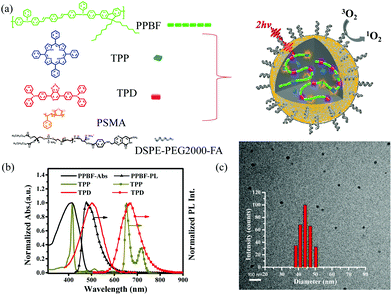
Red emitting conjugated polymer based nanophotosensitizers for selectively targeted two-photon excitation imaging guided photodynamic therapy†
Xiangyan
Duan
a,
Xiao-Fang
Jiang
ad,
Dehua
Hu
a,
Peng
Liu
a,
Shuang
Li
*a,
Fei
Huang
 a,
Yuguang
Ma
a,
Yuguang
Ma
 a,
Qing-Hua
Xu
a,
Qing-Hua
Xu
 *bc and
Yong
Cao
a
*bc and
Yong
Cao
a
aInstitute of Polymer Optoelectronic Materials and Devices, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: lishuang618@scut.edu.cn
bDepartment of Chemistry, National University of Singapore, Singapore 117543. E-mail: chmxqh@nus.edu.sg
cNational University of Singapore (Suzhou) Research Institute, Suzhou 215123, China
dSchool of Physics and Telecommunication Engineering, South China Normal University, Guangzhou 510006, China
First published on 15th November 2018
Abstract
Two-photon excitation (2PE) photodynamic therapy (PDT) is a non-invasive technique for the treatment of cancer. However, its clinical applications are limited by small two-photon absorption cross section values of conventional photosensitizers. Here we designed multifunctional conjugated polymer based nanoparticles consisting of a conjugated polymer, a photosensitizer and a red-emitting dye, which can realize simultaneous 2PE red emission imaging and 2PE-PDT activities. The working principle is based on a 2PE fluorescence resonance energy transfer strategy from the conjugated polymer to photosensitizing and imaging agents. In these nanoparticles (NPs), the conjugated polymer, PPBF, was chosen as a two-photon light-harvesting material while the photosensitizer (tetraphenylporphyrin, TPP) and the red-emitting dye (TPD) were chosen as energy acceptors. The 2PE emission of TPP and TPD was enhanced by up to ∼161 and ∼23 times, respectively. The 2PE-PDT activity of these NPs was significantly improved compared with those NPs without PPBF by up to ∼149 times. Further surface-functionalization with folic acid (FA) groups allows these nanoparticles to exhibit selective affinity toward KB cancer cells. These NPs could act as novel 2PE conjugated polymer based nanoparticles combined with the advantages of low dark cytotoxicity, selective targeting and imaging-guided 2PE-PDT activities.
Introduction
Photodynamic therapy (PDT) is a non-invasive medical treatment technique for many diseases.1,2 It uses light in combination with drug molecules which are generally known as photosensitizers (PSs). Upon activating the PS molecules by absorbing light of a particular wavelength, the excitation energy is eventually transferred to the nearby oxygen molecules after a series of photo-physical processes to generate cytotoxic reactive oxygen species, mostly singlet oxygen, for cell apoptosis.3,4 PDT activity is a synergistic effect of three key components: light, PS and tissue oxygen.5 Conventional PDT is generally performed by using ultraviolet or visible light as the excitation source to activate the PS molecules by one-photon excitation (1PE). Two-photon excitation photodynamic therapy (2PE-PDT) using near-infrared (NIR) light as the excitation source offers many advantages such as less interference from the surrounding biological environment and deeper tissue penetration to allow minimal invasive treatment.6–8 In addition, 2PE-PDT also benefits from great precision in treatment owing to the 3-dimensionally confined focal volume of 2PE, which is much smaller than that of 1PE.9However, the clinical applications of 2PE-PDT are limited by small two-photon absorption (2PA) cross section values (δ) of current clinically approved PSs.5 Moreover, most PSs used in PDT are hydrophobic and form aggregates easily in aqueous environment, resulting in low biocompatibility and reduced singlet oxygen yield, and consequently unsatisfactory therapeutic effects.10 In addition to generating cytotoxic singlet oxygen for killing cancer cells, photoluminescence of the PS is useful for fluorescence imaging applications, such as planning, assessment, and monitoring of the PDT response.3 However, it is difficult to realize both high singlet oxygen generation efficiency and high brightness from a molecular PS because fluorescence emission and singlet oxygen generation are two competing processes. Generally, most