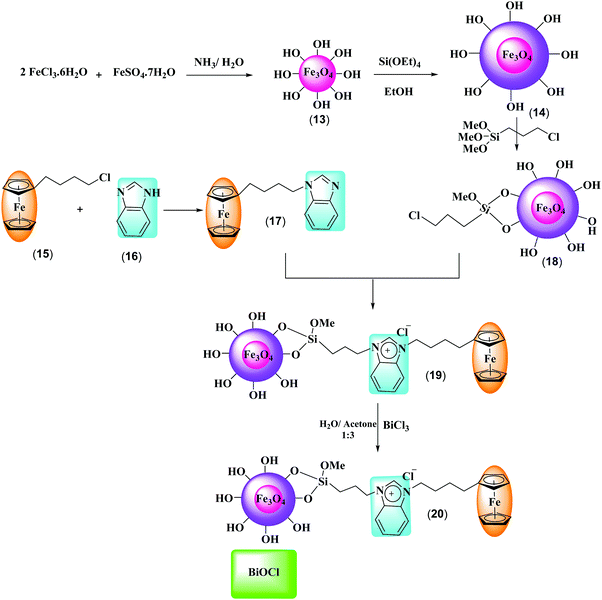
Synthesis and characterization of a novel Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite and its efficient catalytic activity in the ultrasound-assisted synthesis of diverse chromene analogs†
Reza
Mohammadi
 *,
Somayeh
Esmati
,
Mahdi
Gholamhosseini-Nazari
and
Reza
Teimuri-Mofrad
*,
Somayeh
Esmati
,
Mahdi
Gholamhosseini-Nazari
and
Reza
Teimuri-Mofrad
Department of Organic and Biochemistry, Faculty of Chemistry, University of Tabriz, Tabriz 5166614766, Iran. E-mail: r.mohammadi@tabrizu.ac.ir; Fax: +98 413 334 0191; Tel: +98 413 339 3117
First published on 13th November 2018
Abstract
In this study, a novel magnetically recoverable Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite was synthesized using a simple chemical co-precipitation method. This is the first time that a magnetic nano-catalyst bearing ionic liquid, ferrocene and BiOCl is synthesized. The Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite was characterized by Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX) and field emission scanning electron microscopy (FE-SEM) techniques. The catalytic activities of the novel magnetic nano-composite were evaluated in one-pot three-component synthesis of a wide variety of 2-amino-3-cyano-4H-chromene derivatives under ultrasound irradiation. A simple, facile and highly efficient ultrasound-assisted method was developed to synthesize 4H-chromene derivatives via one-pot, three-component reaction of aldehyde, malononitrile and active phenolic compounds (2-naphthol, 1-naphthol, 3-(dimethylamino)phenol, resorcinol and orcinol) at room temperature. The reaction of aldehyde, malononitrile and orcinol is newly introduced in this paper. The ultrasound-assisted synthesis protocol that was studied in this article exhibits some notable advantages such as short reaction times, operational simplicity, green reaction conditions, high yields and easy work-up and purification steps. In addition, the novel magnetic nano-composite could be easily recovered by an external magnetic field and reused for six-reaction cycles without significant loss of its catalytic activity.
1. Introduction
Heterocyclic compounds containing a 4H-chromene moiety are present in a wide variety of drugs, many natural products and numerous biologically and pharmaceutically active compounds. 2-Amino-4H-chromene derivatives are known to exhibit anticancer,1–3 antioxidant,4,5 anti-inflammatory,6,7 antimicrobial,8,9 anti-tuberculosis,10 antiviral,11 anti-proliferative,12 antibacterial and antifungal,13 anticoagulant and anti-HIV activities.14 Hence, the design and synthesis of such compounds is a hot research topic for chemists. Synthetic methods using different catalysts such as K2CO3,15,16 MW/DUB,17 TFE,18 amino-silane modified Fe3O4 nanoparticles (MNPs-NH2),19 potassium phthalimide-N-oxyl (POPINO),20 NaBr,21 cetyltrimethylammoniumchloride (CTAC),22 glycine,23 Fe3O4-chitosan nano-particles,24 4-dimethylaminopyridine (DMAP),25 piperidine26 and Mg/Al hydrotalcite,27 2-ethyl imidazolium acetate,28 and tris-hydroxymethylaminomethane (THAM)29 were reported. However, because of the importance of 2-amino-4H-chromene derivatives, more efficient and environmentally benign methods are still in demand. In order to introduce a simple, green and efficient strategy for the synthesis of these compounds, it is important to use an applied heterogeneous catalyst, an eco-friendly medium for the reactions, and an appropriate source of input energy in order to optimize the reaction duration and product yield and avoid undesirable side-products.On the other hand, supported heterogeneous catalysts as reusable and environmentally friendly materials play a key role in modern science and technology, especially in organic synthesis.30–32 Due to the advantages of the immobilization of the catalyst on the solid supports, such as easily handling, non-toxicity, low solubility and increasing the selectivity of the reactions, these types of heterocatalysts are widely used.33 Catalysts immobilized on nanostructure supports are more attractive, because they exhibit higher activity and selectivity.34–36 Recent studies show that magnetite (Fe3O4) nano-particles have unique properties such as superparamagnetism, high surface area, thermal stability, low toxicity, low cost, easy separation from the reaction mixture using an external magnetic field, potential to immobilize different functional groups and excellent recyclability, which make them very applicable and useful supports to design and synthesize reusable heterogeneous catalysts.37,38 Furthermore, benzimidazolium based ionic liquids (ILs) have received much attention and have been used as environmentally friendly catalysts and solvents, due to their special properties such as negligible volatility, thermal stability, non-inflammability, high activity and selectivity and easy recyclability. Benzimidazole based ILs introduced a new possibility to develop new, efficient and environmentally friendly catalysts for different organic reactions.39
In recent years, the development of ultrasound-assisted synthesis reactions has been focused in many scientific and industrial investigations. Sonochemical synthesis has many benefits such as convenience, improved yields and reaction rates, simplicity and controllability of the reaction compared to traditional heating methods which generally require longer reaction times and higher temperatures. Therefore, a large number of organic reactions have been carried out under ultrasonic irradiation in higher yields, shorter reaction times or milder conditions in accordance with the green chemistry principles.40,41
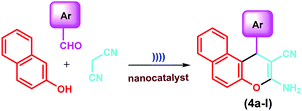
In the continuation of our interest towards the development of new efficient synthetic methodologies for various multi-component reactions using nano-magnetic heterogeneous catalysts,42–46 herein, we report a simple, facile and highly efficient ultrasound-assisted method for the synthesis of 2-amino-4H-chromene derivatives. One-pot three-component reactions of aldehyde, malononitrile and active phenolic compounds (2-naphthol, 1-naphthol, 3-(dimethylamino)phenol, resorcinol and orcinol) catalyzed by the Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite under ultrasonic conditions were investigated (Scheme 1).
