Thin supported MOF based mixed matrix membranes of Pebax® 1657 for biogas upgrade†
Javier
Sánchez-Laínez
 ,
Inés
Gracia-Guillén
,
Beatriz
Zornoza
,
Inés
Gracia-Guillén
,
Beatriz
Zornoza
 ,
Carlos
Téllez
,
Carlos
Téllez
 and
Joaquín
Coronas
and
Joaquín
Coronas
 *
*
Chemical and Environmental Engineering Department, Instituto de Nanociencia de Aragón (INA) and Instituto de Ciencia de Materiales de Aragón (ICMA), Universidad de Zaragoza-CSIC, 50018 Zaragoza, Spain. E-mail: coronas@unizar.es
First published on 19th November 2018
Abstract
This work shows the preparation of thin mixed matrix membranes (MMMs) with a 2–3 μm thick Pebax® 1657 layer on two different supports: a porous asymmetric polyimide P84® and dense polytrimethylsilylpropyne (PTMSP). Nanoparticles of metal–organic frameworks (MOFs) ZIF-8, MIL-101(Cr), UiO-66 and ZIF-7/8 core–shells were selected as fillers for the Pebax® 1657 based MMMs, all of them being MOFs with high CO2 adsorption capacity but different pore size distribution. All the membranes were characterized by SEM, FTIR, Raman, TGA and XRD analyses, showing in all cases a perfect compatibility of the Pebax® layer with both supports and also a good dispersion of the fillers in the polymeric matrix. These membranes were applied for the separation of equimolar CO2/CH4 mixtures at 35 °C under feed pressures between 3 and 5 bar, where an improvement in the gas separation performance with increasing pressure was noticed, thanks to the favored solubility of CO2. The synergistic compatibility between Pebax® 1657 and P84® gave rise to a 470% enhancement in CO2/CH4 selectivity, reaching a maximum value of 114 while the CO2 permeance increased by 40% up to 7.5 GPU. The addition of fillers in the Pebax® polymeric phase produced an improvement in the gas separation performance of the membranes, especially in terms of permeance, where the MMMs containing a 10 wt% loading of UiO-66 reached the optimum value of 11.5 GPU of CO2 (together with a CO2/CH4 selectivity of 55.6).
1. Introduction
Biogas production from renewable sources (e.g., from agriculture, landfills, or sewage plants) is one of the fields where membrane technology can show its greatest potential.1,2 The main components of biogas are methane (CH4, the combustible component) and carbon dioxide (CO2, the non-combustible component), although it also typically contains traces of H2O, N2, H2S and other organic aromatics.3 The high concentration of CO2 and CH4 in the mixture, basically in the same proportion, makes biogas upgrading appropriate to be carried out with polymeric membranes, a technology that offers advantages such as low energy costs and environmental benignity,4 and that can be an alternative to other existing approaches, such as cryogenic upgrading or liquefaction.5 For example, PVAm/PVA blends have shown a CH4 recovery of 99% at a low running cost in a 2-stage recycled process.6 Besides purifying the CH4 flow, the captured CO2 is also suitable for its conversion into high value-added products, such as MeOH.7The major materials used for membranes are polyimides and fluoropolymers.8 To obtain membranes with a good gas separation performance (i.e. high CO2 permeation flux and CO2/CH4 selectivity), materials with intrinsic separation capacity for the target mixture are necessary. Poly(ether-block-amide), best known under the trademark Pebax®, constitutes a family of polymers that possess these advantageous properties. These polymers combine linear chains of rigid polyamide with flexible, CO2-philic polyether segments, building crystalline/amorphous structures that show the properties of both thermoplastics and rubbers. It is believed that the hard amide block provides mechanical strength, whereas gas selective transport occurs primarily through the soft ether block.9 The polyamide/polyetheroxide proportion in the blend determines the Pebax® grade. The membranes in this work were prepared with Pebax® 1657, consisting of 40 wt% polyamide.10
Membranes with high permeance are essential for large-scale applications, such as biogas upgrading.11 This variable is not only related to the membrane permeability but also to the thickness of the membrane, and membranes consisting of a very thin selective layer are necessary to achieve this goal. Such high performance membranes can be prepared as composite materials, where the selective layer is deposited on a highly porous support that provides mechanical stability.12 Pebax® 1657 can be found in the literature in the form of thin film composites on several polymeric supports, such as polyvinylidene fluoride (PVDF),13,14 polyacrylonitrile (PAN)15,16 and polysulfones.17,18 The CO2 permeances vary from 13 to 670 GPU according to the membrane morphology and the CO2/CH4 selectivities are found to be between 13.6 and 18.0. These works also provide CO2/N2 selectivities, which show highly dispersed values (between 32 and 70).