PS molecules are optimized for singlet oxygen generation and thus display a low fluorescence quantum yield. In order to realize an effective imaging-guided 2PE-PDT, recently lots of research efforts have been devoted to the development of PSs possessing large 2PA cross section values, high singlet oxygen generation efficiency, high brightness in the far-red/NIR range, and target specificity.11,12 Various nanophotosensitizers with dual capabilities of imaging and PDT have been developed, including polymeric NPs,13 up-conversion NPs,14 gold NPs,15,16 mesoporous silica NPs,17 quantum dots,18 and nanocomposites.19 These nanophotosensitizers have attractive advantages such as amphiphilicity, enhanced permeability and retention effect, and target-specific recognition moieties through surface biological modification and functionalization.20
Among these nanophotosensitizers, conjugated polymers have attracted extensive attention due to their large extinction coefficients, high fluorescence quantum yields and good biocompatibility.21–23 They can be utilized as two-photon light harvesting materials to improve the singlet oxygen production of the PS molecules.24 Conjugated polymer based nanoparticles (CPNs) also possess good biocompatibility for potential clinical applications.20 A lot of attention has been drawn to the development of CPNs for 2PE-PDT over the past few years.13,25–27 Chen et al. utilized a 2PA block copolymer to encapsulate a hydrophobic porphyrin to improve the singlet oxygen generation efficiency through energy transfer processes.28 Chang et al. designed CPNs by covalently incorporating porphyrin into the polymer backbone, which exhibit high singlet oxygen generation and overcome the leaching problem as encountered in the PS-doped CPNs.29 In previous work from our research group, Shen et al. developed CPNs (PFEMO/TPP NPs) with significantly enhanced two-photon-induced singlet oxygen generation capability through two-photon excitation fluorescence resonance energy transfer (2PE-FRET).13 Lately, we have synthesized a new conjugated polymer with large 2PA cross section values, which improved the singlet oxygen generation capability of the doped PSs by up to hundreds of times. Further surface modification with folic acid on the NPs helps to specifically target the KB cancer cells and thus realize targeted 2PE-PDT.25
Herein, based on our previous research work,13,21,25,30 we developed a donor–acceptor type of conjugated polymer based nanophotosensitizer with highly efficient 2PE-PDT activities and two-photon imaging capability at the same time by encapsulating a PS (tetraphenylporphyrin, TPP) and a red-emitting dye, 4,4′-([1,2,5]thiadiazolo [3,4-c]pyridine-4,7-diyl)-bis(N,N-diphenylaniline) (TPD, Fig. 1a), into a hydrophobic conjugated polymer, poly[1,4-bis(4-phenyl-phenylamino-styryl)-benzene-alt-2,7-dioctylfluorene] (PPBF, Fig. 1a). This nanophotosensitizer (PPBF/TPP/TPD NPs) consists of one energy donor (PPBF) and two energy acceptors (TPP, TPD). The incorporation of a non-emitting co-polymer poly(styrene-co-maleic anhydride) (PSMA) imparts hydrophilicity to the CPNs and helps to stabilize the NPs and prevent self-quenching of PPBF in the CPNs. The 2PA cross section value of CPNs is estimated to be 8.6 × 106 GM per NP at 750 nm. These PPBF/TPP/TPD NPs combine the advantages of three different materials through the 2PE-FRET strategy to offer simultaneous NIR excitation, far-red/NIR emission imaging and 2PE-PDT activities. In the as-prepared PPBF/TPP/TPD NPs, 2PE emissions of molecular TPP and TPD are enhanced by up to ∼161 and ∼23 times, respectively. The two-photon induced singlet oxygen generation efficiency of these PPBF/TPP/TPD NPs was improved by up to ∼149 times compared with those NPs without PPBF. Folic acid (FA) molecules have a strong binding affinity to folate receptors,31 which are overexpressed in some cancer cells.32,33 These PPBF/TPP/TPD NPs have been further surface-functionalized with DSPE-PEG2000-FA to introduce FA onto the nanoparticle surface for specific targeting capability toward the KB cancer cells. We have further demonstrated the application of these FA-PPBF/TPP/TPD NPs in 2PE-PDT and simultaneous 2PE cell imaging of the KB cancer cells with an excellent contrast ratio.
