Ultra-smooth and space-filling mineral films generated via particle accretion processes†
Joe
Harris‡§
a,
Ingo P.
Mey§
b,
Corinna F.
Böhm
a,
Thi Thanh Huyen
Trinh
a,
Simon
Leupold
a,
Carsten
Prinz
c,
Philipp
Tripal
d,
Ralf
Palmisano
de and
Stephan E.
Wolf
 *af
*af
aInstitute for Glass and Ceramics (WW3), Department of Materials Science and Engineering (WW), Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Martensstrasse 5, 91058 Erlangen, Germany. E-mail: stephan.e.wolf@fau.de
bInstitute for Organic and Biomolecular Chemistry, Tammannstrasse 2, 37077 Göttingen, Germany
cFederal Institute for Materials Research and Testing (BAM), Richard-Willstätter-Straße 11, 12489 Berlin, Germany
dOptical Imaging Centre, University of Erlangen-Nuremberg, Erlangen, Germany
eInterdisciplinary Center for Nanostructured Films (IZNF) Friedrich-Alexander University Erlangen-Nürnberg (FAU), 91058 Erlangen, Germany
fInterdisciplinary Center for Functional Particle Systems (FPS), Friedrich-Alexander University Erlangen-Nürnberg (FAU), Haberstrasse 9a, 91058 Erlangen, Germany
First published on 17th June 2019
Abstract
Nonclassical crystallization typically yields materials with pronounced roughness and porosity as it is driven by nanoparticle self-organization. Here, we demonstrate that bio-inspired nonclassical mineralization via magnesium-doped polymer-induced liquid precursors (PILP) can yield ultra-smooth and space-filling CaCO3 films featuring an unprecedented low roughness of 0.285 nm.
New conceptsNonclassical crystallization, fueled by nanoparticle accretion rather than by an ion-wise growth, paradigmatically changes our current concepts of solid-state material synthesis. A key trait of the so-synthesized mesocrystalline materials are their high porosity and specific surface area. At first-sight, these are appealing hallmarks of mesocrystals but represent also distinct limitations as no dense material state is accessible by nonclassical routes; they are governed by the supremum of packing densities. Although generated via nonclassical routes, a range of biominerals are nevertheless space-filling, a trait crucial for functionality. Here, we demonstrate for the first time that this constraint of nonclassical crystallization can be overcome by bioinspired, nonclassical mineralization routes relying on the self-assembly of liquid-condensed mineral precursors whose fluidity and aging/solidification time is well-adjusted to the boundary conditions of the experimental setup. With this approach we are able to generate ultra-smooth and dense calcium carbonate thin films. Although fabricated under chemically trivial conditions, they feature an root-mean-square roughness of 0.285 nm and are on par with standard ultraflat substrates. Our contribution sheds new light on the role of magnesium in biomineralization of calcareus species and provide the first concept how to lift a key limitation of nonclassical mineralization pathways. |
The concept of nonclassical crystallization encompasses mineralization pathways in which the growth of the inorganic solid-state material is governed by aggregation of colloids, e.g., solid nanoparticles. These pathways are in contrast with classical processes which are driven by the attachment of smaller building units, such as ions or molecules.1,2 Over the last few decades, a wide range of reactive agents have been shown to participate in nonclassical pathways. Aggregation of nanocrystals drives mineralization in so-called oriented attachment processes while aggregation of solid-amorphous and even liquid-like colloidal intermediates fuel alternative nonclassical mineralization pathways such as the polymer-induced liquid-precursor (PILP) process.1,3–7 As nonclassical mineralization is characterized by self-organization of nanoparticles, it is of no surprise that inorganic solid-state materials synthesized by nonclassical routes are highly porous8–10 and express granular and rough surfaces reflecting their fundamental building blocks.8,11–14 The marked porosity of mesocrystals10,15,16 which have a specific surface area of more than 250 m2 g−1 essentially arises from a packing problem:17 solid particles can only reach a packing equal to unity in the rare case of truly monodisperse and geometrically well-defined building units which are able to tile space, i.e., plesiohedra, which are space-filling bodies such as cubes.18
To date, there is no synthetic example of a mineral body formed via a particle-accretion mechanism that is fully dense and space-filling. In contrast, the biosynthesis of inorganic solid-state materials, i.e. biominerals, typically yields non-porous and space-filling bodies.19 The space-filling character of biominerals is critical as it facilitates their functionalities for instance as load-bearing or light-guiding materials. The absence of porosity is in contrast with the current and broadly accepted notion that nonclassical processes are dominant in biomineral formation.1,11,20–23 The involvement of particle accretion processes in biomineral formation has been explicitly demonstrated in the bivalve P. nobilis, in which nacre develops from the nanoscale assembly of individual mineral nanoparticles yielding a space-filling solid-state material.21 Moreover, nanogranularity, an apparent hallmark of a nonclassical process, is ubiquitous in biominerals.23,24
In this contribution we reconcile these conflicting views. We demonstrate that a nonclassical crystallization pathway, which involves a liquid-like colloidal intermediate, is indeed able to generate dense mineralization products. By using the air/water-interface as a exceptionally flat substrate, we were able to generate mineral films which are space-filling and feature unprecedented low roughness of 0.285 nm.