In general, the gas separation performances of polymeric membranes can be enhanced through the concept of mixed matrix membranes (MMMs), consisting of the dispersion of inorganic fillers within a polymeric matrix so that either or both permeability and selectivity of the membrane can be improved through the synergistic combination of the two components.19 Metal–organic frameworks (MOFs) are materials that have been widely used as fillers in MMMs. In the case of the Pebax® 1657 polymer, ZIF-8 has been used as the filler by Xu et al.20 and Zheng et al.21 The former found an increase in the CO2 permeability from 79.2 to 156 Barrer as the ZIF-8 loading increased from 0 to 20 wt%, but the CO2/N2 selectivity decreased until 40.5. The latter showed fluctuating CO2 permeabilities between 55.8 and 179 Barrer and practically constant CO2/N2 and CO2/CH4 selectivities. Within the ZIF family, ZIF-7 has also been used as the filler in Pebax® 1657 membranes. Li et al.22 prepared thin Pebax® 1657 based MMMs supported on PAN that showed the best performance results at 34 wt% loading with a CO2 permeance of 39 GPU, and CO2/N2 and CO2/CH4 selectivities of 105 and 44, respectively. ZIF-7 has also been used as a filler by Sutrisna et al.13 who prepared MMMs on PVDF hollow fibers with optimum values of 300 GPU of CO2, and with CO2/N2 and CO2/CH4 selectivities of 47.5 and 17.0, respectively. Other MOF-Pebax® 1657 combinations for dense MMMs included MOFs ZIF-94, NH2-MIL-53(Al), MIL-69(Al) and MIL-96(Al), with the latter giving rise to the best CO2/N2 performance: permeability and selectivity were enhanced by 25 and 18%, respectively, as compared to the pure polymer.23 Interestingly, the effect of the MOF functionalization (comparing the use of MIL-53(Al) and NH2-MIL-53(Al) with better CO2 permeability and CO2/CH4 selectivity values for the latter) has been recently studied on dense MMMs with Pebax® 1657.24 There is no doubt that 1657 is the most used Pebax® code in the MMM field.
This work shows the preparation of thin film composite membranes with a thin mixed-matrix selective top layer of MOF/polymer Pebax® 1657 for biogas upgrade. The membranes have been prepared on different polymeric supports and the influence of the feed pressure on the gas separation performance has been studied. Different MOFs (ZIF-8, ZIF-7/8 core–shells, UiO-66 and MIL-101(Cr)) have been embedded in Pebax® 1657, dissolved in a water–ethanol mixture,25 as fillers to obtain thin supported MMMs. Materials with a high CO2 uptake (see Table 1) have been selected to favor the solubility of this gas in the membrane composite and thus enhance its CO2/CH4 separation performance.
| MOF | Adsorption conditions | CO2 uptake, (mmol g−1) | Pore aperture (nm) | Cavity (nm) | Ref. |
|---|---|---|---|---|---|
| ZIF-8 | 273 K, 1 bar | 1.3 | 0.34 | 1.16 | 26,27 |
| 298 K, 30 bar | 35 | ||||
| UiO-66 | 273 K, 1 bar | 2.4 | 0.80 | 2.1 | 28–30 |
| 300 K, 35 bar | 7.0 | ||||
| MIL-101(Cr) | 303 K, 1 bar | 1.6 | 1.2–1.6 | 2.9–3.4 | 31–33 |
| 304 K, 50 bar | 40 | ||||
| ZIF-7/8 core–shells | 273 K, 1 bar | 2.5 | 0.29–0.34 | 0.43–1.16 | 26 |
2. Experimental section
2.1 Synthesis of MOF nanoparticles
Four different MOFs were synthesized to be used as fillers in the MMMs of this work. The synthesis of ZIF-8 was performed following a method based on a MeOH–water mixture as the solvent.34 UiO-66 was synthesized solvothermally in N,N-dimethylformamide (DMF, 99.8%, Sigma-Aldrich).35 The synthesis of MIL-101(Cr) was microwave assisted, with DI water as the solvent for the metal source and the ligand.36 And finally, the ZIF-7/8 core–shells were prepared via post-synthetic modification of the previously described explained ZIF-8 nanoparticles.26 The experimental details are described in the ESI.†2.2 Membrane preparation
Before testing the gas separation performance, several membranes were treated with PDMS (Sylgard® 184, Dow Corning) by dip coating. The coating solution was prepared by mixing the PDMS polymer base and the hardener (dimethyl, methylhydrogen siloxane) provided with the Sylgard® kit with a weight ratio of 10 : 1. The mixture was added to n-hexane to obtain a 3 wt% solution. The membranes were immersed in the coating solution for 5 min, and then allowed to evaporate at room temperature for 2 h. Finally, the membranes were cured in an oven at 100 °C for 18 h.
