Taro leaf-inspired and superwettable nanonet-covered nanofibrous membranes for high-efficiency oil purification†
Jichao
Zhang
a,
Jianlong
Ge
a,
Yang
Si
ab,
Feng
Zhang
a,
Jianyong
Yu
ab,
Lifang
Liu
a and
Bin
Ding
 *ab
*ab
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Textiles, Donghua University, Shanghai 201620, China. E-mail: binding@dhu.edu.cn
bInnovation Center for Textile Science and Technology, Donghua University, Shanghai 200051, China
First published on 7th May 2019
Abstract
Membranes with high permeance and low energy consumption are highly desirable for efficient oil/water emulsion separation. However, creating such materials still remains a great challenge. Here, we report a novel electrohydrodynamic assembly strategy to create a thin nanonet-covered skin with a taro leaf-like micro/nano hierarchical surface and submicron pores on a fibrous membrane. The resultant biomimetic composite membrane exhibits integrated properties of robust under-oil superwettability, low water adhesion, and high water intrusion pressure. Consequently, the membrane can effectively separate various surfactant-free and surfactant-stabilized emulsions under ultralow pressure (<10 kPa), with high permeation flux (maximum of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994 L m−2 h−1), excellent separation efficiency (water contents in filtrates below 50 ppm), and outstanding antifouling properties.
994 L m−2 h−1), excellent separation efficiency (water contents in filtrates below 50 ppm), and outstanding antifouling properties.
New conceptsWe develop a novel assembly strategy, that is, finely tuning the electrohydrodynamic ejection configuration into droplet/jet composite mode, to fabricate a taro leaf-like skin on a nanofibrous membrane. Such a biomimetic skin combines the advantages of ejection products derived from three conventional electrohydrodynamic technologies (i.e. electrospinning, electrospraying, and electrospinning/netting), possessing a good channel connectivity, multiscale roughness, and fully-covered nanonet. Moreover, this skin layer is a 5 μm thick, free-standing, defect-free and transparent entity, which is significantly different from previously reported electrosprayed microsphere layers that are extremely thick (>50 μm) and lack structural integration. These promising structural characteristics together with excellent superwettability and low water adhesion properties allow the resultant biomimetic membrane to separate water-in-oil emulsions under ultralow driving pressure (<10 kPa), which can satisfy the demands for dealing with actual emulsions generated in industry processes and daily life. We also construct a novel device with a traditional design to realize continuous and effective oil purification. This work may open up new avenues to develop next-generation energy-efficient membranes for oil spill cleanup and oil recovery. |
Introduction
Frequent oil spills severely threaten the marine ecosystem and the health of coastal inhabitants.1–3 For example, the Deepwater Horizon disaster discharged 636 million liters of crude oil into the marine environment and contaminated ocean of an area of 3200 km2. In our daily life, the manufacturing and catering industry also produce large quantities of oil/water mixtures every day. Environmental conservation and economic development have raised the need for effective technologies for separating oil/water mixtures to recycle the waste oil resources. Owing to the minor droplet size (<20 μm) and easily deformable property of the emulsified droplets, oil/water emulsion separation has been considered as a challenging task and is highly urgent.4–6 Many separation techniques in industry, such as centrifugation and sedimentation, are inefficient to separate oil/water emulsions and are time-consuming.7 Membrane-based filtration technology has been successfully applied to treat oil/water emulsions. Since industrial processing involves large quantities of emulsions, the membrane should have high permeance to control the processing within a reasonable time and consume less energy.8 However, traditional membrane materials such as ultrafiltration (UF) membranes with a low pore interconnectivity require a high driving force (>1 bar) for operation, and they suffer from fast decline of flux due to poor antifouling properties.6,9,10 Therefore, creating a membrane with high permeance under low energy input still remains difficult to achieve.The systematic design of the desired membranes for separating emulsions needs to parameterize two critical physical characteristics: (i) surface wettability, which affects the selectivity and antifouling performance of the membrane, and (ii) pore structure, which dictates the permeation rate of continuous phase flowing through the membrane.9,11,12 Nature has demonstrated how multiscale surface texture and surface chemistry can be used to design surfaces with superwettability, such as taro leaves, lotus leaves, and fish scales.13–16 As for the pore structure, according to the Hagen–Poiseuille law, an ideal oil/water emulsion separation membrane is expected to have a separating layer, which not only possesses small pore size to obtain a remarkable retention of the dispersed phase but should be as thin as possible to maximize the continuous phase permeability.8,17 Based on these two principles, the past years have seen the exploration of various membranes such as short fiber deposited films (a pure carbon nanotube (CNT) film,18 a polymer decorated CNT film,19 and a Au nanorod doped CNT film20), which possess surfaces with selective superwettability and thin separating layers, and have been used for emulsion separation. However, the intrinsic limitations of the close-packed structure, combined with a bonding structure derived from the essential additive agents that are significant for their mechanical stability and selective wetting properties, result in low porosity. Furthermore, the challenges in scale-up production, including high materials cost and complex fabrication procedures, should also be addressed before they are applied extensively.21,23
Currently, electrohydrodynamic ejection technology has attracted much attention in constructing membranes for oil/water emulsion separation because of its capacity to fabricate products with controllable structure, high porosity and ease of scalable synthesis from various materials.24–29 Based on the ejection mode, this promising ejection technology can be categorized into three major types, involving electrospinning, electrospraying, and electrospinning/netting.30 The first one generates traditional electrospun fibrous membranes (EFMs) featuring high porosity and good channel connectivity.31,32 Despite these superior characteristics, the pervasive issue associated with electrospun fibers is their pseudo-nanoscale diameters of >100 nm (usually 0.2–2 μm), which results in the absence of nanoscale roughness and leads to a hard trade-off between small pore size and thin thickness.33,34 Electrospraying utilizes a low-molecular-weight polymer dilute solution to generate polymeric spheres, but it is limited as a surface modification method to increase the number of scales of surface texture, such as the previously reported polymer sphere layer35–38 and polymer–inorganic sphere layer.39 Because these sphere layers lacked structural integration, were distributed discontinuously, and even exhibited large gaps, the resultant materials could not effectively work on submicron-sized emulsions.35 Electrospinning/netting could produce discontinuous pieces of nanowire networks randomly interspersed in pseudo-nanoscale nanofiber membranes. However, the fatal empty space and large gaps between the pieces of networks in different depths of the membranes may lead to emulsified droplet leakage, thus significantly limiting the intercept capacity.40,41 We thus expected that membranes could achieve promising permeance under low energy consumption if they exhibit integrated merits of the above three ejection products in a thin layer, i.e., high porosity along with a good channel connectivity, multiscale roughness, and nanowire network with a small pore size.