To address this issue, we employed an established biomimetic mineralization protocol, the so-called polymer-induced liquid-precursor (PILP) process.7 It is a simplistic but efficient mimesis of calcareous biomineral formation, in which short-chained polyelectrolytes such as polyaspartate or polyacrylate imitate the action of acidic biomineralization proteins.3,25 In our biomimetic setup, the polymeric additives prevent nucleation of a crystalline phase and, upon sufficient supersaturation of the mother solution, trigger the phase separation of a transient, highly hydrated calcium carbonate precursor phase.3,4,26–28 In its very early stages, the precursor is a liquid and able to flow which allows, for example, generation of nanowires by capillary infiltration.29,30 The mineral precursor gradually dehydrates and thus solidifies with time:26 mineral bodies generated by the PILP process are initially highly hydrated and amorphous; after completed dehydration they transform with preservation of their morphology by a so-called pseudomorphic transformation into a crystalline material, similar to the archetypal calcification in biomineralizing organisms.22,26–31 The nonclassical mineralization route is reflected in the nanoscopic organization of the synthesized mineral bodies as they feature a nanogranular organization barely distinguishable from their biogenic counterparts.3,22,31,32 Under kinetically suitable conditions, the colloidal droplets can serve as building blocks of the growing mineral body by attaching on exposed substrates.
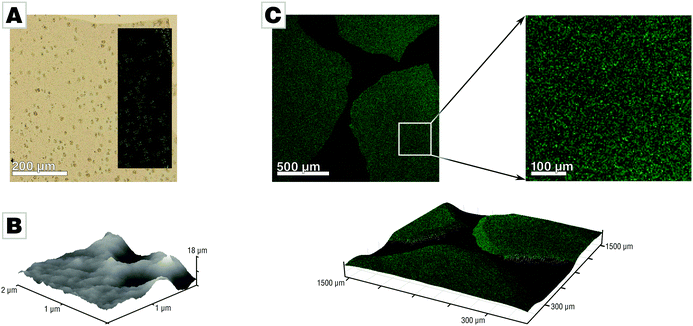
We conducted mineralization experiments using the established slow-diffusion technique,33,34 which involves incubation of a calcium chloride solution (10 mM) in the vapor of decomposing ammonium carbonate. We employed the air/water-interface as a simple but ultra-smooth mineralization substrate which conveniently allows for generation of thin and free-standing mineral films.31 The film forms at the air/water-interface where the supersaturation of calcium carbonate, is the highest. This approach simplifies sample characterization and reduced sample preparation to a minimum as neither sectioning nor polishing was required. Mineralization via the PILP process under standard conditions (10 mM CaCl2, 200 μg mL−1 PAA Mw 5100, 21 L desiccator;33 for further details see ESI†) yields mineral films at the air/water interface, in agreement with earlier reports.3,31,32 The films precipitated after 42 h in presence of PAA and absence of Mg were predominantly amorphous calcium carbonate (ACC) containing spherulitic calcite patches (Fig. 1A). The presence of PAA is crucial for film formation, as evidenced in control experiments (see Fig. S1 provided in the ESI†).31 The films generated under standard conditions showed distinct roughness and nanogranularity in atomic force microscopy (AFM) analyses, providing evidence for their nonclassical origin (Fig. 1B). Thermogravimetric analysis of the spherulites showed that they essentially contain only a few percent of water (Fig. S2, ESI†).31 The spatial distribution of PAA within the amorphous calcium carbonate films was assessed by confocal fluorescence microscopy measurements using a fluorescein-tagged polymer which showed that the polymer was evenly distributed throughout the films (Fig. 1C), within the limits of the chosen approach (for more details, see Fig. S6, ESI†).