2.3 Membrane characterization
Thermogravimetric analyses (TGA) were carried out using a Mettler Toledo TGA/STDA 851e. Samples (10 mg) placed in 70 μL alumina pans were heated under a 40 cm3(STP) min−1 air flow from 25 to 900 °C at a heating rate of 10 °C min−1. Differential scanning calorimetry (DSC) analyses were performed on a Mettler Toledo DSC822e. Samples (10 mg) placed in 70 μL aluminum pans were heated under a 40 cm3(STP) min−1 of nitrogen flow from 25 to 500 °C at a heating rate of 10 °C min−1. The scanning electron microscopy (SEM) images of the MOFs and membranes were obtained using a FEI Inspect F50 model SEM, operated at 20 kV. Cross-sections of the membranes were prepared by freeze-fracturing after immersion in liquid N2 and subsequently coated with Pt. Fourier transform infrared spectroscopy (FTIR) was performed for the MOF powders and for the different membrane samples, using a Bruker Vertex 70 FTIR spectrometer equipped with a DTGS detector and a Golden Gate diamond ATR accessory. The spectra were recorded on the Pebax® side by averaging 40 scans in the 4000–600 cm−1 wavenumber range at a resolution of 4 cm−1. Membranes were also characterized by Raman spectroscopy using a WiTec Alpha300 Confocal Raman Microscope, with a 785 nm laser excitation beam. X-ray diffraction (XRD) patterns of the MOFs and MMMs were obtained using Panalytical Empyrean equipment, using CuKα radiation (λ = 1.540 Å), taking data from 2θ = 2.5° to 40° at a scan rate of 0.03° s−1.2.4 Gas separation analysis
The membrane samples were placed in a module consisting of two stainless steel pieces and a 316LSS macroporous disk support of 3.14 cm2 (from Mott Co.) with a 20 μm nominal pore size, and gripped inside with silicon O-rings. The permeation module was placed in a UNE 200 Memmert oven to control the temperature of the experiments. Gas separation measurements were carried out by feeding a CO2/CH4 equimolar mixture (25/25 cm3(STP) min−1) at 3–5 bar to the feed side by means of two mass-flow controllers (Alicat Scientific, MC-100CCM-D), while the permeate side of the membrane was swept with a 1 cm3(STP) min−1 mass-flow controlled stream of He at 1 bar (Alicat Scientific, MC-5CCM-D). The concentrations of CO2 and CH4 in the outgoing streams were analyzed using an Agilent 3000A online gas microchromatograph equipped with a thermal conductivity detector. Permeances were calculated in GPU (10−6 cm3(STP) cm−2 s−1 cmHg−1) once the steady-state of the membrane module exit stream was reached (for at least 3 h), and the separation selectivity was calculated as the ratio of permeances. At least 2–3 membrane samples of each type were fabricated and measured to provide the corresponding error estimations.3. Results and discussion
3.1 Membrane characterization
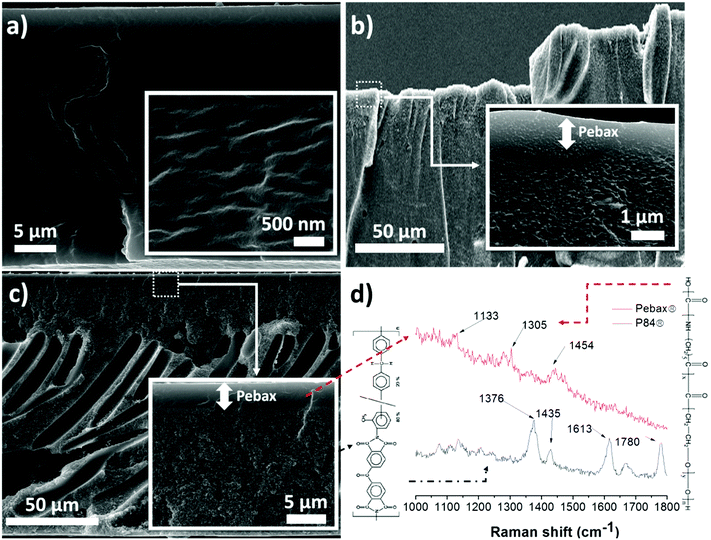
Fig. 1 shows the cross-sections of three different membranes based on Pebax® 1657: a self-supported dense Pebax® 1657 membrane of around 80 μm thickness (Fig. 1a) and two supported Pebax® 1657 membranes prepared on dense PTMSP and asymmetric P84® supports (Fig. 1b and c, respectively). The cross-section of the Pebax® 1657/P84® composite shows a thickness of 120 μm for the P84® support, of which 15 μm corresponds to the denser top layer. Moreover, it can be observed in the inset at a higher magnification that the Pebax® 1657 layer is approximately 3 μm thick and shows a good adhesion to the polyimide support. A good compatibility can also be observed in the composite membrane prepared on PTMSP, the Pebax® 1657 layer in this case being 2 μm thick.
Fig. 1d shows the Raman spectra of the cross-section of the Pebax® 1657/P84® membrane. Two different points on the zones corresponding to the Pebax® 1657 layer and the P84® support were measured. Although the Pebax® 1657 Raman spectrum shows weak signals owing to its fluorescence, three peaks can be distinguished at 1133, 1305 and 1454 cm−1 related to the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration modes.38 Signals in the 1300–1800 cm−1 range can be seen in the P84® spectrum. The signals at 1376 and 1435 cm−1 correspond to the C
O vibration modes.38 Signals in the 1300–1800 cm−1 range can be seen in the P84® spectrum. The signals at 1376 and 1435 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in-phase stretching mode. The band at 1613 cm−1 is related to the aromatic ring stretching mode, and that at 1780 cm−1 corresponds to the aromatic C–N stretching.39
O in-phase stretching mode. The band at 1613 cm−1 is related to the aromatic ring stretching mode, and that at 1780 cm−1 corresponds to the aromatic C–N stretching.39
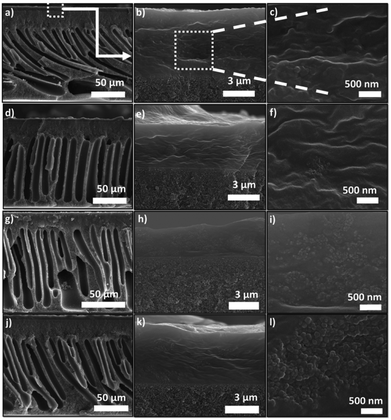
Pebax® 1657 MMMs are shown in Fig. 2. Membranes containing a 10 wt% loading of ZIF-8, UiO-66, MIL-101(Cr) and ZIF-7/8 core–shell particles can be seen at three different magnifications. By visual inspection a good dispersion of the different fillers in the Pebax® thin layer can be observed, resulting in homogeneous membranes where a good filler–polymer adhesion is noticeable. The SEM images of the fillers are also provided (see Fig. S1 from the ESI†), from which the cumulative and differential particle size distributions were obtained using the ImageJ 1.49b software, together with median particle sizes of 150, 25, 33 and 124 nm for ZIF-8, UiO-66, MIL-101(Cr) and ZIF-7/8 core–shell particles, respectively (see Fig. S2 and Table S1 in the ESI†).
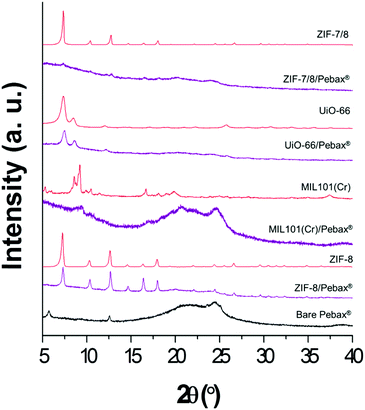
Fig. 3 shows the XRD patterns of the different membranes, MOFs and of the pure polymeric Pebax® 1657 membrane for comparison. Pristine Pebax® 1657 is a semicrystalline copolymer which consists of both crystalline and amorphous PEO and PA6 phases, showing characteristic peaks at 2θ = 5.8°, 12.6° and 24.4°.40 These signals are also noticeable in the patterns of the MMMs, although with a lower intensity due to the higher crystallinity of the fillers. It is also clear that ZIF-8 and UiO-66 maintain their crystallinity in the polymer matrix since their XRD reflections dominate over the amorphous band of the polymer. In the case of the other two MOFs, the peaks are not so well defined. This is due to the lower crystallinity of MIL-101(Cr) and the fact that the ZIF-7/8 core–shells are not as crystalline as the original ZIF-8 from which they are synthesized, according to our previous study.26 Besides, after the incorporation of the MOFs, the peak positions of Pebax® 1657 remained almost unaltered, proving that there were no changes in the d-spacing of the polymer.
The FTIR spectra were recorded to further characterize and analyze the Pebax® 1657 MMMs (see Fig. S3 in the ESI†). The observed peak at 1094 cm−1 is attributed to the stretching vibration of the C–O–C group of the soft segment part of PEO.40 Regarding the hard segment of PA chains, the peak corresponding to the –N–H– linkages is found at 3298 cm−1 and the characteristic peak at 1636 cm−1 is assigned to the H–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.41 The most intense signals of each MOF can be found in the corresponding MMM spectrum. However, none of the membranes show new absorbance peaks, suggesting weak chemical interactions between the filler nanoparticles and the polymer chains or that the filler loading is too low for their visualization.
O group.41 The most intense signals of each MOF can be found in the corresponding MMM spectrum. However, none of the membranes show new absorbance peaks, suggesting weak chemical interactions between the filler nanoparticles and the polymer chains or that the filler loading is too low for their visualization.