Herein, we present a robust strategy for creating a taro leaf-like and thin nanonet-covered nanofibrous skin on the surface of a conventional electrospun membrane. The keys of our design were the synergy of the biomimetic multiscale structure and low surface energy of the utilized fluorinated polymer, which endows the resultant composite membrane with under-oil superhydrophobicity and excellent fouling resistance. Moreover, the porous nanonets comprised polymeric nanowires (average diameter of ∼45 nm) that allow the biomimetic membrane to efficiently remove minute water droplets (water contents in the filtrates below 50 ppm). Additionally, the thin characteristic of the functional skin (thickness of ∼5 μm) ensures fast oil permeation up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994 L m−2 h−1 under ultralow pressure (<10 kPa), which is superior to that found in commercially available membranes with high driving pressure (>100 kPa). Our biomimetic design strongly demonstrates its potential for energy efficiency in separation and purification.
994 L m−2 h−1 under ultralow pressure (<10 kPa), which is superior to that found in commercially available membranes with high driving pressure (>100 kPa). Our biomimetic design strongly demonstrates its potential for energy efficiency in separation and purification.
Results and discussion
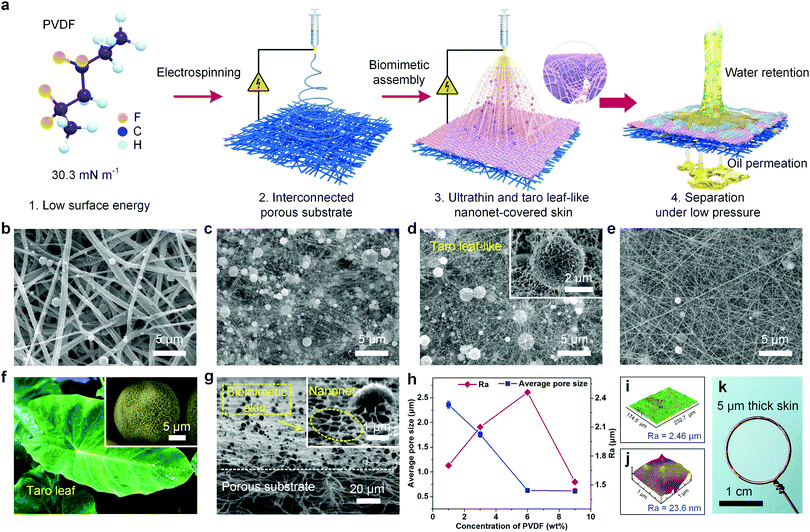
Biomimetic and thin nanonet-covered membrane design
We designed the membrane on the basis of three decisive criteria: (i) the membranes must achieve robust selective superwettability and extremely low water adhesion in a water–oil–solid three-phase system, (ii) the membranes must possess a separating layer with a low thickness and open-cell cellular structure to facilitate oil transport, and (iii) the membrane surface must be endowed with submicron pores to guarantee the enhanced interception of tiny droplets. The first requirement was satisfied by using a taro leaf-like architecture, on which the fluorinated polymer was selected to endow appropriate surface energy. To satisfy the other two requirements, we regulated the electrohydrodynamic ejection configuration into a composite mode (i.e., droplet and jet coexistence) to assemble into a thin nanonet-covered layer.Fig. 1a displays the synthesis pathway. The fabrication began with the construction of pristine polyvinylidene fluoride (PVDF) EFM as a substrate by electrospinning, which exhibited a randomly oriented three-dimensional nonwoven architecture with an average fiber diameter of 421 nm (Fig. S1a, ESI†). The obvious interpenetrating micron porous structure among the fibers was beneficial for medium transportation (Fig. S2, ESI†). Subsequently, dilute precursor PVDF solutions with various concentrations were prepared to fabricate the skin layer on the top of the substrate, and the as-prepared composite membranes exhibited a clear difference in morphology and structure (Fig. 1b–e). The skin layer formed from 1 wt% PVDF only possessed numerous spheres with an average diameter of 710 nm scattered on the surface of the substrate, which was the result of the conventional electrospraying process (Fig. 1b and Fig. S3a, ESI†). The principle for the formation of the microspheres was due to relatively low solution viscosity (Table S1, ESI†), which resulted in the weak entanglements among the macromolecule chains, and thus the solution streams broke up into droplets during their flight and finally solidified into spheres.42 On dramatic increasing the PVDF concentration to 9 wt%, many of the microspheres disappeared and turned into one-dimensional nanofibers (average diameter of 74 nm, the maximum diameter of 125 nm) due to the increased solution viscosity resulting in stable jets (Fig. 1d). Excitingly, by finely modulating the structural evolution between the critical formation thresholds for droplets and jets from PVDF, the microsphere/nanowire composite nets were assembled. As shown in Fig. 1c, nanowires with an average diameter of 33 nm, one order of magnitude less than that of the common electrospun fibers, began to grow out of the surface of the spheres (average diameter of 790 nm), sparsely connected with each other and stacked on the porous substrate. More significantly, increasing the PVDF concentration to 6 wt%, a great number of nanowires (average diameter of 45 nm) with interspersed nanobeads (average diameter of 85 nm) on their axes were randomly deposited on the microsphere surface (inset of Fig. 1d), assembling into taro leaf-like micro-/nanoarchitectures (Fig. 1f and Fig. S4, ESI†), which was critical to acquire superwettability in accordance with Cassie's law.43Fig. 1g revealed that the biomimetic composite membrane displayed an asymmetric cross-section with top-down hierarchical networks of pores from the nano to micron levels, and there was a thin skin layer with a thickness of ∼5 μm on the top of it (Fig. S5, ESI†). The dissymmetric design is effective to enhance the fluid flow due to the significantly reduced channel length. Besides, the titled-view image showed that the skin layer was fully covered with stable and continuous nanonets with a small aperture (inset of Fig. 1g), which endowed more robust size interception capacity than conventional electrospun materials.