Results and discussion
Fig. 1a shows the schematic illustration of the preparation process for FA-PPBF/TPP/TPD NPs. In these NPs, the conjugated polymer (PPBF) acts as a two-photon light-harvesting complex (energy donor), while the PS molecule (TPP) and the red-emitting dye molecule (TPD) function as photosensitizing and imaging agents. PPBF was chosen as the two-photon light-harvesting material due to its large 2PA cross section values (δ750 nm = 529 GM per repeat unit) and high fluorescence quantum yield (∼84% in THF). The 2PA cross section value of each FA-PPBF/TPP/TPD NP was estimated to be 8.6 × 106 GM per NP at 750 nm (see the calculation method in the ESI†). TPP was chosen as the photosensitizing agent due to its high singlet oxygen generation efficiency (∼63%).34 The singlet oxygen quantum yield of the TPP doped polymer NPs was found to be ∼70% based on the singlet oxygen emission at 1270 nm using TMPyP4 as the reference standard. TPD was known as an excellent imaging contrast agent with a high fluorescence quantum yield (∼44% in hexane) in the far-red/NIR range (570–830 nm).35 The emission spectrum of PPBF has a reasonable overlap with the absorption spectra of TPP and TPD (Fig. 1b), which ensures efficient energy transfer from PPBF to TPP and TPD. The energy transfer efficiency (ET) can be estimated according to ET (%) = (1 − F/F0) × 100(%), where F0 and F are the emission intensities of the donor (PPBF) in the absence and presence of the acceptor molecules (TPP and/or TPD), respectively.36 Poly(styrene-co-maleic anhydride) (PSMA) acted as a stabilizing agent through their negatively charged carboxyl groups on the surface of the nanoparticles. The incorporation of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate (polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000-FA) allowed specific targeting to cancer cells.In addition to spectral overlap, another critical requirement for the efficient FRET is the close distance between the donor and acceptor molecules. The average distance between the donor and acceptor molecules can be adjusted by controlling the doping concentration of the acceptor molecules. Increasing the doping concentration of the acceptors will lead to smaller donor–acceptor separation distances and consequently efficient energy transfer. To fully utilize the excitation energy of PPBF under 2PE, the doping concentrations of the acceptors should not be too high to avoid energy dissipation via self-aggregation and energy transfer between the adjacent acceptor molecules themselves. The doping concentrations of TPP and TPD will therefore influence the overall two-photon imaging and therapeutic capabilities. The FRET from PPBF to TPD and the FRET from PPBF to TPP are two competing processes since both TPD and TPP take up the energy from PPBF. The efficiencies of both FRET processes are expected to be highly dependent on TPD and TPP concentrations, which were optimized separately.
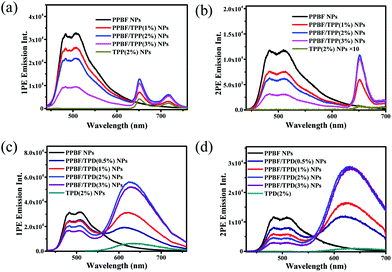
The FRET process in TPP-doped PPBF NPs (PPBF/TPP NPs) was first investigated. The 1PE and 2PE emission spectra of PPBF/TPP NPs in H2O with different TPP/PPBF molar ratios (from 0 to 3%) are shown in Fig. 2a & b. The 1PE emission spectra were recorded under excitation at 400 nm. As the molar ratio of TPP increased, the fluorescence intensity of PPBF (445–630 nm) decreased while the TPP emission intensity (635–750 nm) gradually increased (Fig. 2a). The observed increased TPP emission (635–750 nm) mainly results from intraparticle energy transfer from PPBF to TPP, because TPP has little absorption at λ = 400 nm and can barely be directly excited. The 2PE emission spectra were recorded under excitation using femtosecond laser pulses at 750 nm, where PPBF has the largest 2PA cross section values (529 GM per repeat unit, Fig. S1†). The trend of the 2PE emission spectra of PPBF/TPP NPs versus the molar ratio of TPP is similar to the corresponding 1PE emission spectra: as the molar ratio of TPP increased, the 2PE emission intensity of PPBF in PPBF/TPP NPs steadily decreased while the 2PE emission intensity of TPP (635–700 nm) steadily increased. Under both 1PE and 2PE, PPBF/TPP NPs with a molar ratio of 2% TPP give an optimum TPP emission intensity, under which the PPBF to TPP energy transfer efficiency (ET) is 48% (for 2PE). The higher doping concentration of TPP causes a reduced TPP emission intensity due to self-aggregation induced quenching.
The FRET process in PPBF/TPD NPs has also been investigated. Both 1PE and 2PE emission spectra of PPBF/TPD NPs displayed a similar trend as those of PPBF/TPP NPs (Fig. 2c & d). Compared to PPBF/TPP NPs and PPBF/TPD NPs containing the same amount of TPP and TPD, bare TPP and TPD NPs display a low 2PE emission intensity due to their smaller 2PA cross section values (δTPP = 12 GM,13δTPD = 97 GM, Fig. S1 in the ESI†).