The key to tuning the morphology and roughness of the film lies in controlling the lifetime of the liquid state of the mineral precursor phase. Thus, to ensure that the precursor colloids are still sufficiently liquid and are still able to flow upon attachment, one has to slow down the precursor's dehydration rate. Under standard conditions, the solidification of the building blocks, i.e., the droplets of the liquid-condensed phase, is already considerably advanced when they adhere to the substrate. As the particles are (near-to) solid they are incapable of extensively flowing which preserves their particulate morphology upon attachment. A straightforward approach for decreasing the dehydration rate of the liquid-condensed phase is to increase the hydration of the involved ions. Calcium has a varying number of water molecules in its first hydration shell ranging from six to ten,35 where a coordination number of eight seems to dominate.36 The mean lifetime of a water molecule in the first coordination shell of calcium is about 10−9 s, comparable to Sr2+ or Ba2+. Magnesium, prevalent in calcareous biomineral formation, features residence times two orders of magnitude longer than those shown by its earth alkaline homologues.37 This behavior is due to the different enthalpies of hydration, as Mg2+ releases approximately 350 kJ mol−1 more energy than Ca2+ upon hydration.38 The resulting increased barrier to dehydration of magnesium relative to calcium is also reflected in magnesium's capability to considerably increase the stability of hydrated ACC.39–41 In fact, Cheng and Gower were the first to propose that the large dehydration barrier of magnesium may provide a possibility to enhance the liquid-like character of the PILP phase.28
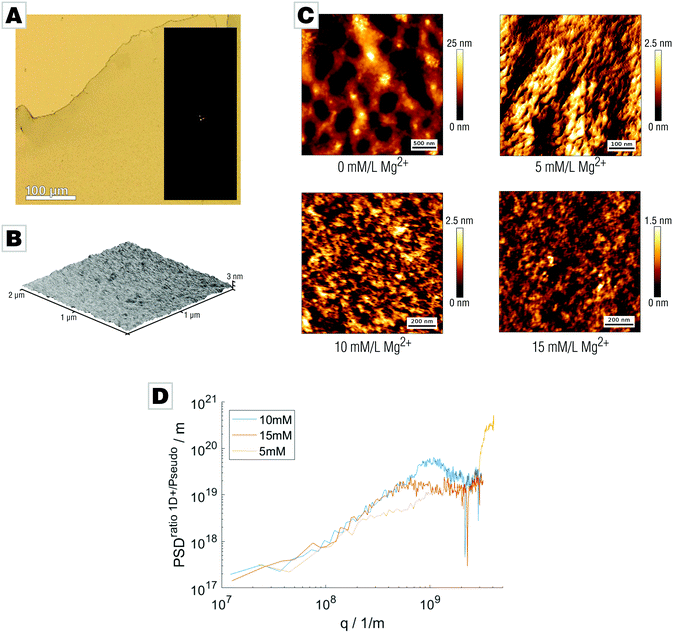
We tested this hypothesis by probing the effect of magnesium on the surface roughness of the mineral films by addition of 5 to 15 mM Mg2+ to the mineralizing solution. After 42 hours, a transparent and fully X-ray amorphous film covering the air/water-interface formed (Fig. 2A and Fig. S3, ESI†). The water content of the final films depends on their magnesium content: films with high Mg content bear more than 15 wt% water even after air-drying (Fig. S4, ESI†). The increased water content of the Mg-bearing films also efficiently suppressed pseudomorphic crystallization. Release of bound water by heat treatment (400 °C, 2 h) allowed formation of Mg-calcite. Atomic force micrographs demonstrated that the smoothness of the films was successfully increased by Mg addition; only marginal irregularities or grain-like features were found (Fig. 2B). We further employed more advanced AFM techniques using intermittent contact mode to characterize the surface morphology of the mineral films at higher resolution (Fig. 2C). To analyze the roughness, images ranging from 5 × 5 μm2 to 0.5 × 0.5 μm2 were acquired showing magnesium-free films to exhibit a demonstrably rough surface structure with deep grooves and notches. Magnesium doping results in pronounced changes to the surface topology of the films; addition of only 5 mM Mg partially smooths the surface albeit with discernable roughness still present. Increasing Mg concentration further reduces the films’ unevenness. In the case of 10 mM Mg and 15 mM Mg, the surface topology is remarkably smooth. We determined the root mean square roughness sq for all micrographs applying different methods. First, we calculated sq along the fast scanning axis which is insensitive to thermal drift. We received the one-dimensional roughness of each scanline demonstrating that film smoothness increases with Mg content: sq (15 mM) = (274 ± 41) pm, sq (10 mM) = (601 ± 71) pm, and sq (5 mM) = (636 ± 121) pm. Second, we calculated the two-dimensional roughness for entire AFM micrographs showing an identical trend in smoothness: sq2D (15 mM) = (276 ± 62) pm, sq2D (10 mM) = (608 ± 82) pm, and sq2D (5 mM) = (636 ± 180) pm. The