The pore structure and surface texture of the resultant composite membranes were further investigated quantitatively. The additive nanowires effectively reduced the average pore size from 2.36 to 0.62 μm with increasing PVDF concentration from 1 to 9 wt%, revealing a significant improvement in the pore structure (Fig. 1h and Fig. S7, ESI†). Moreover, the arithmetic average roughness (Ra) of the membrane surface increased with increasing precursor solution concentration, and the maximum value of 2.46 μm for the taro leaf-like membrane derived from 6 wt% PVDF was reached. The three-dimensional (3D) surface profile of the biomimetic membrane indicated a relatively uniformly distributed microsphere/nanowire composite net (Fig. 1i). The surface of the microspheres on the taro leaf-like skin also showed Ra value of 23.6 nm as detected by atomic force microscopy (AFM) (Fig. 1j), which was attributed to the covered beads-on-string nanowires as observed in Fig. 1d. These results indicated that the taro leaf-like skin surface possessed micro/nanoroughness, which contributed a lot to the superwettability of the prepared membrane.
In addition, considering practical applications, the mechanical properties of the prepared membranes were also investigated. The stress–strain (σ–ε) behavior was illustrated in Fig. S8 (ESI†). In contrast to pristine EFM, the introduction of biomimetic skin caused a dramatical increase in the mechanical properties, which was improved from 4.55 to 7.15 MPa. This indicated a good interfacial compatibility between the taro leaf-structured layer and porous substrate, which could minimize the fluid transport resistance at the interface of the composite membrane.44 Interestingly, the taro leaf-like skin layer could be peeled off and transferred onto a wire lasso because of the more abundant bonding points between the nanowires and microspheres than those between the skin layer and substrate. Although the thickness of the skin layer is only 5 μm, it formed an integral surface across the whole 1.2 cm diameter of the lasso, indicating that the thin skin was a robust, free-standing, defect-free and transparent entity, which was totally different from the other reported works that were extremely thick (>50 μm)45 with the absence of structural integration.35,36,39
Surface selective wettability analysis
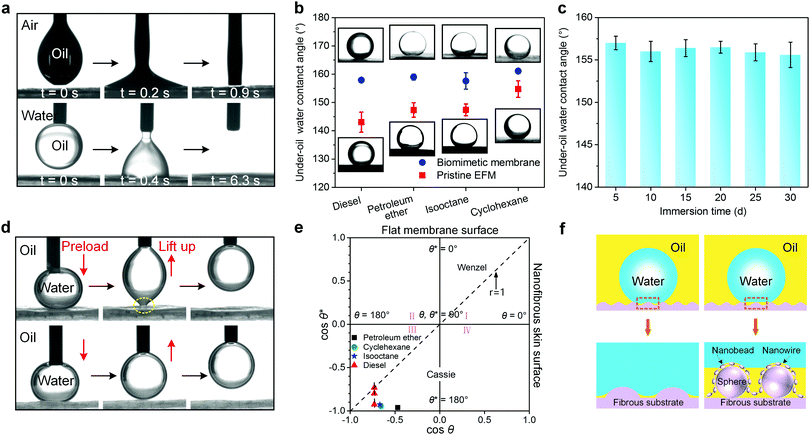
Since selective wettability plays a critical role in oil/water separation, we systematically investigated the wetting property of the biomimetic nanofibrous membrane.46 A high-speed photographic system was utilized to monitor the spreading of a liquid droplet. As demonstrated in Fig. 2a (top), as soon as a 3 μL oil droplet (take diesel as an example) contacted the biomimetic skin layer, it rapidly spread and a nearly zero oil contact angle (OCA) was achieved within less than 1 s due to the penetration of the capillary forces, indicating an excellent oleophilic wettability. It should also be noted that the membrane still exhibited a robust oleophilicity with OCA of 0° even pre-immersed in an aqueous condition (Fig. 2a bottom), which made it possible to replace water with oil even covered by water first. This excellent oil wettability indicated that the biomimetic membrane did not need an oil-prewetting process to form a water repellent layer, which was significantly important for energy efficient separation.47When treating water-in-oil emulsions by using hydrophobic/oleophilic membranes, the actual separation process proceeds in the oil–water–solid system due to the oil wetting membrane, so under-oil water contact angle (UOWCA) represents a more realistic situation than the water contact angle (WCA) in air.48 Based on Cassie theory, it is clear that multiscale roughness is necessary for intensifying the surface lyophobicity in air. This law was also applicable to the under-oil hydrophobicity because the entrapped air inside the surface pores was equivalently substituted by oil. As shown in Fig. 2b, in contrast to pristine EFM, a noteworthy increment of UOWCA of the biomimetic membrane was observed, and all UOWCAs exhibited a high value above 150° for a series of oils, demonstrating that the as-prepared membrane had universal under-oil superhydrophobic properties disregarding the oil kinds. In order to evaluate the environmental stability of the under-oil superhydrophobicity, UOWCA of the biomimetic membrane was measured after being exposed to oil (take diesel as an example) for a prolonged period of time (Fig. 2c). It was apparent that the membrane could retain a good under-oil superhydrophobic property (UOWCA > 150°) even after soaking for 30 days, indicating the stable water-repellence performance of the biomimetic membrane in an oil environment. This promising superwettability could ensure high permeability to oil and avoid direct contact between the water and membrane at the same time, thereby enormously improving the separation performance.9
Simultaneously, the under-oil dynamic water wetting behavior of the membrane was investigated to evaluate the water adhesion property. Fig. 2d (top) displays a water droplet (3 μL) as a detecting probe to be forced to contact and squeeze with the pristine PVDF EFM surface and then lifted from it. A great shape distortion was observed during the leaving process, confirming the high water adhesion for pristine EFM under-oil. By contrast, when the compressed water droplet was lifted up from the surface of the biomimetic membrane, almost no deformation could be observed (Fig. 2d bottom), demonstrating the excellent under-oil anti-water-adhesion property of the membrane. This characteristic was a benefit for the antifouling performance of the biomimetic membrane.22 Meanwhile, a water droplet could easily roll on the biomimetic membrane with a sliding angle of 3° (Fig. S9, ESI†), which is similar to the taro leaf surface.15 To further assess the adhesion quantitatively, a highly sensitive microelectromechanical balance system was employed to acquire the real-time force–distance curves of water on the membranes under-oil. The water adhesion force of pristine PVDF EFM was 59.9 μN, while it sharply decreased to 4.6 μN after being modified by the taro leaf-like layer (Fig. S10, ESI†), which was much less than the previous work.48 Besides, the same experiment was conducted on the PVDF flat membrane, and the real adhesive force could not be precisely measured but must be much larger than the former two membranes because the networks of the water molecules were broken up (Fig. S11, ESI†).13