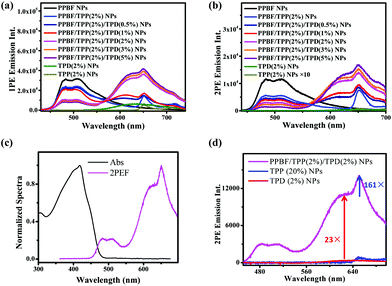
We fixed the TPP doping concentration at 2% to further introduce different amounts of the red-emitting dye, TPD, into the CPNs. The corresponding 1PE and 2PE emission spectra are shown in Fig. 3. The new broad emission bands from 550 to 700 nm in both 1PE and 2PE emission spectra of PPBF/TPP/TPD NPs mainly arise from energy transfer from PPBF to TPD as bare TPD NPs containing the same amount of TPD molecules exhibited negligible emission under the same experimental conditions. As the TPD doping concentration increased, the intensity of the new emission bands steadily increased and started to slow down when the doping concentration increased above 2%. It is interesting to note that further increase in the TPD doping concentration from 2% to 5% results in the reduction of the sharp peaks centered at 650 nm (arising from the FRET from PPBF to TPP), which indicated a competition between the FRET to TPD and TPP.
The enhancement factors of the 1PE and 2PE emission of TPP and TPD in PPBF/TPP/TPD NPs with different TPD molar ratios are summarized in Fig. S2 & S3,† respectively. 2PE emission intensities of TPP and TPD could be enhanced by factors of up to 225 ± 2 and 51 ± 1 upon incorporation of PPBF into the NPs. When the doping concentrations of TPP and TPD are both selected as 2% molar ratio of PPBF, the 1PE and 2PE emission intensities of TPD were enhanced by factors of 4.9 ± 0.1 and 23 ± 1, respectively (Fig. 3d). Meanwhile, the 1PE and 2PE emission of TPP was enhanced by factors of 1.1 ± 0.1 and 161 ± 4, respectively. PPBF/TPP/TPD NPs with doping concentrations of TPP and TPD were both selected as 2% for further investigation to achieve dual imaging and therapy capabilities. Under these conditions, the molecular photosensitizer (TPP) loading efficacy and loading capacity of the nanoparticles are 98.7% and 8.2%, respectively (see the detailed calculation in the ESI†).
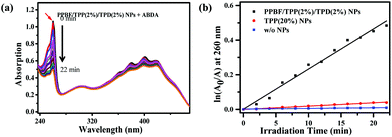
The two-photon-induced singlet oxygen generation capability of PPBF/TPP/TPD NPs was evaluated by using a photochemical method to monitor the photo-oxidation of 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA). ABDA can interact with singlet oxygen to convert into its endoperoxide, resulting in decreased absorption at 260 nm. In the presence of PPBF/TPP(2%)/TPD(2%) NPs the absorbance of ABDA at 260 nm decreases steadily with the irradiation time under irradiation with femtosecond laser pulses at 750 nm (Fig. 4). ABDA was reduced by >70% after irradiating the sample for ∼20 min. In the control experiments for ABDA incubated with 20% TPP NPs or without any nanoparticles, the decrease in the ABDA absorption intensity is much slower under the same irradiation conditions (Fig. S6 in the ESI†). The photo-oxidation rate of ABDA in the presence of PPBF/TPP(2%)/TPD(2%) NPs was 149 ± 3 times that of bare TPP NPs containing the same amount of TPP, which is quite close to the enhancement factor of the 2PE emission intensity of TPP (161 ± 4). This observation demonstrated that the generation of singlet oxygen could be significantly enhanced via the efficient FRET from PPBF to TPP. The photophysical properties of the nanoparticles remain nearly unchanged after the introduction of FA groups by mixing DSPE-PEG2000-FA in the feeding solution.
The physiological properties of FA-PPBF/TPP(2%)/TPD(2%) NPs were investigated before further studies in cells. The obtained NPs displayed an average diameter of 43.7 nm with a polydispersity index value of 0.367 based on dynamic light scattering measurements (inset of Fig. 1c). TEM results give a smaller diameter of 27 ± 3 nm due to particle shrinkage during the drying process on copper grids. The zeta potential of FA-PPBF/TPP(2%)/TPD(2%) NPs is −17.1 ± 1.4 mV, which is slightly less negative than that of PPBF/TPP/TPD NPs (−43 ± 4.3 mV) due to the introduction of DSPE-PEG2000-FA into the NPs. The stability of these NPs was tested in cell culture media for 24 h and there was no obvious change in the zeta potential, hydrodynamic diameter, and color of the NPs (see the ESI, Fig. S2†). No significant decrease in cell viability was observed in the presence of FA-PPBF/TPP/TPD NPs at a concentration range of up to 4 μM (in PPBF repeat units, Fig. S5†) in the dark.