Thermogravimetric analyses (TGA) in flowing air were used to elucidate the thermal stability of the different membranes prepared in this work. As seen in Fig. S4 in the ESI,† while the P84® support shows an onset temperature of 592 °C, Pebax® 1657 was less stable since it started to degrade at around 400 °C. This is consistent with a slightly reduced thermal stability of the supported Pebax® 1657/P84® composite. Regarding the MMMs, the thermograms indicate that all the MOFs started to decompose over 300 °C. Besides, these TGA analyses helped to verify that the actual MOF content in the mixed matrix thin layer (12.5 wt% for ZIF-8, 8.2 wt% for MIL-101(Cr), 10.9 wt% for UiO-66 and 13.4 wt% for ZIF-7/8 MMMs) fits with the nominal (10 wt%). The thermal properties of Pebax® 1657 were further investigated by DSC (see Fig. S5 in the ESI†). Pristine Pebax® 1657 shows two endothermic peaks whose maxima occur approximately at 40 and 130 °C. These can be attributed to the fusion of the crystalline fraction of the blocks of poly(ethylene oxide) and polyamide, and limit the operating temperature of the membranes.42
3.2 Gas separation performance
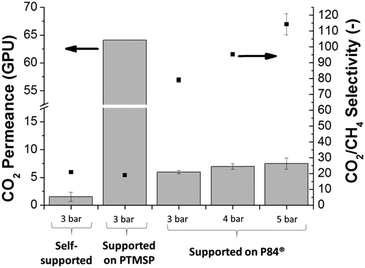
The different membranes prepared were tested for the separation of the CO2/CH4 equimolar mixtures at 35 °C and under different feed pressures ranging from 3 to 5 bar.Fig. 4 depicts the gas separation performance of pristine Pebax® 1657 membranes. Three different types of membranes were studied, self-supported Pebax® 1657 membranes and supported Pebax® 1657 using supports of two different polymers: a dense PTMSP and an asymmetric porous P84®. Thick self-supported Pebax® 1657 and thin Pebax® 1657 supported on PTMSP showed similar CO2/CH4 selectivities, with values around 20. However, the difference in CO2 permeance was much more noticeable since the former showed only 1.5 GPU while that of the latter increased up to 64 GPU. This is consistent with the difference in thickness between both membranes: 80 μm of the self-supported membrane vs. 2 μm of the supported membrane. Taking into account the corresponding value of this parameter for each membrane, the calculated CO2 permeability would be around 120 Barrer in both cases. This highlights the reliability of the membrane permeation characterization system.
When testing the Pebax® 1657 supported on P84® also at 3 bar, the CO2 permeance was 6.0 GPU, the flow increase being smaller than for the previous PTMSP supported membrane. Nevertheless, the CO2/CH4 selectivity increased considerably, reaching a value of 79.2, four-fold higher than that of the self-supported membrane. This behavior indicates that the P84® support affects the gas separation performance of the composites, increasing the membrane selectivity and simultaneously decreasing the gas permeability. For a better understanding of the role that the P84® support was playing in the gas separation, the support itself was tested for the CO2/CH4 separation (see Table S2 from the ESI†). The results showed that the P84® support exhibited a CO2 permeance of 270 GPU but showed no CO2/CH4 selectivity. When the P84® was coated with PDMS the permselectivity was enhanced by defect healing, but only the inherent CO2/CH4 selectivity of PDMS was noticeable (5.5), along with its CO2 permeance (55.1 GPU).43 This fact means that P84® and Pebax® 1657 possess a specific compatibility, giving a composite whose gas separation performance is much better than that of the bare polymers. Besides, coating the polyimide P84® support with a more selective polymer such as Pebax® 1657 may lead to a healing effect and the selectivity of the polyimide would approach values found in the literature for this polymer (CO2/CH4 selectivity of 33.4).44
The effect of the feed pressure on the gas separation performance of the CO2/CH4 mixture was also studied. As seen in Fig. 4, the supported Pebax® 1657/P84® membranes were tested from 3 to 5 bar, showing that the increase in pressure implied an augment in both the CO2 permeance and the CO2/CH4 selectivity, reaching optimum values at 5 bar with 7.5 GPU and 114, respectively. The higher permeance of CO2 results from its smaller molecular diameter in combination with its enhanced solubility due to its high quadrupole moment (4.30 D Å for CO2vs. 0.02 D Å for CH4), which enables strong specific interactions with the polar polyether groups in Pebax®.10 Moreover, the CH4 permeance showed a contrary tendency, decreasing at the higher feed pressures tested. A similar reduction of permeation flux resulting from compression has been reported for N2 and CH4 in rubbery polymers such as PDMS and poly(octylmethylsiloxane) (POMS).45,46 Besides, as seen in Fig. S6 in the ESI,† both CO2 and CH4 permeances follow an exponential tendency as a function of feed pressure as described by Stern et al.47 (eqn (S1)†), with beta (the constant characteristic of the penetrant–membrane system at the testing temperature, 35 °C in this case) values positive for CO2 (0.11 bar−1) and negative for CH4 (−0.18 bar−1).