Researchers have revealed that the wettability and the solid/liquid adhesion characteristics of the porous surface were dominated by the geometric structure and chemical composition.13 In this work, all the prepared membranes presented similar surface chemical constituents, indicating that the surface texture played a critical role in determining the surface wetting behavior. Nowadays, three theoretical models, including Wenzel, Cassie, and Wenzel–Cassie metastability, have been proposed to explain this difference. The wetting state of the membranes under various oils could be evaluated using a wetting diagram (Fig. 2e).49 The coordinate points of the biomimetic membrane were all at the bottom-left corner of region III for all oils, indicating a stable Cassie wetting state of the as-prepared membrane under various oils.49 Besides, the coordinate points of the flat membrane, pristine EFM and biomimetic membrane were finely fitted with the Cassie equation (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ* = f(1 + cos
θ* = f(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) − 1, where f is the area fraction of solid), the fractions of the membrane surface contacting with water under-oil were 1, 0.75 and 0.27, respectively. The surface of the flat membrane exhibited ultrahigh water adhesive force under-oil, which originated from its low surface roughness (Fig. S12a, ESI†), large water–solid contact region and continuous contact line between the water droplet and membrane. Benefiting from the enhanced roughness of the other two membranes, oil could be retained in the porous structure on the surfaces and the three-phase solid–oil–water interface formed, thus resulting in a small water–solid contact region and a discrete contact line. Furthermore, as the increased number of oil molecules entered the surface cavity, the three-phase composite interface possessed higher oil fraction, which induced the surface wetting state to change into the Cassie state. As a result, the flat membrane (Wenzel state in Fig. S12b, ESI†) displayed a low UOWCA less than 137° (Fig. S13, ESI†) and high adhesion. Pristine EFM (the Wenzel–Cassie metastability state in Fig. 2f left) demonstrated a higher UOWCA and medium adhesion, and the biomimetic membrane with the nanonet-covered microspheres layer (the Cassie state in Fig. 2f right) showed a comprehensively superwetting state under all oils and low adhesion.50 These results indicated that the surface possessing a multiscale microstructure has significantly higher apparent CA and lower water adhesion than only a single scale of texture.43,51
θ) − 1, where f is the area fraction of solid), the fractions of the membrane surface contacting with water under-oil were 1, 0.75 and 0.27, respectively. The surface of the flat membrane exhibited ultrahigh water adhesive force under-oil, which originated from its low surface roughness (Fig. S12a, ESI†), large water–solid contact region and continuous contact line between the water droplet and membrane. Benefiting from the enhanced roughness of the other two membranes, oil could be retained in the porous structure on the surfaces and the three-phase solid–oil–water interface formed, thus resulting in a small water–solid contact region and a discrete contact line. Furthermore, as the increased number of oil molecules entered the surface cavity, the three-phase composite interface possessed higher oil fraction, which induced the surface wetting state to change into the Cassie state. As a result, the flat membrane (Wenzel state in Fig. S12b, ESI†) displayed a low UOWCA less than 137° (Fig. S13, ESI†) and high adhesion. Pristine EFM (the Wenzel–Cassie metastability state in Fig. 2f left) demonstrated a higher UOWCA and medium adhesion, and the biomimetic membrane with the nanonet-covered microspheres layer (the Cassie state in Fig. 2f right) showed a comprehensively superwetting state under all oils and low adhesion.50 These results indicated that the surface possessing a multiscale microstructure has significantly higher apparent CA and lower water adhesion than only a single scale of texture.43,51
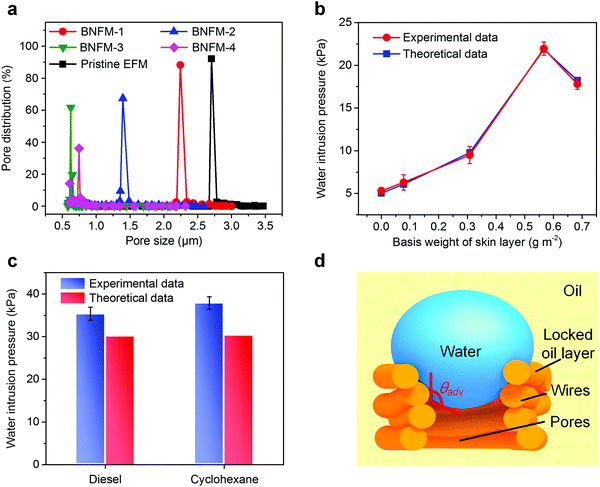
Pore size and water intrusion pressure investigation
To reveal the influence of the nanowire networks on the pore size of the membrane, a series of biomimetic nanofibrous membranes with various skin layer basis weights (0.0778, 0.3084, 0.5667, and 0.6833 g m−2) were prepared, which were named as BNFM-1, BNFM-2, BNFM-3 and BNFM-4. With increasing basis weight of the biomimetic skin, the pore size decreased drastically (Fig. 3a and Fig. S14, ESI†). By progressively enlarging the weight of the skin to 0.5667 g m−2, the average pore size of the membranes significantly decreased from 2.71 to 0.62 μm, and the maximum pore size reduced from 3.50 to 2.17 μm, respectively. However, further increasing the basis weight to 0.6833 g m−2, both the average pore size and maximum pore size slightly increased to 0.75 μm and 2.41 μm, respectively. This could be attributed to the fact that the excessive accumulation of microspheres tended to exhibit more charges, thus creating a stronger repulsive force among the spheres.52A high water intrusion pressure provides a robust separation capacity.53 As shown in Fig. 3b, the biomimetic membrane with a skin weight of 0.5667 g m−2 displayed a high water intrusion pressure of 22.0 kPa, which was ten times higher than that of the previously reported membrane.54 However, as the weight of the skin increased to 0.6833 g m−2, the water intrusion pressure decreased to 17.8 kPa because of the increase in the maximum pore size. To further study the hydrostatic pressure mechanism of the nanofibrous membrane, the theoretical oil intrusion pressure was calculated using the Young–Laplace equation (see details in Supplementary methods, ESI†). As shown in Fig. 3b, the experimental water intrusion pressure in air finely agreed with the theoretical data, demonstrating that the water repellency of the biomimetic membranes greatly depends on the surface wetting property and maximum pore size. However, the experimental water intrusion pressures under-oil were slightly higher than the theoretical data (take diesel and cyclohexane as examples, see details in the Supplementary methods, ESI†) (Fig. 3c). This was attributed to the interaction of the PVDF oleophilic group (–CF2–, –CH2–) and oil molecules leading to the formation of a thin locked oil layer, which strongly adhered on the nanowires and reduced the membrane pore size distribution. Moreover, the time that water droplets took to penetrate through the membrane was extended due to the much higher viscous resistance derived from the oil in the channel than that originated from the air, during which the applied pressure gradually went up (Fig. 3d). Consequently, the experimental water intrusion pressure could be higher than the true pressure at the right time.53
Emulsion separation mechanism analysis
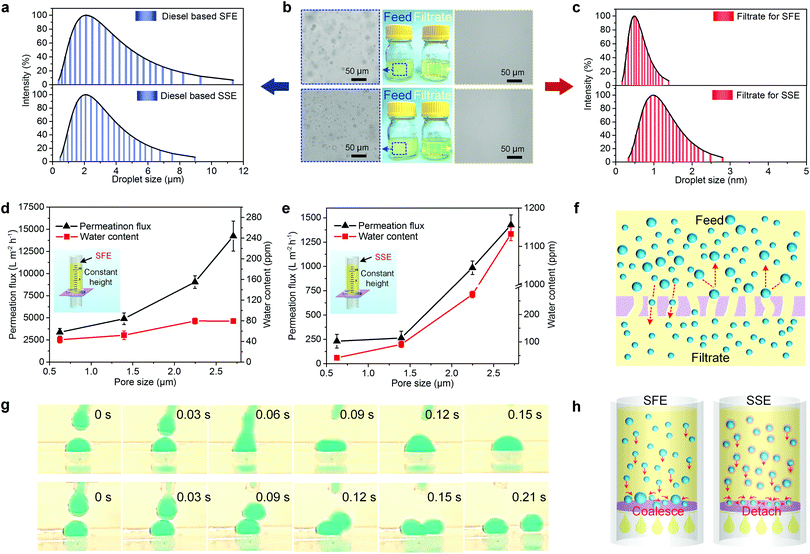
Deeper understandings of the separation mechanism of the biomimetic membrane for water-in-oil emulsions would be useful for the rational design of porous membranes as well as its practical applications. The correlation between the pore size and separation performance should be analyzed in detail. Therefore, two kinds of water-in-oil (take diesel as an example) emulsions, surfactant-free emulsion (SFE) and surfactant-stabilized emulsion (SSE) with similar droplet size, were elaborately prepared as model emulsions. The emulsion droplet sizes were determined by dynamic light scattering (DLS) and presented in Fig. 4a, demonstrating a similar average droplet size and size distribution in the two emulsions. The minor differences in size range could be due to the fact that water is more difficult to disperse in SFE than in SSE. Fig. 5a demonstrates the gravity driven system for oil/water separation. We poured fresh emulsions into the reservoir with the desired height of 12.2 cm to maintain an equivalent gravitational pressure of 1 kPa.The biomimetic membrane (take BNFM-3 as an example) exhibited a high separation performance (Fig. 4b). The feed emulsions were cloudy consisting of numerous water droplets, but the filtrates featured visual transparency and no droplets were invisible in the whole view, indicating that the membrane had successfully removed water beyond any optical discernable droplet in both SFE and SSE. The relevant DLS curves for these two filtrates showed that the signal in the SSE filtrate (0.34 to 2.81 nm) was wider than that in the SFE filtrate (0.17 to 1.46 nm), which may be attributed to the surfactant molecules dissolved in oil (Fig. 4c).
In addition, the pristine EFM and the biomimetic membranes with various pore size were applied for the emulsion separation to investigate the separation performance as a function of the pore size. As shown in Fig. 4d and e, with decreasing the average pore size of the membranes, the permeation flux for SFE and SSE decreased, which is consistent with the Hagen–Poiseuille law. It was noticed that all the fluxes for SSE were much lower than that for SFE, which was due to the permeability that was mainly determined by the demulsification rate, and the SFE was much easier to be broken than the SSE.10 Besides, the membrane contaminated by the surfactant could be another crucial reason to result in a lower oil flux for the SSE. Fig. 4d also demonstrated that the water contents in all the filtrates remained relatively stable for the membranes with different average pore size (from 79.4 to 43.3 ppm). In dramatic contrast to the SFE separation, the water contents in the filtrates of SSE showed a sharp decline (from 1132.3 to 41.8 ppm) as the average pore size of the membrane decreased (Fig. 4e), which demonstrated that the separation efficiency for SSE strongly depended on the pore structure. The difference in the separation efficiency for SFE and SSE could be attributed to two reasons: (i) the difference in the collision–coalescence process of the droplets for SFE and SSE, and (ii) the interception effect of the membrane can be activated for the droplets larger than the pore size on the basis of the oil/water selectivity (Fig. 4f).55
The real-time collision process of the water droplets in an oil environment during the contacting process was recorded in situ by a high-speed camera (Fig. 4g). Here, cyclohexane (Movies S1 and S2, ESI†) and diesel (Movies S3 and S4, ESI†) were used as the model oils to prove our concept (see details in Supplementary methods, ESI†). During the contacting process in pure oil, the top tiny water droplet quickly merged with the bottom small one, coalescing into a large one within only 0.15 s (Fig. 4g top). However, the top droplet was detached away from the bottom one in the oil with surfactants (Fig. 4g bottom). These results demonstrated that the water droplets in SFE could easily coalesce into a large droplet than that in SSE, which is considered to be caused by decreased interfacial tension by the surfactant at oil/water interface.56 The postulated illustrations of the emulsion separation mechanism for SFE and SSE are shown in Fig. 4h. Once contacting the biomimetic membrane surface, the oil instantly spread and actively penetrated through the membrane owing to the low surface energy of PVDF and multiscale roughness, thereby forming a stable oil–solid interface, which dramatically lowered the water wetting hysteresis and water adhesion property. Consequently, the tiny water droplets in SFEs could readily roll on the skin and quickly aggregated with each other to generate larger droplets on the composite surface, resulting in the capture by the skin layer according to the synergistic effect of size exclusion and water repellent pressure (Fig. 4f left). By contrast, the water droplets in SSE were hard to coalesce during the separation and formed into a filter cake comprising tiny droplets on the skin (Fig. 4f right). Eventually, the emulsified droplets much smaller than the membrane pore size would easily leak into the filtrate.
Comprehensive separation performance evaluation
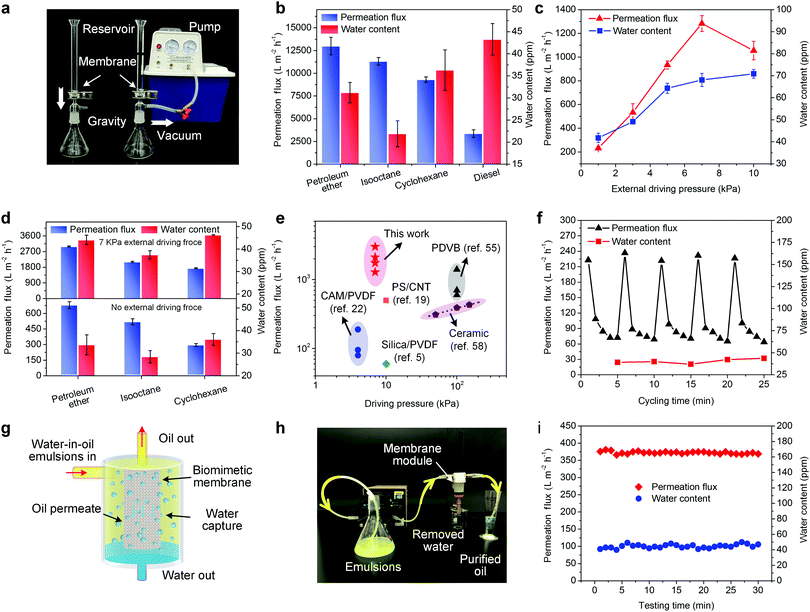
The outstanding under-oil superhydrophobicity, anti-water-adhesion and small pore size of the biomimetic membrane (take BNFM-3 as an example) make it a promising candidate for separating water-in-oil emulsions. Various SFEs were separated under the gravity of ∼1 kPa (Fig. 5a) and the results are shown in Fig. 5b. The water contents were all below 45 ppm, revealing a high separation efficiency of the as-prepared membrane. The permeation fluxes for water-in-petroleum ether, water-in-isooctane, water-in-cyclohexane, and water-in-diesel SFEs were 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994, 11
994, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 322, 9305, and 3370 L m−2 h−1 respectively, which were much superior in comparison to the previously reported membranes.10 The flux difference among these water-in-oil SFEs can be attributed to the fact that flux is inversely proportional to oil viscosity (Table S2, ESI†).
322, 9305, and 3370 L m−2 h−1 respectively, which were much superior in comparison to the previously reported membranes.10 The flux difference among these water-in-oil SFEs can be attributed to the fact that flux is inversely proportional to oil viscosity (Table S2, ESI†).
Compared to SFEs, SSEs were more challenging to be treated, in which surfactants significantly increase the emulsion stability. The biomimetic membrane showed a flux of 232 L m−2 h−1 for water-in-diesel SSE without external driving pressure. Further increasing the external driving pressure from 3 to 7 kPa, the membrane could achieve higher oil flux of 533, 934 and 1282 L m−2 h−1, while the values of the water contents in the filtrates still remained low (49.1, 64.3 and 68.1 ppm, respectively) (Fig. 5c). Interestingly, the flux dropped abruptly under 10 kPa driving pressure, which did not conform to the Hagen–Poiseuille law. This abnormal phenomenon was due to the driving pressure forcing minute water droplets to be deformable and partially inserted into the porous nanonet of the skin layer, leading to plugging of the permeation channel.56,57 We believed that changing the dead-end mode to cross-flow mode would eliminate the boundary layer and further promote the flux.58,59 The membrane was also applied to separate SSEs containing various oils for evaluating the applicability (Fig. 5d). The water contents in all filtrates were below 50 ppm, revealing an extremely superior separation efficiency of the biomimetic membrane. The membrane presented permeation fluxes were 679, 520 and 296 L m−2 h−1 for water-in-petroleum, water-in-isooctane and water-in-cyclohexane SSEs without external driving pressure, indicating lower viscosity for oil leading to a faster permeation (Table S2, ESI†). On further increasing the external driving pressure to 7 kPa, the permeation fluxes went up to 2994, 2107, and 1743 L m−2 h−1, suggesting that the use of an ultralow pressure can greatly benefit the flux promotion. Considering that the oil permeation was performed at ultralow pressure (<10 kPa), our biomimetic membrane was more attractive than a series of state-of-the-art membranes from an energy conservation point of view (Fig. 5e). In addition, the performance stability of the PVDF biomimetic membrane was investigated by testing the separation performance of the membranes soaked in different organic solvents for one week. As shown in Fig. S15 (ESI†), the PVDF biomimetic membranes soaked in the organic solvents exhibited steady permeation fluxes and no sharp decline was observed. Meanwhile, no significant changes in water contents were observed for all the soaked membranes. These results indicated good performance stability of the PVDF biomimetic membrane. Furthermore, compared with the pristine biomimetic membrane, the morphology structure of the soaked membranes could remain almost unchanged and no obvious swelling can be seen (Fig. S16, ESI†), which further suggested high chemical and structural stability of the biomimetic membrane.
The antifouling performance of the biomimetic membrane was demonstrated in Fig. 5f. The oil flux exhibited a drastic drop during the first minute in one cycle and then displayed a moderate decrease during the remaining time. This was due to the rapid formation of a water barrier on the membrane surface which greatly obstructed the oil permeation. Notably, the flux can completely convert to the original value after simply cleaning with alcohol and pure oil with purity over 99.995% was collected during the entire process, suggesting durability of the biomimetic membrane and excellent antifouling property for long-term usage.
From the practical point of view, if the water barrier can be in situ eliminated without interrupting the separation process, a continuous and time-saving treatment would be realized. We put up an oil purification module consisting of a vertically placed hollow structured membrane and a peristaltic pump (Fig. 5g). The keys to the design lay in (i) converting the horizontal mode into the vertical mode to utilize the gravity to make the droplets roll away from the membrane surface, and (ii) the hollow structured architecture to reduce the footprint. As shown in Fig. 5h and Movie S5 (ESI†), the emulsion was pumped into the module by a peristaltic pump, and only oil could pass through the membrane and flow along pipes to the collecting device, while water was rejected and settled on the bottom. The whole separation process does not need any cleaning process. Moreover, Fig. 5i showed that the membrane maintained a stable permeation flux (∼350 L m−2 h−1) and constant low water content (<50 ppm) during the whole testing time, indicating that the vertical hollow-structured membrane could effectively avoid water-pinning and pore clogging. Our novel strategy allowed the separation of water-in-oil emulsions in a quick, easy and continuous way. Besides, a large-scale (50 cm × 60 cm) biomimetic membrane was easily obtained (Fig. S15, ESI†). It is possible to realize massive industrial production by further enlarging the relevant equipment, which is of immense importance for practical applications.
Conclusions
In summary, we have developed a biomimetic assembly strategy for scalable fabrication of a taro leaf-like thin skin, which possessed good channel connectivity, multiscale roughness, and a porous nanonet with a small pore size, on a highly interconnected fibrous membrane. Benefiting from these intriguing structure and under-oil superhydrophobicity, the resultant biomimetic nanofibrous membrane can successfully separate various water-in-oil emulsions with the droplet size ranging from nanometer to micrometer under ultralow driving pressure (<10 kPa), and display promising flux (maximum of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994 L m−2 h−1) and high separation efficiency (water contents in the filtrates below 50 ppm), which may satisfy the requirements for real-world emulsion separation on a large scale. Additionally, the resultant membrane also exhibited superwetting durability, excellent mechanical properties, and impressive recyclability. We envision that this new kind of membrane will be a great candidate in fuel purification, oil recovery, spill oil cleanup and separation of commercial emulsions.
994 L m−2 h−1) and high separation efficiency (water contents in the filtrates below 50 ppm), which may satisfy the requirements for real-world emulsion separation on a large scale. Additionally, the resultant membrane also exhibited superwetting durability, excellent mechanical properties, and impressive recyclability. We envision that this new kind of membrane will be a great candidate in fuel purification, oil recovery, spill oil cleanup and separation of commercial emulsions.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51773033 and 51673037), and the Fundamental Research Funds for the Central Universities (No. 17D310107).Notes and references
- M. Schrope, Nature, 2011, 472, 152–154 CrossRef CAS PubMed.
- C. H. Peterson, Science, 2003, 302, 2082–2086 CrossRef CAS PubMed.
- R. M. Atlas and T. C. Hazen, Environ. Sci. Technol., 2011, 45, 6709–6715 CrossRef CAS PubMed.
- D. L. Valentine, G. B. Fisher, S. C. Bagby, R. K. Nelson, C. M. Reddy, S. P. Sylva and M. A. Woo, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 15906–15911 CrossRef CAS PubMed.
- Y. Liao, M. Tian and R. Wang, J. Membr. Sci., 2017, 543, 123–132 CrossRef CAS.
- H. S. Kang, H. Cho, W. Panatdasirisuk and S. Yang, J. Mater. Chem. A, 2017, 5, 18762–18769 RSC.
- R. Zolfaghari, Sep. Purif. Technol., 2016, 170, 377–407 CrossRef CAS.
- S. Karan, Z. Jiang and A. G. Livingston, Science, 2015, 348, 1347–1351 CrossRef CAS PubMed.
- Y. Si, Q. Fu, X. Wang, J. Zhu, J. Yu, G. Sun and B. Ding, ACS Nano, 2015, 9, 3791–3799 CrossRef CAS PubMed.
- W. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071–2076 CrossRef CAS PubMed.
- Q. L. Ma, H. F. Cheng, A. G. Fane, R. Wang and H. Zhang, Small, 2016, 12, 2186–2202 CrossRef CAS PubMed.
- L. Hou, N. Wang, J. Wu, Z. Cui, L. Jiang and Y. Zhao, Adv. Funct. Mater., 2018, 1801114 CrossRef.
- M. Liu, S. Wang, Z. Wei, Y. Song and L. Jiang, Adv. Mater., 2009, 21, 665–669 CrossRef CAS.
- X. Wang, B. Ding, J. Yu and M. Wang, Nano Today, 2011, 6, 510–530 CrossRef CAS.
- J. Ma, Y. Sun, K. Gleichauf, J. Lou and Q. Li, Langmuir, 2011, 27, 10035–10040 CrossRef CAS PubMed.
- J. Lin, Y. Cai, X. Wang, B. Ding, J. Yu and M. Wang, Nanoscale, 2011, 3, 1258–1262 RSC.
- H. Huang, Z. Song, N. Wei, L. Shi, Y. Mao, Y. Ying, L. Sun, Z. Xu and X. Peng, Nat. Commun., 2013, 4, 2979 CrossRef PubMed.
- Z. Shi, W. Zhang, F. Zhang, X. Liu, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2422–2427 CrossRef CAS PubMed.
- J. Gu, P. Xiao, J. Chen, F. Liu, Y. Huang, G. Li, J. Zhang and T. Chen, J. Mater. Chem. A, 2014, 2, 15268–15272 RSC.
- L. Hu, S. Gao, X. Ding, D. Wang, J. Jiang, J. Jin and L. Jiang, ACS Nano, 2015, 9, 4835–4842 CrossRef CAS PubMed.
- L. Yu, S. C. Ruan, X. T. Xu, R. J. Zou and J. Q. Hu, Nano Today, 2017, 17, 79–95 CrossRef CAS.
- L. Chen, Y. Si, H. Zhu, T. Jiang and Z. Guo, J. Membr. Sci., 2016, 520, 760–768 CrossRef CAS.
- K.-K. Yan, L. Jiao, S. Lin, X. Ji, Y. Lu and L. Zhang, Desalination, 2018, 437, 26–33 CrossRef CAS.
- X. Wang, J. Yu, G. Sun and B. Ding, Mater. Today, 2016, 19, 403–414 CrossRef CAS.
- M. Ge, C. Cao, J. Huang, X. Zhang, Y. Tang, X. Zhou, K. Zhang, Z. Chen and Y. Lai, Nanoscale Horiz., 2018, 3, 235–260 RSC.
- J. Song, X. Wang, J. Yan, J. Yu, G. Sun and B. Ding, Sci. Rep., 2017, 7, 1636 CrossRef PubMed.
- Y. Cai, J. Song, X. Liu, X. Yin, X. Li, J. Yu and B. Ding, Environ. Sci.: Nano, 2018, 5, 2631–2640 RSC.
- X. Tang, Y. Si, J. Ge, B. Ding, L. Liu, G. Zheng, W. Luo and J. Yu, Nanoscale, 2013, 5, 11657–11664 RSC.
- J. Lin, B. Ding and J. Yu, ACS Appl. Mater. Interfaces, 2010, 2, 521–528 CrossRef CAS PubMed.
- Y. Huang, N. Bu, Y. Duan, Y. Pan, H. Liu, Z. Yin and Y. Xiong, Nanoscale, 2013, 5, 12007–12017 RSC.
- C.-L. Zhang and S.-H. Yu, Mater. Horiz., 2016, 3, 266–269 RSC.
- S. Jiang, B. C. Ma, W. Huang, A. Kaltbeitzel, G. Kizisavas, D. Crespy, K. A. I. Zhang and K. Landfester, Nanoscale Horiz., 2018, 3, 439–446 RSC.
- J. Ge, D. Zong, Q. Jin, J. Yu and B. Ding, Adv. Funct. Mater., 2018, 1705051 CrossRef.
- Y. Liao, C.-H. Loh, M. Tian, R. Wang and A. G. Fane, Prog. Polym. Sci., 2018, 77, 69–94 CrossRef CAS.
- J. Wu, Y. Ding, J. Wang, T. Li, H. Lin, J. Wang and F. Liu, J. Mater. Chem. A, 2018, 6, 7014–7020 RSC.
- L. Jiang, Y. Zhao and J. Zhai, Angew. Chem., Int. Ed., 2004, 43, 4338–4341 CrossRef CAS PubMed.
- R. Zheng, Y. Chen, J. Wang, J. Song, X.-M. Li and T. He, J. Membr. Sci., 2018, 555, 197–205 CrossRef CAS.
- Z. Zhu, Z. Liu, L. Zhong, C. Song, W. Shi, F. Cui and W. Wang, J. Membr. Sci., 2018, 563, 602–609 CrossRef CAS.
- J. L. Ge, J. C. Zhang, F. Wang, Z. L. Li, J. Y. Yu and B. Ding, J. Mater. Chem. A, 2017, 5, 497–502 RSC.
- X. Wang, B. Ding, G. Sun, M. Wang and J. Yu, Prog. Mater. Sci., 2013, 58, 1173–1243 CrossRef CAS.
- B. W. Cheng, Z. J. Li, Q. X. Li, J. G. Ju, W. M. Kang and M. Naebe, J. Membr. Sci., 2017, 534, 1–8 CrossRef CAS.
- H. Gao, Y. Yang, O. Akampumuza, J. Hou, H. Zhang and X. Qin, Environ. Sci.: Nano, 2017, 4, 864–875 RSC.
- G. Kwon, E. Post and A. Tuteja, MRS Commun., 2015, 5, 475–494 CrossRef CAS.
- Y. S. Jiang, J. W. Hou, J. Xu and B. T. Shan, Carbon, 2017, 115, 477–485 CrossRef CAS.
- G. Jiang, L. Luo, L. Tan, J. Wang, S. Zhang, F. Zhang and J. Jin, ACS Appl. Mater. Interfaces, 2018, 10, 28210–28218 CrossRef CAS PubMed.
- M. Huang, Y. Si, X. Tang, Z. Zhu, B. Ding, L. Liu, G. Zheng, W. Luo and J. Yu, J. Mater. Chem. A, 2013, 1, 14071–14074 RSC.
- D. Ge, L. Yang, C. Wang, E. Lee, Y. Zhang and S. Yang, Chem. Commun., 2015, 51, 6149–6152 RSC.
- M. Tao, L. Xue, F. Liu and L. Jiang, Adv. Mater., 2014, 26, 2943–2948 CrossRef CAS PubMed.
- A. Tuteja, W. Choi, M. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618–1622 CrossRef CAS.
- Y. K. Lai, J. Y. Huang, Z. Q. Cui, M. Z. Ge, K. Q. Zhang, Z. Chen and L. F. Chi, Small, 2016, 12, 2203–2224 CrossRef CAS PubMed.
- A. K. Kota, W. Choi and A. Tuteja, MRS Bull., 2013, 38, 383–390 CrossRef CAS.
- S. M. S. Shahabadi, H. Rabiee, S. M. Seyedi, A. Mokhtare and J. A. Brant, J. Membr. Sci., 2017, 537, 140–150 CrossRef.
- S. K. Hong, S. Bae, H. Jeon, M. Kim, S. J. Cho and G. Lim, Nanoscale, 2018, 10, 3037–3045 RSC.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef CAS.
- W. Zhang, N. Liu, Y. Cao, Y. Chen, L. Xu, X. Lin and L. Feng, Adv. Mater., 2015, 27, 7349–7355 CrossRef CAS PubMed.
- T. Yamaguchi, S.-H. Park and S. Nakao, Chem. Eng. Sci., 2001, 56, 3539–3548 CrossRef.
- E. Benet, A. Badran, J. Pellegrino and F. Vernerey, J. Membr. Sci., 2017, 535, 10–19 CrossRef CAS.
- N. W. Gao, Y. Q. Fan, X. J. Quan, Y. W. Cai and D. W. Zhou, J. Mater. Sci., 2016, 51, 6379–6388 CrossRef CAS.
- Y. Dou, D. Tian, Z. Sun, Q. Liu, N. Zhang, J. H. Kim, L. Jiang and S. X. Dou, ACS Nano, 2017, 11, 2477–2485 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed description of the fabrication procedure of the membranes, Fig. S1–S17, Movies S1–S5, and Tables S1 and S2. See DOI: 10.1039/c9nh00166b |
| This journal is © The Royal Society of Chemistry 2019 |