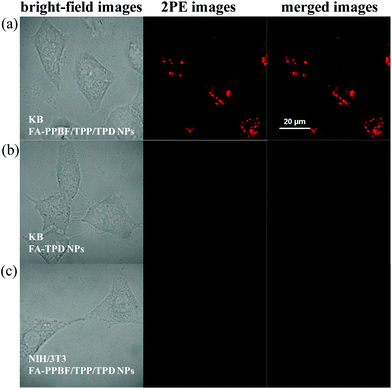
The 2PE fluorescence imaging capability of various NPs was tested in KB cancer cells after incubation for 12 h (Fig. 5a). The working concentration of FA-PPBF/TPP(2%)/TPD(2%) NPs is 0.5 μM in repeat units of PPBF for 2PE fluorescence imaging experiments. 2PE fluorescence imaging experiments were conducted by two-photon laser scanning confocal microscopy by collecting red emission signals from 610 to 700 nm under femtosecond laser excitation at 750 nm. Strong 2PE red emission signals were observed in KB cancer cells incubated with FA-PPBF/TPP/TPD NPs (Fig. 5a, middle), while almost no 2PE fluorescence signals were observed from KB cancer cells incubated with FA-TPD NPs (Fig. 5b, middle) due to their small 2PA cross section values. This result confirms that FA-PPBF/TPP/TPD NPs can act as promising 2PE red emission imaging agents. In the control experiments, no obvious 2PE fluorescence signals were observed for normal NIH/3T3 fibroblast cells (with low folate receptor expression) incubated with FA-PPBF/TPP/TPD NPs under the same conditions (Fig. 5c, middle), which confirmed the target specificity of FA functionalized CPNs. The overexpression of the folate receptors allowed specific targeting of cancer cells. FA-PPBF/TPP(2%)/TPD(2%) NPs were found mainly accumulated in the lysosome around the nuclei region of KB cancer cells.
Several commercial trackers emitting in the green spectral region were further utilized to co-stain KB cancer cells with FA-PPBF/TPP(2%)/TPD(2%) NPs following the protocol in the manufacturer's brochure. The co-localization images using a lysosome tracker are shown in Fig. S7.† In comparison with other two commercial trackers, mitochondria tracker and lipid dyes BODIPY 493/503, the merged figures confirmed that FA-PPBF/TPP(2%)/TPD(2%) NPs were mainly accumulated in the lysosome.
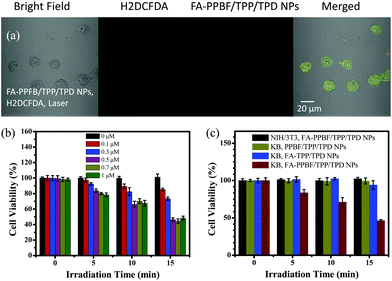
Reactive oxygen species (ROS) generation from FA-PPBF/TPP(2%)/TPD(2%) NPs in the cells was verified using an ROS indicator H2DCFDA. As shown in Fig. 6a, a strong green emission from KB cancer cells containing FA-PPBF/TPP(2%)/TPD(2%) NPs after laser irradiation (Fig. S8a†) and the change in the cell shape prove the generation of ROS. We have investigated the 2P-PDT activities of the NPs in different concentrations (0, 0.1, 0.3, 0.5, 0.7, and 1 μM in repeat units of PPBF). As can be seen from Fig. 6b, the cell viability of KB cancer cells incubated with FA-PPBF/TPP(2%)/TPD(2%) NPs after laser irradiation for 10 min drops quickly when the particle concentration increased from 0.1 to 0.5 μM. 2P-PDT activities were not obviously increased as the particle concentration further increased to 1 μM. This is probably ascribed to the ineffective (saturated) cell uptake of the NPs. Taking dark cytotoxicity, 2P-imaging capability and 2P-PDT activities into consideration, we finally chose 0.5 μM for both 2P-imaging and 2P-PDT investigations. The 2PE-PDT capabilities of various CPNs were examined by using the MTT assay on KB cancer cells (Fig. 6c), under the illumination of femtosecond laser pulses with a power density of about 0.91 W cm−2 at λ = 750 nm, the viability of KB cancer cells treated with FA-PPBF/TPP/TPD NPs rapidly decreased to about 46% after 15 min of irradiation (Fig. 6c). In contrast, the viability of KB cancer cells incubated with FA-TPP(2%)/TPD(2%) NPs (without PPBF) remained nearly 100% under the same experimental conditions. The result further confirms that the incorporation of PPBF into the NPs significantly enhanced two-photon-induced singlet oxygen generation, and consequently dramatically improved 2PE-PDT effects. This result is also consistent with the experimental results of the photo-oxidation of ABDA (Fig. 4). Compared with those incubated with the FA-PPBF/TPP(2%)/TPD(2%) NPs, the cell viability of normal NIH/3T3 cells in the control experiments incubated with FA-PPBF/TPP(2%)/TPD(2%) NPs still remained nearly 100% after 15 min of irradiation under the same conditions, showing little 2PE-PDT activity. This result (Fig. 5 and 6) further confirms that the incorporation of the surface FA significantly facilitated the uptake of FA-PPBF/TPP(2%)/TPD(2%) NPs in KB cancer cells. FA-PPBF/TPP(2%)/TPD(2%) NPs have an advantage for the target specific imaging-guided 2PE-PDT.