good agreement of one- and two-dimensional roughness parameters suggests isotropy. To verify this, we calculated power spectral densities (PSD) from radial averaging of the two-dimensional PSD and an averaged PSD from every scan line along the fast scanning axis, as the ratio of both PSDs is a direct measure of isotropy.42 We were therefore able to conclude that the sample is isotropic within the observable range. The small deviation at low q-values might indicate an anisotropy at larger length scales. Films prepared with 5 mM Mg addition show a deviation at lower q values suggesting anisotropy which might be correlated to the segmentation observed within the AFM images. For 15 mM and 10 mM films, the ratio of power spectral densities shows linear behavior until a critical q value; this cutoff actually originates from the finite cantilever tip size and not from the sample (Fig. 2C).
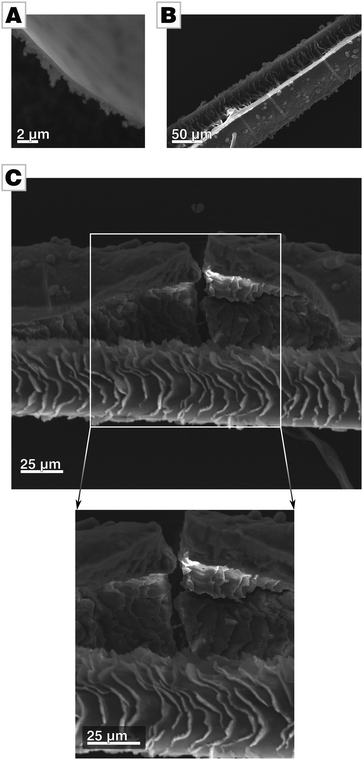
A micrograph of a film's cross-section shows that the films are apparently free of internal pores (Fig. 3A and Fig. S3C, ESI†). To further probe for porosity, we conducted BET analysis of the ultra-smooth Mg-containing films, showing a BET surface area of approx. 35 m2 g−1.¶ Compared to biogenic calcite prisms extracted from P. nobilis, which we use as a biological standard in addition to geological calcite as a geological standard, the effective surface area of our films is still one order of magnitude higher than the space-filling standards, i.e., calcite and biogenic calcite prisms (approx. 1 m2 g−1).19 We attribute the larger surface area to the pronounced aspect ratio of the thin films. Still, the films’ values are well below those reported for mesocrystalline materials which are typically in the range of hundreds of square meters per gram.10,15,16 We further determined the density of the films by helium pycnometry. As amorphous calcium carbonate is known to show a varying degree of hydration, the films were ripened to calcite before measurements. The biomimetic calcite films showed a density of 2.6621 g cm−3 which is in accordance with the expected theoretical value of 2.6788 g cm−3, when taking into account the 2% of polymer incorporated into these films.31 Taken together, these findings evidence that the films are essentially pore-free and thus space-filling.
The micrograph of a film's cross-section further reveals that only the air-facing side is ultra-smooth (Fig. 3A). The side facing the mother solution is rough, resembling the morphology of standard films and provide evidence for a growth mode of the film by particle attachment. We assume that the roughness of the solution-facing side is due to two independent issues. Firstly, shadowing causes so-called columnar growth during film formation by ballistic aggregation, as typically observed in physical vapor deposition processes.43 The columnar morphology in the cross-sectional micrograph in Fig. 3A evidences that similar processes are active in film formation processes in solutions. Secondly, the employed slow-diffusion setup is a one-pot synthesis with reaction parameters (e.g., pH, ion concentration, polymer concentration, ionic strength) which can be only controlled at the very beginning of the reaction but which change in the later stages of the reaction (Fig. S5, ESI†).34,44 For instance, the constituents of the forming film are consumed during film formation without being replenished. Thus, not only does supersaturation decline but also polymer and Mg concentration decrease as they are incorporated into the film. Thus, we assume that precursor colloids formed at later time points have a lower degree of hydration and are therefore already more solidified and less able to spread during attachment. This is reflected in the gradual change of morphology within the film, from an initially dense and monolithic film to a relatively porous morphology in which the individual building blocks of the films are discernible.