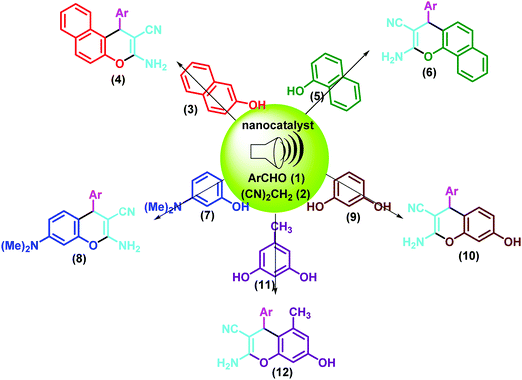 | ||
| Scheme 1 Ultrasound-assisted synthesis of diverse 2-amino-4H-chromenes via Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl catalyzed three-component reactions. | ||
2. Experimental
2.1. Materials and apparatus
The chemical reagents used in synthesis were purchased from Merck and Sigma-Aldrich Co. Melting points were determined with a MEL-TEMP model 1202D and are uncorrected. FT-IR spectra were recorded on a Bruker Tensor 27 spectrometer as KBr disks. The 1H NMR spectra were recorded using a Bruker Spectrospin Avance 400 spectrometer with DMSO-d6 as solvent and TMS as an internal standard. 13C NMR spectra were determined on the same instrument at 100 MHz. All chemical shifts were reported as d (ppm) and coupling constants (J) are given in Hz. Elementary analyses (C, H, N) were performed on a Vario EL III analyzer. X-ray diffraction patterns of samples were taken on a Siemens D500 X-ray powder diffraction diffractometer (CuK radiation, λ = 1.5406 Å). FE-SEM images of the products were visualized by a TESCAN MIRA3 Field Emission Scanning Electron Microscope. A multiwave ultrasonic generator (Sonicator 3200; Bandelin, MS 73, Germany), equipped with a converter/transducer and titanium oscillator (horn), 12.5 mm in diameter, operating at 20 kHz with a maximum power output of 10–60 W, was used for the ultrasonic irradiation. The ultrasonic generator automatically adjusted the power level.2.2. Synthesis of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, to give 1-(4-ferrocenylbutyl)-1H-benzimidazole as a brown viscous oil; FT-IR: 3102, 2959, 2877, 1693, 1651, 1526 cm−1; 1H NMR: δ 1.46–1.54 (m, 2H, –CH2–), 1.84–1.91 (m, 2H, –CH2–), 2.34 (t, J = 7.5 Hz, 2H, –CH2–), 4.01 (m, 2H, –CH2–), 4.04 (m, 7H, Cp-H), 4.14–4.17 (m, 2H, Cp-H), 7.24–7.30 (m, 2H, benzimid-H), 7.47 (m, 2H, benzimid-H), 7.73 (m, 1H, benzimid-H), 8.09 (s, 1H, benzimid-H); 13C NMR: δ 27.2, 27.9, 28.5, 44.0, 66.0, 66.9, 67.3, 86.9, 108.8, 119.4, 120.7, 121.7, 134.6, 144.8 ppm.
1, to give 1-(4-ferrocenylbutyl)-1H-benzimidazole as a brown viscous oil; FT-IR: 3102, 2959, 2877, 1693, 1651, 1526 cm−1; 1H NMR: δ 1.46–1.54 (m, 2H, –CH2–), 1.84–1.91 (m, 2H, –CH2–), 2.34 (t, J = 7.5 Hz, 2H, –CH2–), 4.01 (m, 2H, –CH2–), 4.04 (m, 7H, Cp-H), 4.14–4.17 (m, 2H, Cp-H), 7.24–7.30 (m, 2H, benzimid-H), 7.47 (m, 2H, benzimid-H), 7.73 (m, 1H, benzimid-H), 8.09 (s, 1H, benzimid-H); 13C NMR: δ 27.2, 27.9, 28.5, 44.0, 66.0, 66.9, 67.3, 86.9, 108.8, 119.4, 120.7, 121.7, 134.6, 144.8 ppm.
2.3. General procedure for the synthesis of 2-amino-4H-chromenes derivatives under ultrasound irradiation
A mixture of an aldehyde (1 mmol), malononitrile (1.1 mmol), an appropriate C–H acid 3, 5, 7, 9 or 11 (1 mmol), and Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl (10 mg) in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 5 mL) was sonicated at ambient temperature. When the reaction was completed [monitored by thin layer chromatography (TLC), using n-hexane/ethyl acetate (3
2, 5 mL) was sonicated at ambient temperature. When the reaction was completed [monitored by thin layer chromatography (TLC), using n-hexane/ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent], the catalyst was separated using an external magnet and the reaction mixture was cooled and the precipitate was filtered, washed and dried. The crude product was crystallized from ethanol. The structures of the new compounds 12a–f were characterized by IR, 1H NMR, 13C NMR and CHN analysis.
1) as the eluent], the catalyst was separated using an external magnet and the reaction mixture was cooled and the precipitate was filtered, washed and dried. The crude product was crystallized from ethanol. The structures of the new compounds 12a–f were characterized by IR, 1H NMR, 13C NMR and CHN analysis.