Membranes based on Pebax® 1657 using ZIF-8, UiO-66, MIL-101(Cr) and ZIF-7/8 core–shell particles as fillers have been prepared on P84® supports, forming thin supported MMMs. These MOFs have been selected because of their high CO2 uptake (1.3–2.5 mmol g−1 at 1 bar, see Table 1) in order to favor the solubility of this gas over CH4 in the Pebax® 1657 based MMMs. Only MIL-101(Cr) has cavities in the mesoporous range, while other MOFs are microporous materials (see Table 1). Fig. 5a shows the gas separation performance of these MMMs at 35 °C. Two different feed pressures of 3 and 5 bar were tested showing that, as in the previous separation with pristine Pebax® 1657 (see Fig. 4), both the CO2 permeance and the CO2/CH4 selectivity enhanced with increasing pressure. In terms of CO2 permeance, the gas separation performance of the membranes improved with the incorporation of MOFs into the polymeric matrix. MMMs showed an average increase in CO2 permeance of 6%, except for the UiO-66 MMMs, which showed a much greater improvement with a maximum value of 11.5 GPU at 5 bar, almost twice that of pristine Pebax® 1657 at the same feed pressure. Regarding the CO2/CH4 selectivity, its value decreased to one half when any of the fillers were incorporated into Pebax® 1657. Nevertheless, CO2/CH4 selectivities remained high with values between 50 and 60 for different MMMs, making them still very attractive. The best value was obtained for the ZIF-8 MMMs, with a CO2/CH4 selectivity of 65.1 (with 7.7 GPU of CO2) at 5 bar. This result is logical since ZIF-8, besides having a moderate CO2 adsorption, is the MOF with the narrowest pore access (0.34 nm), between the kinetic diameters of CO2 and CH4 (0.33 and 0.36 nm, respectively). The narrowest porosity of the ZIF-7/8 material (see Table 1), which is the worst performer in terms of CO2/CH4 selectivity, may hinder the transport of CO2 in comparison to other MOFs.
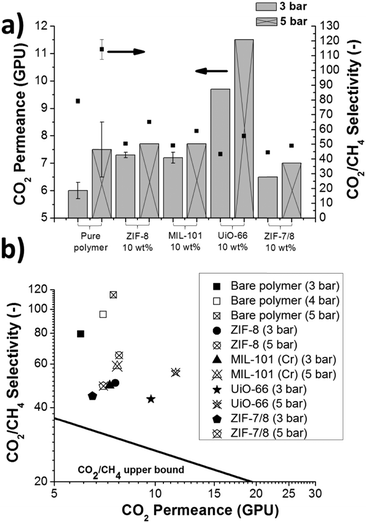 | ||
| Fig. 5 Comparison of the gas separation performance of pristine Pebax® 1657 and the different supported on P84® MMMs in the form of histogram (a) and upper bound type graph (b). | ||
Considering separately the effect of the diffusivity and selectivity of the MOFs in the gas separation performance of the membranes, ZIF-8 and ZIF-7/8 core–shells are expected to have a greater effect on the diffusivity thanks to their narrower pore distribution (see Table 1). In contrast, UiO-66 and MIL-101(Cr) may have a greater effect on the contribution of the solubility due to their higher CO2 uptake (see Table 1).
The gas separation performances of all MMMs were plotted on a selectivity–permeance graph (Fig. 5b). Since the Robeson's upper bound was originally defined in Barrer48(see the values in Table S3 from the ESI†), a new upper bound was calculated in GPU to obtain a more accurate comparison (Fig. S7 from the ESI†). The Robeson's upper bound, revisited in 200848 was defined from the pure component permeability data of dense membranes, allowing the determination of the state-of-the-art limits for gas separation with polymeric membranes. The upper bound relationship is expressed by Pi = k·αnij, where Pi is the permeability of the more permeable gas, α is the separation factor (Pi/Pj) and n is the slope of the log–log limit. It was observed that the representation of −1/n vs. dij (where dij is the difference between the gas molecular diameters (dj − di)) yielded a straight line relationship. Since the gas permeability was defined for the explained purpose in Barrer, a new CO2/CH4 upper bound relationship in GPU has been calculated here. This used the values from the literature that defined the original upper bound but changing permeabilities in Barrer by permeances in GPU (see Table S3 from the ESI†), as done in a previous work for H2/CO2 mixtures.37 The thicknesses used are those reported in the publications cited in Table S3 in the ESI,† although possible inaccuracies in the ex situ measurement of this length, such as experimental errors or membrane swelling, might affect such values. These values are represented in Fig. S7 in the ESI† and fitted to a logarithmic equation, resulting in the following upper bound relationship: 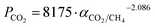 . A factor k of 8175 GPU was obtained and the slope n of −2.086 was not far from the value found in the original publication (−2.636). Fig. 5b shows that all the membranes prepared in this work clearly surpassed the new calculated upper bound, reaching the so-called commercially attractive region. UiO-66 MMMs exhibited the highest CO2 permeances, followed by MIL-101(Cr) MMMs, thanks to their wide porosity (see Table 1). In contrast, ZIF-7/8 MMMs are the least permeable and they also contain fillers with the narrowest pore distribution. ZIF-8 MMMs are the best balanced membranes, showing a great CO2/CH4 selectivity with high CO2 permeance.
. A factor k of 8175 GPU was obtained and the slope n of −2.086 was not far from the value found in the original publication (−2.636). Fig. 5b shows that all the membranes prepared in this work clearly surpassed the new calculated upper bound, reaching the so-called commercially attractive region. UiO-66 MMMs exhibited the highest CO2 permeances, followed by MIL-101(Cr) MMMs, thanks to their wide porosity (see Table 1). In contrast, ZIF-7/8 MMMs are the least permeable and they also contain fillers with the narrowest pore distribution. ZIF-8 MMMs are the best balanced membranes, showing a great CO2/CH4 selectivity with high CO2 permeance.