The fluid state of the mineral precursor not only allows for the generation of ultra-smooth topologies by utilizing the smoothness of the air/water-interface. The liquid state of the precursor also permits the coating of highly corrugated substrates with high fidelity; the mineral surface can even adapt to substrates with a complicated and rippled shape. In order to demonstrate this, we used hairs as a substrate mimicking a biological mineralization substrate with rough and craggy surface features. The complex and corrugated hair morphology is remarkably well re-produced by the mineral body, hence, a close match/bond between the substrate and the forming mineral was established (see Fig. 3B and C).
Conclusions
In this contribution we evidenced that well-controlled nonclassical mineralization can yield dense, space-filling and ultra-smooth mineral films when relying on hydrated intermediates rather than solid nanoparticles.With our mild nonclassical mineralization approach, in which no harsh conditions or hazardous chemicals are employed, we can generate Mg-doped calcium carbonate thin films featuring a root mean square roughness of 274 ± 41 pm, a value which is unprecedented for minerals generated via nonclassical pathways and on par with state-of-the-art template-stripping methods.45 Other standard “ultra-flat” surfaces such as Si wafers show RMS roughness values in the range of one nanometer down to 0.110 nm, depending on the applied post-processing such as chemical polishing. An overview of established methods and their RMS values is provided in Table S1 in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 Such ultra-smooth surfaces may serve as substrates for imaging and probing the interaction of small biological molecules with mineral surfaces.
46 Such ultra-smooth surfaces may serve as substrates for imaging and probing the interaction of small biological molecules with mineral surfaces.
Moreover, we demonstrated that the morphosynthesis concept is not restricted to flat substrates: optimizing the plasticity of the mineral precursor phase allows for close matching of wrinkled and complex substrates—key issues in joining disparate materials, e.g., when applying mineral coatings for biomedical applications.
This contribution experimentally substantiates the claim that hydrated amorphous colloids which are still able to flow19,22 can serve as space-filling building blocks en route towards monolithic biominerals and synthetic solid-state materials. Our results suggest that one potential function of magnesium in biomineralization processes is to contribute to a long-lived highly hydrated state of the transient amorphous mineral precursors; according to the results presented herein a precondition for generating a space-filling and dense mineral body by particle accretion. Besides magnesium, a range of other agents might also be capable of contributing to the long-lasting high hydration state of the precursor, such as biopolymers or longer-chained poly-electrolytes which act as sufficiently strong water sorbents.47
In the last decade, nonclassical crystallization has emerged as a mild synthesis approach for the formation of inorganic–organic hybrid functional materials with complex structures.2,23 Our contribution introduces for the first time an approach to overcome the inherent supremum of packing densities, a key limitation of current nonclassical crystallization routes. It further demonstrates limitations of currently widely used mineralization approaches and that, in future, a much more elaborate control of mineralization conditions has to be established, e.g., by flow chemistry approaches, in order to tap the full potential of nonclassical crystallization approaches.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
SEW acknowledges financial support by an Emmy Noether starting grant issued by the German Research Foundation (DFG, grant no. WO1712/3-1). Support in lab work by Benedikt Demmert, Alexander Fink, and Frank Bayer is acknowledged.Notes and references
- J. J. De Yoreo, P. U. P. A. Gilbert, N. A. J. M. Sommerdijk, R. L. Penn, S. Whitelam, D. Joester, H. Zhang, J. D. Rimer, A. Navrotsky, J. F. Banfield, A. F. Wallace, F. M. Michel, F. C. Meldrum, H. Cölfen and P. M. Dove, Science, 2015, 349, aaa6760 CrossRef PubMed.
- D. Gebauer and S. E. Wolf, J. Am. Chem. Soc., 2019, 141, 4490–4504 CrossRef CAS PubMed.
- L. B. Gower, Chem. Rev., 2008, 108, 4551–4627 CrossRef CAS PubMed.