3. Results and discussion
The new magnetic nano-catalyst Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl was prepared following the multistep protocol shown in Scheme 2. A literature survey showed that Fe3O4/BiOCl nano-composites were previously synthesized and their photocatalytic efficiency was studied,47–49 but the catalytic activity of these nano-composites has not been investigated. Following our recent studies on the design and synthesis of novel ferrocene containing ionic liquids supported on Fe3O4 nano-particles, here we report novel generation of this nano-catalyst as a Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite. Initially, the chemical co-precipitation of Fe2+ and Fe3+ ions in NH4OH solution was performed in order to prepare magnetic Fe3O4 nano-particles. Subsequently, silica-coated magnetic nano-particles (Fe3O4@SiO2) were easily achieved using the known Stober method. Then, the Fe3O4@SiO2 nano-particles were allowed to react with (3-chloropropyl)trimethoxysilane to obtain chloropropyl containing nano-particles.50–52 On the other hand, 1-(4-ferrocenylbutyl)-1H-benzimidazole was prepared by the reaction of benzimidazole with 4-chlorobutylferrocene. Subsequently 1-(4-ferrocenylbutyl)-1H-benzimidazole reacted with Fe3O4@SiO2–(CH2)3Cl to afford Fe3O4@SiO2@BenzIm-Fc[Cl] nanoparticles. Finally, the Fe3O4@SiO2@BenzIm-Fc[Cl]-coupled BiOCl nano-composite was prepared by using a simple chemical co-precipitation method using BiCl3 at room-temperature. Chemical analysis of the prepared magnetic nano-particles and Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite was studied by Fourier transform infrared spectroscopy (FT-IR), field emission scanning electron microscopy (FE-SEM), energy dispersive X-ray (EDX) and X-ray diffraction (XRD) analysis.The FT-IR spectra of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@(CH2)3Cl, (d) Fe3O4@SiO2@BenzIm-Fc[Cl] and (e) Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl are shown in Fig. 1. In the FT-IR spectroscopy of the nano-particles, the absorption peaks at about 580 cm−1 are related to Fe–O bond vibrations. The absorption peaks at about 1080 cm−1 in all Fe3O4@SiO2 core–shell MNPs are linked to the asymmetric stretching vibrations of Si–O–Si. In the FT-IR spectroscopy of Fe3O4@SiO2–(CH3)3Cl MNPs more than Fe–O and Si–O–Si bond vibrations, an absorption peak at 2918 cm−1 appeared which is related to the asymmetric stretching vibration of aliphatic C–H. The FT-IR spectroscopy of Fe3O4@SiO2@BenzIm-Fc[Cl] MNPs shows an absorption peak at above 3000 cm−1 related to the stretching vibration of aromatic C–H on benzimidazole and ferrocene groups. The absorption peaks at 1643 and 1549 cm−1 are also linked to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds on aromatic rings. Finally, in the FT-IR spectroscopy of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite, the absorption peak at about 530 cm−1 is assigned to Bi–O stretching vibration and the absorption peak at 1460 cm−1 is assigned to the stretching vibration peak of the Bi–Cl band in the BiOCl structure. These results revealed the successful synthesis of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-catalyst.
C bonds on aromatic rings. Finally, in the FT-IR spectroscopy of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite, the absorption peak at about 530 cm−1 is assigned to Bi–O stretching vibration and the absorption peak at 1460 cm−1 is assigned to the stretching vibration peak of the Bi–Cl band in the BiOCl structure. These results revealed the successful synthesis of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-catalyst.
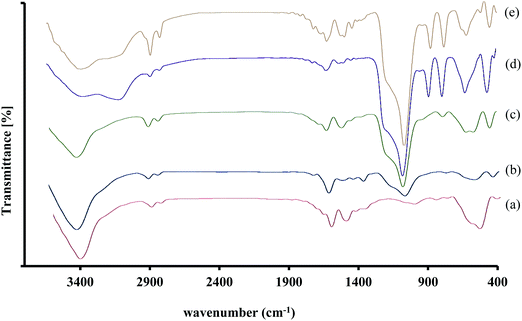 | ||
| Fig. 1 The FT-IR spectra of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@(CH2)3Cl, (d) Fe3O4@SiO2@BenzIm-Fc[Cl] and (e) Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl. | ||
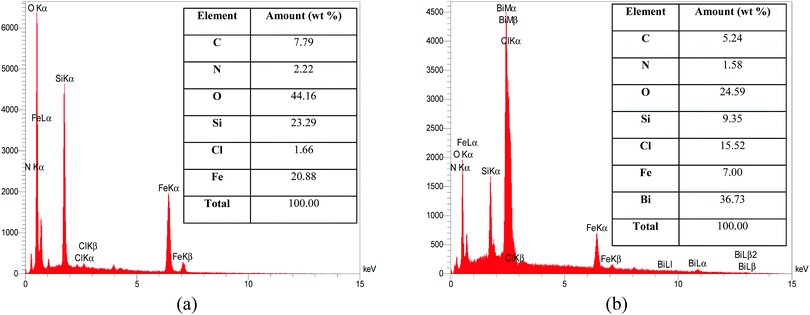
Energy-dispersive X-ray spectroscopy (EDX) analysis of Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles and the newly synthesized one are presented in Fig. 2. The EDX spectrum and data of Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles introduced the presence of the expected elements (C, N, O, Si, Cl and Fe) in their regions. The EDX spectrum of the novel synthesized Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite indicated the presence of the expected elements (C, N, O, Si, Cl, Fe and Bi) and confirmed the structure of the nano-catalyst. The presence of Bi in the EDX spectrum of the final nano-catalyst confirmed the synthesis of a nano-composite.