Conclusion
We designed a type of multifunctional CPN with both enhanced imaging and therapeutic capability under two-photon excitation for selectively targeted imaging-guided two-photon excitation photodynamic therapy (2PE-PDT). The multifunctional NPs are fabricated using PPBF as the two-photon light-harvesting material, which are doped with the photosensitizer tetraphenylporphyrin (TPP) and a red-emitting dye (TPD). The enhanced imaging and PDT effect are realized by the 2PE-FRET from the conjugated polymer PPBF to TPP and TPD. The 2PE emission of TPP and TPD was enhanced by up to ∼161 and ∼23 times, respectively. Singlet oxygen generation and cytotoxicity evaluation results clearly showed that the 2PE-PDT efficiencies of these FA-PPBF/TPP/TPD NPs are remarkably improved compared with those without PPBF, potentially up to about 149 times. Surface-functionalization of the nanoparticles with folic acid groups allows the obtained FA-PPBF/TPP/TPD NPs to display a specific selectivity in targeting KB cancer cells. These NPs could act as novel nanophotosensitizers for simultaneous 2PE-PDT and 2PE fluorescence imaging with the advantages of low dark cytotoxicity and specific targeting toward cancer cells.Experimental section
Materials
TPP, poly(styrene-co-maleic anhydride) (PSMA, average Mn ≈ 1900), and ABDA were purchased from Sigma-Aldrich. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000-FA) and DSPE-PEG2000 were purchased from Avanti Polar Lipids, Inc. H2DCFDA, fetal bovine serum (FBS), streptomycin, penicillin, phosphate buffered saline (PBS), Dulbecco's modified Eagle's medium (DMEM), and FA-free RPMI-1640 medium were purchased from Thermo Fisher. TPD was synthesized as described in the literature.35 PPBF was synthesized as described in the literature.37 Normal NIH/3T3 fibroblast cells were a gift from Prof. Anjun Qin. KB cancer cells were purchased from KeyGen Biotech.Preparation of nanoparticles
PPBF/TPP/TPD NPs were prepared following a modified re-precipitation method.25 Various volumes of TPP (0.2 mM), TPD (0.2 mM), DSPE-PEG2000 (4 μL, 1 mg mL−1) and PSMA (40 μL, 0.2 mM) in tetrahydrofuran (THF) solutions were added into a solution of PPBF in THF (1.0 mL, 40 μM in repeat units). The mixture solution was rapidly injected into 4.0 mL of H2O under vigorous sonication. Vacuum evaporation was subsequently utilized to remove THF and obtain PPBF/TPP/TPD NPs dispersed in aqueous solution. The same preparation method was adopted to prepare folate-functionalized NPs (FA-PPBF/TPP/TPD NPs) by exchanging DSPE-PEG2000 with DSPE-PEG2000-FA (4.0 μL, 1.0 mg mL−1) to the above mixtures. The obtained nanoparticle solutions were transparent with a yellow-green color. PPBF NPs, TPP-doped PPBF NPs (PPBF/TPP NPs) and TPD-doped PPBF NPs (PPBF/TPD NPs) were prepared using the same method. All the concentrations of NPs are in term of PPBF repeat units.Characterization
UV-vis absorption and 1PE fluorescence spectra were obtained using a Hitachi double-beam spectrophotometer (UH5300) and a Jobin Yvon spectrofluorometer (Fluoromax-4), respectively. Particle size distribution was measured by dynamic light scattering (DLS) on a Nano ZS Zetasizer (Malvern). TEM images were obtained on a JEM-2100F TEM operating at an acceleration voltage of 200 kV.2PE fluorescence measurements
The excitation source of 2PE fluorescence measurements was a femtosecond (fs) Ti:sapphire oscillator (Avesta TiF-100 M) which generates laser pulses with a pulse duration of ∼80 fs and a repetition rate of 84.5 MHz. The excitation power was adjusted to 100 mW with a central wavelength of 750 nm. The laser beam was focused onto the sample through a lens with a 3.5 cm focal length. A short pass filter with a cutoff wavelength at 700 nm was positioned before the signal collection lens and optical fibre, which was connected to a monochromator (Acton Spectra Pro 2300i) integrated CCD (Princeton Instruments Pixis 100B) system. The concentrations of NPs in water dispersion were ∼10 μM (in repeat units of PPBF).Detection of singlet oxygen generation
The 2P-1O2 generation capability was evaluated by monitoring the chemical oxidation of ABDA incubated with various NPs. The sample under laser irradiation was dispersed in 0.4 mL of H2O, containing NPs (10 μM) and ABDA (0.01 mM). The laser conditions were set the same as those in the 2PE fluorescence measurement. The decrease in the ABDA absorbance (377 nm) was monitored at 2 min intervals under laser irradiation.Cell culture and cell viability
The cell culture medium of KB cancer cells was prepared using a FA-free RPMI-1640 medium with FBS (10%) and penicillin–streptomycin (1%) at 37 °C under a humidified atmosphere of 5% CO2. Normal NIH/3T3 fibroblast cells were cultured in DMEM with FBS (10%) and penicillin–streptomycin (1%) at 37 °C under a humidified atmosphere of 5% CO2. MTT colorimetric cell proliferation assay (Sarstedt) was utilized to determine the cell viability of cells following the manufacture's manual (see the details in the ESI†).2PE fluorescence imaging of live cells
Cells were separately seeded in a confocal dish (Nest) and grown until 70–80% confluence. A medium containing NPs (0.5 μM in repeat units of PPBF) was added to the dish and incubated for 12 h in the dark at 37 °C. The cells were then washed three times with 1× PBS. Fluorescence images were obtained on an OLYMPUS IX73 microscope system under excitation with fs laser pulses at λ = 750 nm with a power of 5 mW. A hybrid photomultiplier detector (PMA Hybrid 40, PicoQuant) was utilized to detect the fluorescence emission from 610 to 700 nm. The co-localization experiments with FA-PPBF/TPP(2%)/TPD(2%) NPs were performed using a commercial mitochondria tracker (MitoTrackerTM Green FM, Invitrogen), lysosome tracker (LysoTrackerTM Green DND-26, Invitrogen) and lipid dye BODIPY 493/503 green to co-stain KB cancer cells after incubation with the NPs for 12 h. The staining method followed the procedures described in the brochure from the manufacturer. Commercial dyes emitting in the green spectral region were excited by a 488 nm laser and the emission of KB cancer cells stained with commercial dyes was collected from 500 to 550 nm. Images of KB cancer cells incubated with FA-PPBF/TPP/TPD NPs were excited by a 405 nm laser and with emission collected from 610 to 700 nm.2PE-PDT treatment on cancer cells
Cells were seeded in a 96-well plate at a density of 5 × 104 cells per mL. After culturing for 24 h, the cells were then washed with PBS buffer solution and treated with media (100 μL) containing various NPs, followed by culturing for 12 h in the dark. Cells were subsequently washed with PBS buffer solution and re-cultured in FA-free RMPI media for laser irradiation. An unfocused 1 kHz fs laser with a laser beam (0.33 cm2) and a power density of about 0.91 W cm−2 at λ = 750 nm was adopted as the irradiation source. The illumination time varied from 0 to 15 min. The plates after laser irradiation were incubated for 24 h before cell proliferation and the cells were then evaluated by the MTT assay.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the Guangdong Innovative Research Team Program of China (Grant No. 201101C0105067115), the National Natural Science Foundation of China (Grant No. 21673155, 51603069), the Scientific Research Staring Natural Science Foundation of Guangdong Province, China (Grant No. 2016A030310432, 2017A030313287), and the Fundamental Research Funds for the Central Universities (2017BQ012).Notes and references
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS PubMed.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380 CrossRef CAS PubMed.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- H. Abrahamse and M. R. Hamblin, Biochem. J., 2016, 473, 347–364 CrossRef CAS PubMed.
- O. Kazuya and K. Yoshiaki, Anticancer Agents Med. Chem., 2008, 8, 269–279 CrossRef.
- S. Brown, Nat. Photonics, 2008, 2, 394 CrossRef CAS.
- Y. Shen, A. J. Shuhendler, D. Ye, J.-J. Xu and H.-Y. Chen, Chem. Soc. Rev., 2016, 45, 6725–6741 RSC.
- Y. Tanaka, Y. Tsunemi, M. Kawashima, N. Tatewaki and H. Nishida, Clin., Cosmet. Invest. Dermatol., 2013, 6, 167–176 CrossRef PubMed.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Angew. Chem., Int. Ed., 2009, 48, 3244–3266 CrossRef CAS PubMed.
- R. W. Boyle and D. Dolphin, Photochem. Photobiol., 1996, 64, 469–485 CrossRef CAS PubMed.
- Y. Zhou, X. Liang and Z. Dai, Nanoscale, 2016, 8, 12394–12405 RSC.
- M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- X. Shen, F. He, J. Wu, G. Q. Xu, S. Q. Yao and Q.-H. Xu, Langmuir, 2011, 27, 1739–1744 CrossRef CAS PubMed.
- K. Liu, X. Liu, Q. Zeng, Y. Zhang, L. Tu, T. Liu, X. Kong, Y. Wang, F. Cao, S. A. G. Lambrechts, M. C. G. Aalders and H. Zhang, ACS Nano, 2012, 6, 4054–4062 CrossRef CAS PubMed.
- Y. Cheng, A. C. Samia, J. D. Meyers, I. Panagopoulos, B. Fei and C. Burda, J. Am. Chem. Soc., 2008, 130, 10643–10647 CrossRef CAS PubMed.
- T. Zhao, K. Yu, L. Li, T. Zhang, Z. Guan, N. Gao, P. Yuan, S. Li, S. Q. Yao, Q.-H. Xu and G. Q. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 2700–2708 CrossRef CAS PubMed.
- P. Couleaud, V. Morosini, C. Frochot, S. Richeter, L. Raehm and J.-O. Durand, Nanoscale, 2010, 2, 1083–1095 RSC.
- A. C. S. Samia, X. Chen and C. Burda, J. Am. Chem. Soc., 2003, 125, 15736–15737 CrossRef CAS PubMed.
- J. Hu, Y. A. Tang, A. H. Elmenoufy, H. Xu, Z. Cheng and X. Yang, Small, 2015, 11, 5860–5887 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS PubMed.
- S. Li, X.-F. Jiang and Q.-H. Xu, Sci. China: Chem., 2018, 61, 88–96 CrossRef CAS.
- X. Duan, L. Liu, F. Feng and S. Wang, Acc. Chem. Res., 2010, 43, 260–270 CrossRef CAS PubMed.
- L. Feng, C. Zhu, H. Yuan, L. Liu, F. Lv and S. Wang, Chem. Soc. Rev., 2013, 42, 6620–6633 RSC.
- O. S. Finikova, T. Troxler, A. Senes, W. F. DeGrado, R. M. Hochstrasser and S. A. Vinogradov, J. Phys. Chem. A, 2007, 111, 6977–6990 CrossRef CAS PubMed.
- X. Shen, S. Li, L. Li, S. Q. Yao and Q.-H. Xu, Chem. – Eur. J., 2015, 21, 2214–2221 CrossRef CAS PubMed.
- L. Feng, J. Zhu and Z. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 19364–19370 CrossRef CAS PubMed.
- G. Feng, Y. Fang, J. Liu, J. Geng, D. Ding and B. Liu, Small, 2017, 13, 1602807 CrossRef PubMed.
- C.-Y. Chen, Y. Tian, Y.-J. Cheng, A. C. Young, J.-W. Ka and A. K. Y. Jen, J. Am. Chem. Soc., 2007, 129, 7220–7221 CrossRef CAS PubMed.
- K. Chang, Y. Tang, X. Fang, S. Yin, H. Xu and C. Wu, Biomacromolecules, 2016, 17, 2128–2136 CrossRef CAS PubMed.
- S. Li, X. Shen, L. Li, P. Yuan, Z. Guan, S. Q. Yao and Q.-H. Xu, Langmuir, 2014, 30, 7623–7627 CrossRef CAS PubMed.
- W. Xia and P. S. Low, J. Med. Chem., 2010, 53, 6811–6824 CrossRef CAS PubMed.
- P. S. Low, W. A. Henne and D. D. Doorneweerd, Acc. Chem. Res., 2008, 41, 120–129 CrossRef CAS PubMed.
- A. Saha, S. C. Mohanta, K. Deka, P. Deb and P. S. Devi, ACS Appl. Mater. Interfaces, 2017, 9, 4126–4141 CrossRef CAS PubMed.
- R. W. Redmond and J. N. Gamlin, Photochem. Photobiol., 1999, 70, 391–475 CrossRef CAS PubMed.
- J. Jiang, D. Hu, M. Hanif, X. Li, S. Su, Z. Xie, L. Liu, S. Zhang, B. Yang and Y. Ma, Adv. Opt. Mater., 2016, 4, 2109–2118 CrossRef CAS.
- H. Chen, H. L. Puhl, S. V. Koushik, S. S. Vogel and S. R. Ikeda, Biophys. J., 2006, 91, L39–L41 CrossRef CAS PubMed.
- P. Liu, S. Li, Y. Jin, L. Qian, N. Gao, S. Q. Yao, F. Huang, Q.-H. Xu and Y. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 6754–6763 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DETAILS. See DOI: 10.1039/c8nr06957c |
| This journal is © The Royal Society of Chemistry 2019 |