- S. E. Wolf and L. B. Gower, in New Perspectives on Mineral Nucleation and Growth, ed. M. Kellermeier, A. van Driessche, L. Benning and D. Gebauer, Springer International, Cham, Switzerland, 2017, pp. 43–75 Search PubMed.
- M. Niederberger and H. Cölfen, Phys. Chem. Chem. Phys., 2006, 8, 3271 RSC.
- H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef PubMed.
- L. B. Gower and D. Odom, J. Cryst. Growth, 2000, 210, 719–734 CrossRef CAS.
- R.-Q. Song and H. Cölfen, Adv. Mater., 2010, 22, 1301–1330 CrossRef CAS PubMed.
- R.-Q. Song, H. Cölfen, A.-W. Xu, J. Hartmann and M. Antonietti, ACS Nano, 2009, 3, 1966–1978 CrossRef CAS PubMed.
- D. Wang, T. Xie, Q. Peng and Y. Li, J. Am. Chem. Soc., 2008, 130, 4016–4022 CrossRef CAS PubMed.
- C. Rodriguez-Navarro, E. Ruiz-Agudo, J. Harris and S. E. Wolf, J. Struct. Biol., 2016, 196, 260–287 CrossRef CAS PubMed.
- Y.-Y. Kim, A. S. Schenk, J. Ihli, A. N. Kulak, N. B. J. Hetherington, C. C. Tang, W. W. Schmahl, E. Griesshaber, G. Hyett and F. C. Meldrum, Nat. Commun., 2014, 5, 1–14 Search PubMed.
- A. S. Schenk, I. Zlotnikov, B. Pokroy, N. Gierlinger, A. Masic, P. Zaslansky, A. N. Fitch, O. Paris, T. H. Metzger, H. Cölfen, P. Fratzl and B. Aichmayer, Adv. Funct. Mater., 2012, 22, 4668–4676 CrossRef CAS.
- T. Wang, M. Antonietti and H. Cölfen, Chem. – Eur. J., 2006, 12, 5722–5730 CrossRef CAS PubMed.
- T. Wang, H. Cölfen and M. Antonietti, J. Am. Chem. Soc., 2005, 127, 3246–3247 CrossRef CAS PubMed.
- T. Wang, H. Cölfen and M. Antonietti, J. Am. Chem. Soc., 2005, 127, 3246–3247 CrossRef CAS PubMed.
- G. Töth and W. Kuperberg, Handbook of Convex Geometry, Elsevier, 1993, pp. 799–860 Search PubMed.
- B. Grünbaum and G. C. Shephard, Bull. Am. Math. Soc., 1980, 3, 951–973 CrossRef.
- L. Yang, C. E. Killian, M. Kunz, N. Tamura and P. U. P. A. Gilbert, Nanoscale, 2011, 3, 603–609 RSC.
- A. Gal, K. Kahil, N. Vidavsky, R. T. DeVol, P. U. P. A. Gilbert, P. Fratzl, S. Weiner and L. Addadi, Adv. Funct. Mater., 2014, 24, 5420–5426 CrossRef CAS.
- R. Hovden, S. E. Wolf, M. E. Holtz, F. Marin, D. A. Muller and L. A. Estroff, Nat. Commun., 2015, 6, 10097 CrossRef CAS PubMed.
- S. E. Wolf, I. Lieberwirth, F. Natalio, J.-F. Bardeau, N. Delorme, F. Emmerling, R. Barrea, M. Kappl and F. Marin, Faraday Discuss., 2012, 159, 433 RSC.
- S. E. Wolf, C. F. Böhm, J. Harris, B. Demmert, D. E. Jacob, M. Mondeshki, E. E. Ruiz-Agudo, C. Rodriguez-Navarro, C. Rodríguez-Navarro, E. E. Ruiz-Agudo, J. Harris and S. E. Wolf, J. Struct. Biol., 2016, 196, 260–287 CrossRef PubMed.
- J. Harris, C. F. Böhm and S. E. Wolf, Interface Focus, 2017, 7, 20160120 CrossRef PubMed.
- F. Marin and G. Luquet, Unusually Acidic Proteins in Biomineralization, in Handbook of Biomineralization: Biological Aspects and Structure Formation, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2007, vol. 1 Search PubMed.
- S. E. Wolf, J. Leiterer, M. Kappl, F. Emmerling and W. Tremel, J. Am. Chem. Soc., 2008, 130, 12342–12347 CrossRef CAS PubMed.
- S. E. Wolf, J. Leiterer, V. Pipich, R. Barrea, F. Emmerling and W. Tremel, J. Am. Chem. Soc., 2011, 133, 12642–12649 CrossRef CAS PubMed.
- X. Cheng, P. L. Varona, M. J. Olszta and L. B. Gower, J. Cryst. Growth, 2007, 307, 395–404 CrossRef CAS.
- A. S. Schenk, E. J. Albarracin, Y.-Y. Kim, J. Ihli and F. C. Meldrum, Chem. Commun., 2014, 50, 4729–4732 RSC.
- Y.-Y. Kim, N. B. J. Hetherington, E. H. Noel, R. Kröger, J. M. Charnock, H. K. Christenson and F. C. Meldrum, Angew. Chem., Int. Ed., 2011, 50, 12572–12577 CrossRef CAS PubMed.
- J. Harris, I. Mey, M. Hajir, M. Mondeshki and S. E. Wolf, CrystEngComm, 2015, 17, 6831–6837 RSC.
- Y.-Y. Y. Kim, E. P. Douglas, L. B. Gower, M. Science, V. Uni, R. Hall, P. O. Box and V. Gaines, Langmuir, 2007, 23, 4862–4870 CrossRef CAS PubMed.
- J. Harris and S. E. Wolf, Minerals, 2017, 7, 122 CrossRef.
- J. Ihli, P. Bots, A. Kulak, L. G. Benning and F. C. Meldrum, Adv. Funct. Mater., 2013, 23, 1965–1973 CrossRef CAS.
- N. A. Hewish, G. W. Neilson and J. E. Enderby, Nature, 1982, 297, 138–139 CrossRef CAS.
- F. Jalilehvand, D. Spångberg, P. Lindqvist-Reis, K. Hermansson, I. Persson and M. Sandström, J. Am. Chem. Soc., 2001, 123, 431–441 CrossRef CAS.
- L. Helm and A. E. Merbach, Chem. Rev., 2005, 105, 1923–1959 CrossRef CAS PubMed.
- M. Peschke, A. T. Blades and P. Kebarle, J. Phys. Chem. A, 1998, 102, 9978–9985 CrossRef CAS.
- J. K. Berg, T. Jordan, Y. Binder, H. G. Börner, D. Gebauer, H. G. Börner, D. Gebauer, H. G. Börner and D. Gebauer, J. Am. Chem. Soc., 2013, 135, 12512–12515 CrossRef CAS PubMed.
- Y. Politi, D. R. Batchelor, P. Zaslansky, B. F. Chmelka, J. C. Weaver, I. Sagi, S. Weiner and L. Addadi, Chem. Mater., 2010, 22, 161–166 CrossRef CAS.
- S. E. Rodriguez-Cruz, R. A. Jockusch and E. R. Williams, J. Am. Chem. Soc., 1999, 121, 8898–8906 CrossRef CAS PubMed.
- T. D. B. Jacobs, T. Junge and L. Pastewka, Surf. Topogr.: Metrol. Prop., 2017, 5, 013001 CrossRef.
- S. Müller-Pfeiffer, H.-J. Anklam and W. Haubenreisser, Phys. Status Solidi, 1990, 160, 491–504 CrossRef.
- V. Pipich, M. Balz, S. E. Wolf, D. Schwahn, W. Tremel and D. Schwahn, J. Am. Chem. Soc., 2008, 130, 6879–6892 CrossRef CAS PubMed.
- N. Vogel, J. Zieleniecki and I. Köper, Nanoscale, 2012, 4, 3820 RSC.
- C. Teichert, J. F. MacKay, D. E. Savage, M. G. Lagally, M. Brohl and P. Wagner, Appl. Phys. Lett., 1995, 66, 2346–2348 CrossRef CAS.
- H. M. L. Thijs, C. R. Becer, C. Guerrero-Sanchez, D. Fournier, R. Hoogenboom and U. S. Schubert, J. Mater. Chem., 2007, 17, 4864–4871 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00175a |
| ‡ Present address: Johnson Matthey, 250 Science Park, Milton Rd, Cambridge, CB4 0WE, UK. |
| § These authors contributed equally. |
| ¶ It should be noted that BET analysis only gives a measure of the accessible surface area, a value characteristic for a given material. Inaccessible surfaces, e.g., internal and non-connected pores, are not reflected in these values. |
| This journal is © The Royal Society of Chemistry 2019 |