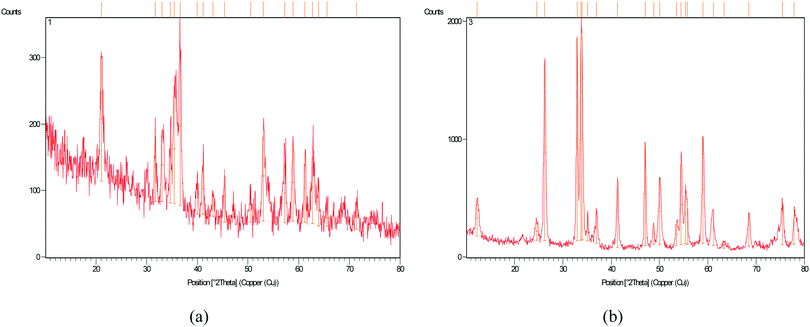
The X-ray diffraction (XRD) pattern of the modified Fe3O4 nano-particles was measured at 0 to 80 degrees. As shown in Fig. 3, the diffusion pattern of Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles shows the crystalline dispersions of Fe3O4 magnetic nano-particles. The XRD pattern of Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles exhibited diffraction peaks at 2θ = 31.6°, 35.4°, 43.1°, 53°, 57.3° and 63.9° which are in good agreement with the standard XRD pattern of Fe3O4 MNPs (JCPDS card no. 85-1436). But the XRD patterns of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite displayed distinctive peaks at 2θ = 12.2°, 24.6°, 26.2°, 32.9°, 33.7°, 36.9°, 41.3°, 47.1°, 50.0°, 54.4°, 55.4°, 58.9°, and 77.8° which are well matched with the tetragonal phase JCPDS-ICDD File No. 01-085-0861. These distinctive peaks were very obvious, suggesting the formation and good crystallinity of the novel synthesized Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite.
The surface morphology and size of the Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles and Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite were evaluated using Field Emission Scanning Electron Microscopy (FE-SEM) analysis (Fig. 4). The FE-SEM images of the Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles showed that the shapes of these particles are mostly spherical with non-smooth surfaces which increase the surface areas and activity of these particles. The FE-SEM images of Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles also displayed that the particles are uniformly distributed and the sizes of the magnetic nano-particles are about 40 nm. The FE-SEM images of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite showed that BiOCl was synthesized on the Fe3O4@SiO2@BenzIm-Fc[Cl] nano-particles as irregular plates which are around 150 nm in width and about 50 nm in thickness.
 | ||
| Fig. 4 FE-SEM images of (a and b) Fe3O4@SiO2@BenzIm-Fc[Cl] and (c and d) Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl. | ||
In continuation, we decided to investigate the catalytic activity of the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite in the synthesis of 2-amino-4H-chromene derivatives. At first, the reaction of 4-chlorobenzaldehyde (1a) (1 mmol), malononitrile (2) (1.1 mmol) and 2-naphthol (3) (1 mmol) under ultrasound irradiation was chosen as a test reaction. The reaction was studied under different conditions and the progress of the reactions was monitored by TLC. In this context, the effects of catalyst loading on the reaction were examined initially. In the absence of catalyst, 3-amino-1-(4-chlorophenyl)-1H-benzo[f]chromene-2-carbonitrile (4a) was obtained after 60 minutes only in 10% yield. With increase in the amount of catalyst to 10 mg, the yield of the desired product (4a) was gradually increased. But more addition of the catalyst to 15 mg didn’t show improvement in the reaction. Then, the subsequent optimization of the reaction conditions affirmed the selection of ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) as the best solvent for the synthesis of the desired product (4a), in an excellent yield. Finally, we tried to test the effect of ultrasound irradiation power on the reaction of 4-chlorobenzaldehyde, malononitrile and 2-naphthol. Various ranges of irradiation powers (30–60 W) were studied and the best results were obtained when the irradiation power was 50 W.
2, v/v) as the best solvent for the synthesis of the desired product (4a), in an excellent yield. Finally, we tried to test the effect of ultrasound irradiation power on the reaction of 4-chlorobenzaldehyde, malononitrile and 2-naphthol. Various ranges of irradiation powers (30–60 W) were studied and the best results were obtained when the irradiation power was 50 W.
Subsequently, the method was developed towards various aldehydes to obtain the related 3-amino-1H-benzo[f]chromene derivatives 4a–l. Accordingly, a number of aromatic aldehydes with electron-withdrawing groups as well as electron-donating groups and a few heteroaromatic aldehydes were subjected to reaction with malononitrile and 2-naphthol in the presence of a catalytic amount of Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nanocomposite under ultrasonic irradiation. In all cases, the corresponding products 4a–l were obtained within 15–20 min in excellent yields (Table 1).
| Entry | Aldehyde | Product | Time (min) | Yield (%) | Obs. m.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde | (4a) | 15 | 96 | 208–210 | 207–20943 |
| 2 | Benzaldehyde | (4b) | 18 | 93 | 280–281 | 279–28143 |
| 3 | 4-Bromobenzaldehyde | (4c) | 18 | 95 | 212–214 | 212–21543 |
| 4 | 4-Fluorobenzaldehyde | (4d) | 18 | 93 | 233–235 | 232–23443 |
| 5 | 4-Methylbenzaldehyde | (4e) | 18 | 92 | 272–274 | 270–27343 |
| 6 | 4-Isopropylbenzaldehyde | (4f) | 20 | 90 | 220–222 | 218–22043 |
| 7 | 4-Methoxybenzaldehyde | (4g) | 20 | 87 | 192–194 | 192–19443 |
| 8 | 4-Nitrobenzaldehyde | (4h) | 15 | 96 | 186–188 | 186–18743 |
| 9 | 3-Nitrobenzaldehyde | (4i) | 18 | 92 | 217–219 | 215–21843 |
| 10 | 2-Chlorobenzaldehyde | (4j) | 20 | 88 | 270–272 | 269–27243 |
| 11 | Thiophene-2-carbaldehyde | (4k) | 20 | 84 | 224–226 | 224–22643 |
| 12 | 4-Pyridinecarboxaldehyde | (4l) | 20 | 82 | 227–229 | 225–22743 |
After this initial success, the generality of the method for the synthesis of 2-amino-4H-benzo[h]chromene derivatives was examined. In this context, replacement of the 2-naphthol with 1-naphthol could be expected to furnish 2-amino-4H-benzo[h]chromenes 6a–l (Table 2). A literature survey revealed that 4H-benzo[h]chromene derivatives possess many biological and pharmacological activities such as cytotoxicity and anticancer activity53 and a number of methods have been reported for the synthesis of these compounds. Here, our new method for the synthesis of these compounds was examined. For this reason three-component reactions between 1-naphthol, malononitrile and a series of aldehydes were performed in the presence of 10 mg of Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite in ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) under ultrasonic irradiation. A group of 2-amino-4H-benzo[h]chromene derivatives (6a–l) were synthesized successfully according to this simple and efficient method in high yields.
2, v/v) under ultrasonic irradiation. A group of 2-amino-4H-benzo[h]chromene derivatives (6a–l) were synthesized successfully according to this simple and efficient method in high yields.
| Entry | Aldehyde | Product | Time (min) | Yield (%) | Obs. m.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde | (6a) | 15 | 96 | 245–247 | 246–24754 |
| 2 | Benzaldehyde | (6b) | 15 | 93 | 217–219 | 216–21754 |
| 3 | 4-Bromobenzaldehyde | (6c) | 18 | 94 | 240–242 | 240–24155 |
| 4 | 4-Fluorobenzaldehyde | (6d) | 15 | 92 | 233–234 | 232–23454 |
| 5 | 4-Methylbenzaldehyde | (6e) | 15 | 90 | 207–209 | 205–20755 |
| 6 | 4-Isopropylbenzaldehyde | (6f) | 18 | 89 | 204–206 | 204–20555 |
| 7 | 4-Methoxybenzaldehyde | (6g) | 20 | 87 | 192–194 | 193–19455 |
| 8 | 4-Nitrobenzaldehyde | (6h) | 15 | 97 | 238–240 | 238–23955 |
| 9 | 3-Nitrobenzaldehyde | (6i) | 18 | 90 | 217–219 | 218–22054 |
| 10 | 2-Chlorobenzaldehyde | (6j) | 20 | 86 | 251–253 | 253–25455 |
| 11 | Thiophene-2-carbaldehyde | (6k) | 25 | 88 | 187–189 | 188–19055 |
| 12 | 2-Furancarboxaldehyde | (6l) | 25 | 86 | 170–172 | 168–17054 |
After establishing a general method for the synthesis of 3-amino-1H-benzo[f]chromenes 4, as well as 2-amino-4H-benzo[h]chromene derivatives 6, we tried to synthesize another important class of chromene-annulated heterocycles, namely 2-amino-7-(dimethylamino)-4H-chromenes 8 (Table 3). To achieve this aim, 3-dimethylaminophenol was chosen as the C–H acid in the three-component reaction. We were interested in the synthesis of 2-amino-4-aryl-7-(dimethylamino)-4H-chromene-3-carbonitrile derivatives because of their numerous medicinal properties especially anticancer activities. Therefore, various aldehydes containing either electron-withdrawing or electron-donating groups were investigated under the optimized reaction conditions. The results given in Table 3 showed that this one-pot three-component condensation completed within 20–28 min with good isolated yields.
| Entry | Aldehyde | Product | Time (min) | Yield (%) | Obs. m.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde | (8a) | 20 | 94 | 200–202 | 202–20556 |
| 2 | Benzaldehyde | (8b) | 22 | 90 | 205–205 | 206–20756 |
| 3 | 4-Bromobenzaldehyde | (8c) | 22 | 89 | 214–216 | 212–21457 |
| 4 | 4-Fluorobenzaldehyde | (8d) | 22 | 90 | 180–182 | 174–17857 |
| 5 | 4-Methylbenzaldehyde | (8e) | 25 | 91 | 242–243 | 240–24256 |
| 6 | 4-Isopropylbenzaldehyde | (8f) | 25 | 87 | 225–227 | 226–22856 |
| 7 | 4-Methoxybenzaldehyde | (8g) | 28 | 86 | 175–177 | 176–17856 |
| 8 | 4-Nitrobenzaldehyde | (8h) | 20 | 95 | 225–227 | 224–22657 |
| 9 | 3-Nitrobenzaldehyde | (8i) | 22 | 90 | 206–208 | 207–20957 |
| 10 | 2-Chlorobenzaldehyde | (8j) | 25 | 86 | 193–195 | 195–19757 |
| 11 | Thiophene-2-carbaldehyde | (8k) | 28 | 84 | 215–217 | 217–22056 |
| 12 | Pyridine-3-carbaldehyde | (8l) | 28 | 85 | 238–240 | 240–24256 |
Encouraged by the above mentioned success of a new Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl catalyzed method, we decided to use resorcinol as another enolizable C–H acid derivative. The literature survey showed that due to the versatile biological properties of these 2-amino-4H-chromene derivatives, the syntheses of these compounds are well studied and numerous methods for their synthesis are reported. In continuation, three component reactions of various aldehydes, malononitrile and resorcinol catalyzed by Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nano-composite under ultrasonic irradiation are investigated in order to develop and explore the scope and generality of this catalytic method. The results are summarized in Table 4. Aromatic aldehydes containing electron-releasing and electron-withdrawing substituents on their aromatic rings react to give products in high to excellent yields in short reaction times. The reaction times of aromatic aldehydes having electron-withdrawing groups are rather faster than those having electron-donating groups.