4. Conclusions
Thin membranes of Pebax® 1657 have been successfully prepared on dense PTMSP and asymmetric porous P84® supports. The obtained supported Pebax® 1657 membranes, with a thickness ranging from 2–3 μm, have been found to exhibit a good compatibility and adhesion between the support and the selective layer. The membranes were tested for CO2/CH4 separation at 35 °C and different feed pressures (3–5 bar), showing an improvement in both the CO2 permeance and the CO2/CH4 selectivity with increasing pressures, thanks to the favored CO2 solubility. While the performance of Pebax® 1657/PTMSP membranes was similar to those of self-supported dense Pebax®, the Pebax® 1657/P84® composites showed a great enhancement in the CO2/CH4 selectivity thanks to the synergistic compatibility between the two polymers. Thin MMMs of Pebax® 1657 containing 10 wt% of ZIF-8, MIL-101(Cr), UiO-66 and ZIF-7/8 core–shell nanoparticles were also prepared supported on P84®. The incorporation of MOFs enhanced the CO2 permeance of the membranes on average 6%, but especially embedding UiO-66, which allowed doubling the permeance of pristine Pebax® 1657 membranes. ZIF-8 MMMs are the best performing composites, maintaining a high CO2 permeance with a good CO2/CH4 selectivity. In any event, it has been demonstrated that the good physicochemical interaction between polymer Pebax® 1657 and P84® support allowed an enhancement in the CO2/CH4 separation. The highest CO2/CH4 selectivity obtained along the work was that of the membrane made of bare Pebax® 1657 on P84®, with a value of 114 (at 7.5 GPU of CO2).Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Spanish MINECO and FEDER (MAT2016-77290-R), the Aragón Government (T43-17R) and the ESF is gratefully acknowledged. J. S.-L. thanks the Spanish Education Ministry Program FPU2014 for his PhD grant. All the microscopy work were carried out in the Laboratorio de Microscopías Avanzadas at the Instituto de Nanociencia de Aragón (LMA-INA). Finally, the authors would like to acknowledge the use of the Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza.References
- M. Poloncarzova, J. Vejrazka, V. Vesely and P. Izak, Angew. Chem., Int. Ed., 2011, 50, 669–671 CrossRef CAS PubMed.
- Y. Zhang, J. Sunarso, S. Liu and R. Wang, Int. J. Greenhouse Gas Control, 2013, 12, 84–107 CrossRef CAS.
- S. Rasi, A. Veijanen and J. Rintala, Energy, 2007, 32, 1375–1380 CrossRef CAS.
- J. C. Chen, X. Feng and A. Penlidis, Sep. Sci. Technol., 2005, 39, 149–164 CrossRef.
- A. Baccioli, M. Antonelli, S. Frigo, U. Desideri and G. Pasini, Appl. Energy, 2018, 217, 328–335 CrossRef CAS.
- L. Deng and M. Hägg, Int. J. Greenhouse Gas Control, 2010, 4, 638–646 CrossRef CAS.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti and E. Tzimas, Appl. Energy, 2016, 161, 718–732 CrossRef.
- B. Li, Y. Duan, D. Luebke and B. Morreale, Appl. Energy, 2013, 102, 1439–1447 CrossRef CAS.
- V. Bondar, B. Freeman and I. Pinnau, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 2051–2062 CrossRef CAS.
- J. H. Kim, S. Y. Ha and Y. M. Lee, J. Membr. Sci., 2001, 190, 179–193 CrossRef CAS.
- G. Valenti, A. Arcidiacono and J. A. N. Ruiz, Biomass Bioenergy, 2016, 85, 35–47 CrossRef CAS.
- Z. Dai, L. Ansaloni and L. Deng, Green Energy Environ., 2016, 1, 102–128 CrossRef.
- P. D. Sutrisna, J. Hou, M. Y. Zulkifli, H. Li, Y. Zhang, W. Liang, D. D'Alessandro and V. Chen, J. Mater. Chem. A, 2018, 6, 918–931 RSC.
- Y. Wang, T. Hu, H. Li, G. Dong, W. Wong and V. Chen, Energy Procedia, 2014, 63, 202–209 CrossRef CAS.
- E. Esposito, G. Clarizia, P. Bernardo, J. C. Jansen, Z. Sedláková, P. Izák, S. Curcio, B. d. Cindio and F. Tasselli, Chem. Eng. Process., 2015, 94, 53–61 CrossRef CAS.
- A. Car, C. Stropnik, W. Yave and K. Peinemann, Sep. Purif. Technol., 2008, 62, 110–117 CrossRef CAS.
- P. Li, Z. Wang, W. Li, Y. Liu, J. Wang and S. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 15481–15493 CrossRef CAS PubMed.
- L. Liu, A. Chakma and X. Feng, Chem. Eng. J., 2004, 105, 43–51 CrossRef CAS.
- G. Dong, H. Li and V. Chen, J. Mater. Chem. A, 2013, 1, 4610–4630 RSC.
- L. Xu, L. Xiang, C. Wang, J. Yu, L. Zhang and Y. Pan, Chin. J. Chem. Eng., 2017, 25, 882–891 CrossRef CAS.
- W. Zheng, R. Ding, K. Yang, Y. Dai, X. Yan and G. He, Sep. Purif. Technol., 2018 DOI:10.1016/j.seppur.2018.04.010.
- T. Li, Y. Pan, K. Peinemann and Z. Lai, J. Membr. Sci., 2013, 425, 235–242 CrossRef.
- A. Sabetghadam, X. Liu, M. Benzaqui, E. Gkaniatsou, A. Orsi, M. M. Lozinska, C. Sicard, T. Johnson, N. Steunou, P. A. Wright, C. Serre, J. Gascon and F. Kapteijn, Chem. – Eur. J., 2018, 24, 7949–7956 CrossRef CAS PubMed.
- S. Meshkat, S. Kaliaguine and D. Rodrigue, Sep. Purif. Technol., 2018, 200, 177–190 CrossRef CAS.
- M. Isanejad, N. Azizi and T. Mohammadi, J. Appl. Polym. Sci., 2017, 134, 44531–44540 CrossRef.
- J. Sánchez-Laínez, A. Veiga, B. Zornoza, S. R. Balestra, S. Hamad, A. R. Ruiz-Salvador, S. Calero, C. Téllez and J. Coronas, J. Mater. Chem. A, 2017, 5, 25601–25608 RSC.
- S. K. Nune, P. K. Thallapally, A. Dohnalkova, C. Wang, J. Liu and G. J. Exarhos, Chem. Commun., 2010, 46, 4878–4880 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- D. Sun, Y. Fu, W. Liu, L. Ye, D. Wang, L. Yang, X. Fu and Z. Li, Chem. – Eur. J., 2013, 19, 14279–14285 CrossRef CAS PubMed.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed.
- J. Benito, S. Sorribas, I. Lucas, J. Coronas and I. Gascon, ACS Appl. Mater. Interfaces, 2016, 8, 16486–16492 CrossRef CAS PubMed.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS PubMed.
- P. L. Llewellyn, S. Bourrelly, C. Serre, A. Vimont, M. Daturi, L. Hamon, G. De Weireld, J. Chang, D. Hong, Y. Kyu Hwang, S. H. Jhung and G. Férey, Langmuir, 2008, 24, 7245–7250 CrossRef CAS PubMed.
- N. Liédana, A. Galve, C. Rubio, C. Téllez and J. Coronas, ACS Appl. Mater. Interfaces, 2012, 4, 5016–5021 CrossRef PubMed.
- L. Hou, L. Wang, N. Zhang, Z. Xie and D. Dong, Polym. Chem., 2016, 7, 5828–5834 RSC.
- N. A. Khan, I. J. Kang, H. Y. Seok and S. H. Jhung, Chem. Eng. J., 2011, 166, 1152–1157 CrossRef CAS.
- J. Sánchez-Laínez, B. Zornoza, C. Téllez and J. Coronas, J. Membr. Sci., 2018, 563, 427–434 CrossRef.
- F. H. Akhtar, M. Kumar and K. Peinemann, J. Membr. Sci., 2017, 525, 187–194 CrossRef CAS.
- J. J. Ge, G. Xue, F. Li, K. W. McCreight, S. Wang, F. W. Harris, S. Z. Cheng, X. Zhuang, S. Hong and Y. Shen, Macromol. Rapid Commun., 1998, 19, 619–623 CrossRef CAS.
- J. H. Kim and Y. M. Lee, J. Membr. Sci., 2001, 193, 209–225 CrossRef CAS.
- A. Ghadimi, M. Amirilargani, T. Mohammadi, N. Kasiri and B. Sadatnia, J. Membr. Sci., 2014, 458, 14–26 CrossRef CAS.
- S. Sridhar, R. Suryamurali, B. Smitha and T. Aminabhavi, Colloids Surf., A, 2007, 297, 267–274 CrossRef CAS.
- K. Berean, J. Z. Ou, M. Nour, K. Latham, C. McSweeney, D. Paull, A. Halim, S. Kentish, C. M. Doherty, A. J. Hill and K. Kalantar-Zadeh, Sep. Purif. Technol., 2014, 122, 96–104 CrossRef CAS.
- S. Sridhar, R. Veerapur, M. Patil, K. Gudasi and T. Aminabhavi, J. Appl. Polym. Sci., 2007, 106, 1585–1594 CrossRef CAS.
- M. Askari, M. L. Chua and T. S. Chung, Ind. Eng. Chem. Res., 2014, 53, 2449–2460 CrossRef CAS.
- J. Schultz and K. Peinemann, J. Membr. Sci., 1996, 110, 37–45 CrossRef CAS.
- S. Stern, J. Mullhaupt and P. Gareis, AIChE J., 1969, 15, 64–73 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: MOF synthesis, membrane characterization and gas separation performance. See DOI: 10.1039/c8nj04769c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |