CAS.
- X.-D. Zhu, Y. Xie and Y.-T. Liu, J. Mater. Chem. A, 2018, 6, 21255–21260 RSC.
- Y. Jiang, X. Yan, W. Xiao, M. Tian, L. Gao, D. Qu and H. Tang, J. Alloys Compd., 2017, 710, 114–120 CrossRef CAS.
- X. Guo, X. Xie, S. Choi, Y. Zhao, H. Liu, C. Wang, S. Chang and G. Wang, J. Mater. Chem. A, 2017, 5, 12445–12452 RSC.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, Appl. Surf. Sci., 2016, 362, 406–417 CrossRef CAS.
- R. Meng, J. Huang, Y. Feng, L. Zu, C. Peng, L. Zheng, L. Zheng, Z. Chen, G. Liu, B. Chen, Y. Mi and J. Yang, Adv. Energy Mater., 2018, 8, 1801514 CrossRef.
- G. Henkelman, A. Arnaldsson and H. Jónsson, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef.
- T. Hu, Z. Li, M. Hu, J. Wang, Q. Hu, Q. Li and X. Wang, J. Phys. Chem. C, 2017, 121, 19254–19261 CrossRef CAS.
- J. Sun, G. Zheng, H. W. Lee, N. Liu, H. Wang, H. Yao, W. Yang and Y. Cui, Nano Lett., 2014, 14, 4573–4580 CrossRef CAS.
- J. Liu, H. B. Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z. Z. Yu, Adv. Mater., 2017, 29, 1702367 CrossRef.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS.
- X. Wang, X. Shen, Y. Gao, Z. Wang, R. Yu and L. Chen, J. Am. Chem. Soc., 2015, 137, 2715 CrossRef CAS.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- J. Lee and Y. Cheng, J. Controlled Release, 2006, 111, 185–192 CrossRef CAS PubMed.
- S. Chen, Y. Zhao, B. Sun, Z. Ao, X. Xie, Y. Wei and G. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 3306–3313 CrossRef CAS.
- D. Wang, Y. Yu, H. He, J. Wang, W. Zhou and H. D. Abruna, ACS Nano, 2015, 9, 1775–1781 CrossRef CAS.
- X. Yang, K. Fan, Y. Zhu, J. Shen, X. Jiang, P. Zhao, S. Luan and C. Li, ACS Appl. Mater. Interfaces, 2013, 5, 997–1002 CrossRef CAS.
- M. Liu, X. Deng, Y. Ma, W. Xie, X. Hou, Y. Fu and D. He, Adv. Mater. Interfaces, 2017, 4, 1700553 CrossRef.
- M. Jing, M. Zhou, G. Li, Z. Chen, W. Xu, X. Chen and Z. Hou, ACS Appl. Mater. Interfaces, 2017, 9, 9662–9668 CrossRef CAS.
- H. Zhang, P. Zhang, W. Zheng, W. Tian, J. Chen, Y. Zhang and Z. Sun, Electrochim. Acta, 2018, 285, 94–102 CrossRef CAS.
- J. Xiong, L. Pan, H. Wang, F. Du, Y. Chen, J. Yang and C. Zhang, Electrochim. Acta, 2018, 268, 503–511 CrossRef CAS.
- B. Ahmed, D. H. Anjum, Y. Gogotsi and H. N. Alshareef, Nano Energy, 2017, 34, 249–256 CrossRef CAS.
- C. Yang, Y. Liu, X. Sun, Y. R. Zhang, L. R. Hou, Q. A. Zhang and C. Z. Yuan, Electrochim. Acta, 2018, 271, 165–172 CrossRef CAS.
- V. Augustyn, J. Come, M. A. Lowe, J. W. Kim, P. L. Taberna, S. H. Tolbert, H. D. Abruna, P. Simon and B. Dunn, Nat. Mater., 2013, 12, 518–522 CrossRef CAS.
- T. Brezesinski, J. Wang, J. Polleux, B. Dunn and S. H. Tolbert, J. Am. Chem. Soc., 2009, 131, 1802–1809 CrossRef CAS PubMed.
- D. Chao, C. Zhu, P. Yang, X. Xia, J. Liu, J. Wang, X. Fan, S. V. Savilov, J. Lin, H. J. Fan and Z. X. Shen, Nat. Commun., 2016, 7, 12122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr04377b |
| This journal is © The Royal Society of Chemistry 2019 |