| Entry | Aldehyde | Product | Time (min) | Yield (%) | Obs. m.p. (°C) | Lit. m.p. (°C) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde | (10a) | 8 | 98 | 235–237 | 239–24158 |
| 2 | Benzaldehyde | (10b) | 10 | 95 | 230–232 | 231–23359 |
| 3 | 4-Bromobenzaldehyde | (10c) | 10 | 92 | 234–236 | 233–23559 |
| 4 | 4-Fluorobenzaldehyde | (10d) | 10 | 93 | 288–290 | 292–29459 |
| 5 | 4-Methylbenzaldehyde | (10e) | 12 | 90 | 186–188 | 185–18759 |
| 6 | 4-Isopropylbenzaldehyde | (10f) | 12 | 87 | 195–197 | 196–19860 |
| 7 | 4-Methoxybenzaldehyde | (10g) | 15 | 88 | 190–192 | 192–19460 |
| 8 | 4-Nitrobenzaldehyde | (10h) | 8 | 97 | 214–216 | 212–21460 |
| 9 | 3-Nitrobenzaldehyde | (10i) | 12 | 86 | 175–177 | 170–17259 |
| 10 | 2-Chlorobenzaldehyde | (10j) | 15 | 84 | 185–187 | 188–18961 |
| 11 | Thiophene-2-carbaldehyde | (10k) | 15 | 85 | 202–204 | 204–20659 |
| 12 | 2-Furancarboxaldehyde | (10l) | 15 | 83 | 190–192 | 189–19162 |
Based on the optimized reaction conditions, the reaction was further extended using various aromatic aldehydes with malononitrile and orcinol to synthesize the corresponding 2-amino-5-methyl-4H-chromene derivatives (12a–f) in excellent yields as shown in Table 5. The new synthesized compounds were characterized by FT-IR, 1H NMR, 13C NMR, and elemental analysis.
Finally, we decided to combine the sonication method with solvent-free and traditional reflux conditions. For this reason, the synthesis of compounds 4a, 6a, 8a, 10a and 12a catalyzed with Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl were studied under different conditions. As summarized in Fig. 5, all 2-amino-4H-chromene derivatives were synthesized more efficiently under ultrasonic irradiation with respect to yields and reaction times.
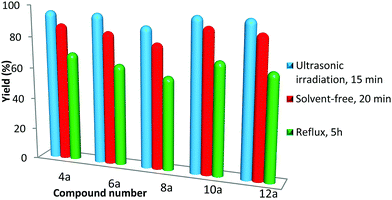 | ||
| Fig. 5 Comparison of the ultrasonic-assisted method with solvent-free and reflux conditions for the synthesis of 2-amino-4H-chromene derivatives catalyzed with Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl. | ||
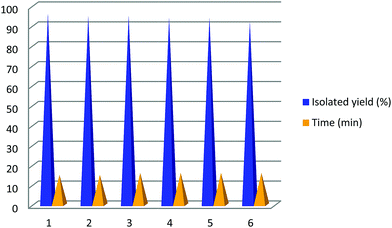
Furthermore, the reusability of the catalyst was investigated with the model reaction under modified conditions and the results are shown in Fig. 6. After completion of the reaction, the catalyst was separated using an external magnet. The collected nano-catalyst was washed several times with EtOH prior to reuse. The recovered nano-catalyst could be reused for six runs without significant decrease in activity.
To highlight the efficiency and applicability of the new method, we compared the results of Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl catalyzed reactions with other reported methodologies. As shown in Table 6, the Fe3O4@SiO2@BenzIm-Fc[Cl]/BiOCl nanocatalyst and new ultrasonic assisted method has a significant impact on the performance of the reactions rather than other catalytic methods.
| Entry | Compound | Catalyst, solvent, condition | Time | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 |
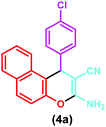
|
Triazine-based porous polymer, H2O, 80 °C | 6 h | 88 | 63 |
| Silica-supported piperazine, CHCl3, reflux | 24 h | 71 | 64 | ||
| [cmmim]Br, solvent-free, 110 °C | 15 min | 90 | 65 | ||
| (SiO2@Im-Fc[OAc]), solvent-free, 90 °C | 20 min | 92 | 42 | ||
| Nanocatalyst, EtOH/H2O, sonication | 15 min | 96 | This work | ||
| 2 |
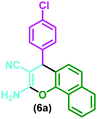
|
Nanostructured diphosphate Na2CaP2O7, H2O, reflux | 4 h | 90 | 66 |
| Nanozeolite clinoptilolite, H2O, reflux | 20 min | 95 | 67 | ||
| Sodium-modified hydroxyapatite, H2O, reflux | 3 h | 96 | 68 | ||
| Silica-supported piperazine, CHCl3, reflux | 7 h | 74 | 64 | ||
| [TEA–PEG800–DIL][Cl], H2O, reflux | 15 min | 95 | 54 | ||
| Nanocatalyst, EtOH/H2O, sonication | 15 min | 96 | This work | ||
| 3 |
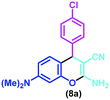
|
Piperidine, EtOH, 35 °C | 12 h | 47 | 69 |
| Tris-hydroxymethylamino methane, EtOH/H2O, R.t. | 2.5 h | 85 | 29 | ||
| Nanocatalyst, EtOH/H2O, sonication | 20 min | 94 | This work | ||
| 4 |
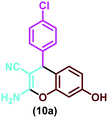
|
Triazine-based porous polymer, solvent-free, 80 °C | 6 h | 85 | 63 |
| Potassium phthalimide-N-oxyl, H2O, reflux | 15 min | 92 | 20 | ||
| Amino-appended β-cyclodextrins, H2O, R.t. | 5 h | 90 | 70 | ||
| Hydrotalcite, H2O, 60 °C | 5 h | 90 | 27 | ||
| 2-Ethylimidazolium acetate, solvent-free, 67.5 °C | 29 min | 94 | 28 | ||
| Nanocatalyst, EtOH/H2O, sonication | 8 min | 98 | This work | ||
4. Conclusions
In summary, a new approach for the synthesis of modified magnetic nano-particles was introduced. A novel Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite was synthesized, characterized and applied as an efficient heterogeneous catalyst for the synthesis of a wide variety of 2-amino-4H-chromene derivatives. The reactions were performed under ultrasonic irradiation which has many benefits such as simple operation, reducing the reaction time and improving the product yield. A series of active phenolic compounds (2-naphthol, 1-naphthol, 3-(dimethylamino)phenol, resorcinol and orcinol) were used for the synthesis of 2-amino-4H-chromene derivatives via one-pot three-component reactions. A general and efficient ultrasound-assisted protocol using a novel recoverable magnetic nano-composite has been demonstrated.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the Iran National Science Foundation (INSF) and the University of Tabriz.References
- M. E. Riveiro, A. Moglioni, R. Vazquez, N. Gomez, G. Facorro, L. Piehl, E. R. de Celis, C. Shayo and C. Davio, Bioorg. Med. Chem., 2008, 16, 2665 CrossRef CAS PubMed.
- M. M. Kandeel, A. M. Kamal, E. K. A. Abdelall and H. A. H. Elshemy, Eur. J. Med. Chem., 2013, 59, 183 CrossRef CAS PubMed.
- S. A. Patil, J. Wang, X. S. Li, J. Chen, T. S. Jones, A. Hosni-Ahmed, R. Patil, W. L. Seibel, W. Li and D. D. Miller, Bioorg. Med. Chem. Lett., 2012, 22, 4458 CrossRef CAS PubMed.
- S. Jabeen, O. A. Chat, G. M. Rather and A. A. Dar, Food Res. Int., 2013, 51, 294 CrossRef CAS.
- G. Melagraki, A. Afantitis, O. Igglessi-Markopoulou, A. Detsi, M. Koufaki, C. Kontogiorgis and D. J. Hadjipavlou-Litina, Eur. J. Med. Chem., 2009, 44, 3020 CrossRef CAS PubMed.
- D. Wlodkowic, J. Skommer, M. M. Matto, M. Eray and J. Pelkonen, Leuk. Res., 2006, 30, 322 CrossRef PubMed.
- G. Melagraki, A. Afantitis, O. Igglessi-Markopoulou, A. Detsi, M. Koufaki, C. Kontogiorgis and D. J. Hadjipavlou-Litina, Eur. J. Med. Chem., 2009, 44, 3020 CrossRef CAS PubMed.
- M. M. Khafagy, A. H. A. El-Wahab, F. A. Eid and A. M. El-Agrody, Farmaco, 2002, 57, 715 CrossRef CAS PubMed.
- N. R. Kamdar, D. D. Haveliwala, P. T. Mistry and S. K. Patel, Anti-Infect. Agents, 2013, 11, 41 CrossRef CAS.
- T. R. Reddy, L. S. Reddy, G. R. Reddy, V. S. Nuthalapati, Y. Lingappa, S. Sandra, R. Kapavarapu, P. Misra and M. Pal, Bioorg. Med. Chem. Lett., 2011, 21, 6433 CrossRef PubMed.
- C. Conti and N. Desideri, Bioorg. Med. Chem. Lett., 2009, 17, 3720 CrossRef CAS PubMed.
- S. Q. Yin, M. Shi, T. T. Kong, C. M. Zhang, K. Han, B. Cao, Z. Zhang, X. Du, L. Q. Tang, X. Mao and Z. P. Liu, Bioorg. Med. Chem. Lett., 2013, 23, 3314 CrossRef CAS PubMed.
- H. M. Aly and M. M. Kamal, Eur. J. Med. Chem., 2012, 47, 18 CrossRef CAS PubMed.
- R. W. Fuller, H. R. Bokesch, K. R. Gustafson, T. C. McKee, J. H. Cardellina, J. B. McMahon, G. M. Cragg, D. D. Soejarto and M. R. Boyd, Bioorg. Med. Chem. Lett., 1994, 4, 1961 CrossRef CAS.
- M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295 CrossRef CAS PubMed.
- M. Kidwai and R. Poddar, Catal. Lett., 2008, 124, 311 CrossRef CAS.
- D. S. Raghuvanshi and K. N. Singh, ARKIVOC, 2010, 10, 305 Search PubMed.
- S. Khaksar, A. Rouhollahpour and S. M. Talesh, J. Fluorine Chem., 2012, 141, 11 CrossRef CAS.
- J. Safari and Z. Zarnegar, J. Mol. Struct., 2014, 1072, 53 CrossRef CAS.
- M. G. Dekamin, M. Eslami and A. Maleki, Tetrahedron, 2013, 69, 1074 CrossRef CAS.
- S. Makarem, A. A. Mohammadi and A. R. Fakhari, Tetrahedron Lett., 2008, 49, 7194 CrossRef CAS.
- N. R. Kamdar, D. D. Haveliwala, P. T. Mistry and S. K. Patel, Eur. J. Med. Chem., 2010, 45, 5056 CrossRef CAS PubMed.
- B. Datta and M. A. Pasha, Ultrason. Sonochem., 2012, 19, 725 CrossRef CAS PubMed.
- J. Safari and L. Javadian, Ultrason. Sonochem., 2015, 22, 341 CrossRef CAS PubMed.
- A. Patra and T. Mahapatra, Synth. Commun., 2013, 43, 1602 CrossRef CAS.
- S. J. Mountford, A. L. Albiston, W. N. Charman, L. Ng, J. K. Holien, M. W. Parker, J. A. Nicolazzo, P. E. Thompson and S. Y. Chai, J. Med. Chem., 2014, 57, 1368 CrossRef CAS PubMed.
- S. R. Kale, S. S. Kahandal, A. S. Burange, M. B. Gawandeb and R. V. Jayaram, Catal. Sci. Technol., 2013, 3, 2050 RSC.
- M. Ghorbani, S. Noura, M. Oftadeh, M. A. Zolfigol, M. H. Soleimani and K. Behbodi, J. Mol. Liq., 2015, 212, 291 CrossRef CAS.
- K. S. Pandit, P. V. Chavan, U. V. Desai, M. A. Kulkarni and P. P. Wadgaonkar, New J. Chem., 2015, 39, 4452 RSC.
- J. E. Mondloch, E. Bayram and R. G. Finke, J. Mol. Catal. A: Chem., 2012, 355, 1 CrossRef CAS.
- P. Munnik, P. E. de Jongh and K. P. de Jong, Chem. Rev., 2015, 115, 6687 CrossRef CAS PubMed.
- J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 198, 317 CrossRef CAS.
- J. M. Thomas and R. Raja, Annu. Rev. Mater. Res., 2005, 35, 315 CrossRef CAS.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371 RSC.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- M. Haruta, Catal. Today, 1997, 36, 153 CrossRef CAS.
- Y. Wei, B. Han, X. Hu, Y. Lin, X. Wang and X. Deng, Procedia Eng., 2012, 27, 632 CrossRef CAS.
- J. Sun, S. Zhou, P. Hou, Y. Yang, J. Weng, X. Li and M. Li, J. Biomed. Mater. Res., Part A, 2007, 80, 333 CrossRef PubMed.
- M. P. Narayan, S. S. Kumar, A. S. Arunrao, P. Karthikeyan and B. P. Rambhau, Res. J. Chem. Environ., 2011, 15, 92 Search PubMed.
- T. Yu, Z. Wang and T. J. Mason, Ultrason. Sonochem., 2004, 11, 95 CrossRef CAS PubMed.
- T. Harifi and M. Montazer, Ultrason. Sonochem., 2015, 27, 543 CrossRef CAS PubMed.
- R. Teimuri-Mofrad, M. Gholamhosseini-Nazari, E. Payami and S. Esmati, Res. Chem. Intermed., 2017, 43, 7105 CrossRef CAS.
- R. Teimuri-Mofrad, M. Gholamhosseini-Nazari, E. Payami and S. Esmati, Appl. Organomet. Chem., 2018, 32, e3955 CrossRef.
- R. Teimuri-Mofrad, S. Esmati, M. Rabiei and M. Gholamhosseini-Nazari, Heterocycl. Commun., 2017, 23, 439 CAS.
- R. Teimuri-Mofrad, S. Esmati, M. Rabiei and M. Gholamhosseini-Nazari, J. Chem. Res., 2018, 42, 7 CrossRef CAS.
- R. Teimuri-Mofrad, M. Gholamhosseini-Nazari, S. Esmati and A. Shahrisa, Res. Chem. Intermed., 2017, 43, 6845 CrossRef CAS.
- L. Zhang, W. Wang, L. Zhou, M. Shang and S. Sun, Appl. Catal., B, 2009, 90, 458 CrossRef CAS.
- H. Chen, X. Wang, W. Bi, Y. Wu and W. Dong, J. Colloid Interface Sci., 2017, 502, 89 CrossRef CAS PubMed.
- C. Tan, G. Zhu, M. Hojamberdiev, C. Xu, J. Liang, P. Luo and Y. Liu, J. Cluster Sci., 2013, 24, 1115 CrossRef.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- K. Nishio, M. Ikeda, N. Gokon, S. Tsubouchi, H. Narimatsu, Y. Mochizuki, S. Sakamoto, A. Sandhu, M. Abe and H. Handa, J. Magn. Magn. Mater., 2007, 310, 2408 CrossRef CAS.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2008, 130, 28 CrossRef CAS PubMed.
- A. M. El-Agrody, H. K. A. El-Mawgoud, A. M. Fouda and E. S. Khattab, Chem. Pap., 2016, 70, 1279 CAS.
- Y. Wang, C. Yue, X. Li and J. Luo, C. R. Chim., 2016, 19, 1021 CrossRef CAS.
- S. Shinde, S. Damate, S. Morbale, M. Patil and S. S. Patil, RSC Adv., 2017, 7, 7315 RSC.
- K. S. Pandit, P. V. Chavan, U. V. Desai, M. A. Kulkarni and P. P. Wadgaonkar, New J. Chem., 2015, 39, 4452 RSC.
- M. Khoobi, T. M. Delshad, M. Vosooghi, M. Alipour, H. Hamadi, E. Alipour and A. Shafiee, J. Magn. Magn. Mater., 2015, 375, 217 CrossRef CAS.
- M. A. Zolfigol, M. Yarie and S. Baghery, Synlett, 2016, 1418 CrossRef CAS.
- M. Ghorbani, S. Noura, M. Oftadeh, M. A. Zolfigol, M. H. Soleimani and K. Behbodi, J. Mol. Liq., 2015, 212, 291 CrossRef CAS.
- B. Şen, N. Lolak, Ö. Paralı, M. Koca, A. Şavk, S. Akocak and F. Şen, Nano-Struct. Nano-Objects, 2017, 12, 33 CrossRef.
- S. Rostamizadeh and N. Zekri, Res. Chem. Intermed., 2016, 42, 2329 CrossRef CAS.
- S. Khaksar, A. Rouhollahpour and S. M. Talesh, J. Fluorine Chem., 2012, 141, 11 CrossRef CAS.
- S. K. Kundu and A. Bhaumik, RSC Adv., 2015, 5, 32730 RSC.
- N. Golari, M. Rahimizadeh, M. Bakavoli and E. R. Seresht, Res. Chem. Intermed., 2015, 41, 6023 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol, O. Khaledian, V. Khakyzadeh, M. H. Beyzavi and H. G. Kruger, Chem. Eng. J., 2014, 248, 122 CrossRef CAS.
- A. Solhy, A. Elmakssoudi, R. Tahir, M. Karkouri, M. Larzek, M. Bousmina and M. Zahouily, Green Chem., 2010, 12, 2261 RSC.
- S. M. Baghbanian, N. Rezaei and H. Tashakkorian, Green Chem., 2013, 15, 3446 RSC.
- Y. Essamlali, O. Amadine, H. Maati, K. Abdelouahdi, A. Fihri, M. Zahouily, R. S. Varma and A. Solhy, ACS Sustainable Chem. Eng., 2013, 1, 1154 CrossRef CAS.
- M. Vosooghi, S. Rajabalian, M. Sorkhi, M. Badinloo, M. Nakhjiri, A. S. Negahbani, A. Asadipour, M. Mahdavi, A. Shafiee and A. Foroumadi, Results Pharma Sci., 2010, 5, 9 CAS.
- Y. F. Ren, B. Yang and X. L. Liao, Catal. Sci. Technol., 2016, 6, 4283 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nj04938f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |