Open Access Article
Open Access ArticleToward an operational methodology to identify industrial-scaled nanomaterial powders with the volume specific surface area criterion†
Claire
Dazon
 a,
Olivier
Witschger
a,
Olivier
Witschger
 *a,
Sébastien
Bau
*a,
Sébastien
Bau
 a,
Vanessa
Fierro
a,
Vanessa
Fierro
 b and
Philip L.
Llewellyn
b and
Philip L.
Llewellyn
 c
c
aLaboratoire de Métrologie des Aerosols, F-54519 Vandoeuvre lès Nancy, France. E-mail: olivier.witschger@inrs.fr
bInstitut Jean Lamour, UMR CNRS 7198, F-88051, Epinal, France
cAix-Marseille Univ., CNRS, Laboratoire MADIREL, 13013, Marseille, France
First published on 9th July 2019
Abstract
Nanoparticulate powders are increasingly found in the workplace. Inhalation exposure to airborne nanoparticles (NPs) is possible throughout the life-cycle of the powders. As the toxicity of NPs has never been demonstrated, it remains essential to evaluate the risks associated with NPs in order to propose preventative measures. The first step of a risk assessment strategy consists in the identification of the ‘nano’ nature of a material, which suffers from a lack of an operational methodology. Here, we present a simplified and operational strategy relying on the volume specific surface area (VSSA) for nanomaterial identification, based on the recommendation stemming from the European Commission and previous work on this topic from the European Project Nanodefine. The proposed strategy was tested on a set of 15 representative industrial powders (TiO2, SiO2, CuO, and ZnO), covering a wide range of properties, and previous published data. The VSSA classification was validated via a comparison with the particle size obtained by transmission electron microscopy (TEM). It was evidenced that the VSSA is in accordance with particle size for nanomaterial powder classification. The proposed methodology involves relatively accessible methods such as thermogravimetric analysis, nitrogen adsorption and helium pycnometry and limits the detection of false negatives. Moreover, it does not imply systematic confirmation of the results with the reference particle size criterion. Our results suggest that the VSSA is a promising parameter to be used for risk assessment and should be further investigated on powder mixings to confirm its relevancy to define nanomaterial powders.
1. Introduction
Nanomaterial powders integrate numerous products in buildings, food, cosmetics, energy, paints and coatings, health, plastics, textiles and papers and so forth, due to the specific physical and chemical properties of the materials at the nanoscale.1–3 The latest R-nano report published in 2017 (ref. 4) related to the French mandatory declaration of nanomaterials produced, imported or distributed indicates that nearly 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of nanomaterials are declared. Half of the involved nanomaterial quantities concern less than 1 ton of nanomaterial declared whereas 27% are related to quantities below 100 kg. These figures suggest that nanomaterials have been spreading widely, mainly in small and medium size enterprises. Even if no explicit state of matter is reported in the declarations, we can put forward the hypothesis that it is in powder form that the most declared products (above 100
000 tons of nanomaterials are declared. Half of the involved nanomaterial quantities concern less than 1 ton of nanomaterial declared whereas 27% are related to quantities below 100 kg. These figures suggest that nanomaterials have been spreading widely, mainly in small and medium size enterprises. Even if no explicit state of matter is reported in the declarations, we can put forward the hypothesis that it is in powder form that the most declared products (above 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons) are found (carbon black, silica, calcium carbonate, titanium dioxide, boehmite (alumina)). Such initiatives for nanomaterials declarations are currently under implementation in other European countries (Belgium, Sweden, Denmark) showing the great concerns entailed by nanomaterials development.
000 tons) are found (carbon black, silica, calcium carbonate, titanium dioxide, boehmite (alumina)). Such initiatives for nanomaterials declarations are currently under implementation in other European countries (Belgium, Sweden, Denmark) showing the great concerns entailed by nanomaterials development.
Therefore, nanomaterial powder aerosols are increasingly found in workplaces.5–8 Indeed, production, conditioning, cleaning can involve lots of operations such as manual spraying, mixing, transferring, embedding, grinding, and blowing of varying, amounts of powders. These actions that can be found throughout the entire life-cycle of the powders can generate airborne particles potentially inhaled by workers. The potential toxicity of nanoparticles has been evidenced since several years.9–12 Consequently, risk assessment related to the inhalation of nanoparticles released in workplaces is necessary to propose preventative measures.
The first step of any risk assessment strategy is hazard identification.13 This area is still lacking of an operational methodology for nanomaterial identification in the workplaces, in particular for the powder cases. In a risk assessment context, it is important to precise the nanomaterial definition used since numerous propositions are available worldwide and notably in Europe (more than 10 definitions14) in the regulatory context. Generally, the European Commission (EC) definition recommendation is chosen.15 This latter is focused on two parameters: the size of the constituent particles as reference criterion and the volume specific surface area (VSSA) as complementary criterion. Despite its regulatory nature, this definition covers the potential risks related to nanomaterials on health, security and environment according to the official text, allowing its application for risk assessment.
According to the EC definition, the “nano” nature of a material is established as soon as more than 50% in the number size distribution of the constituent particles are below 100 nm in at least one dimension. This number size distribution is commonly obtained by electron microscopy (EM) methods (transmission electron microscopy) or scanning electron microscopy (SEM).16 These techniques are interesting since a particle size is directly measured based on microphotographs obtained, and four orders of magnitude in terms of sizes are covered (<1 nm to 10 μm). Other methods providing the size information, such as light scattering methods (indirect measurement) can be used when the constituent powder particles are well dispersed in the liquid samples. These latter are relatively easy to implement and cost-effective compared to EM methods. However, there is no generic protocol for powder dispersion and the results are strongly influenced by sample preparation which limits the relevancy of these approaches for nanomaterial powder identification.17
Concerning the complementary criterion of the VSSA, the concept relies on the ratio of the whole constituent particle surfaces S divided by their whole volume V (eqn (1)). Assuming monomodal and spherical particle shape hypothesis, the VSSA is then related to the constituent particle size d (eqn (2)). The VSSA is therefore an indirect way for constituent particle size determination.
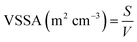 | (1) |
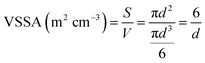 | (2) |
When the VSSA is larger than 60 m2 cm−3, the material is considered as a nanomaterial according EC definition. This threshold (VSSACutoff) is based on the hypothesis of non-porous and monodisperse spherical particles of 100 nm constituent particle size. This threshold can be modified for fibre-like particle shape: 40 m2 cm−3; or 20 m2 cm−3 for platelet-like particle shape.18 However, this is the 60 m2 cm−3 threshold which should be used for nanomaterial identification in the official texts. From an experimental point of view, the VSSA is determined with eqn (3) using the powder external specific surface area (AEX) and the material skeletal density (ρskeletal):
| VSSA (m2 cm−3) = AEx (m2 g−1) × ρskeletal (g cm−3) | (3) |
Two analytical techniques have to be applied to determine VSSA: gas adsorption (nitrogen, argon or krypton), and helium pycnometry for AEX and ρskeletal measurement, respectively.19 These two techniques are well disseminated in research laboratories, but also in industries. These techniques can be applied to all types of powders, including ones whose chemical composition is not clearly known before analysis. Moreover, the protocols of experimental implementation and data analysis are well described in the literature, facilitating their handling. However, they require careful preparation of the samples, including outgassing of the powders prior to analysis to avoid underestimation of the measured values. Nevertheless, these outgassing conditions are the subject of recommendations in literature.20–22 Except this outgassing step, the “powder state” is perfectly adapted for such analysis and no more sample preparation is needed. Recently, the National Institute of Standards and Technology (NIST) performed an inter-laboratory study to evaluate “real-world” precision and bias of specific surface area measurements using a powered material (Standard Reference Material 1898 (titanium dioxide)) containing sub-30 nm” constituent particles.23 The results obtained through a 20 laboratories network evidenced a strong robustness of the gas adsorption method; the biases on the certified specific surface area value did not differ from more than ±5%. Based on these elements, the VSSA appears from an experimental point of view, more accessible than EM methods for nanomaterial identification, in particular for non-specialist of the material characterization domain, more particularly, small, very small enterprises as well as laboratories.
VSSA was recently pointed out as a promising approach for nanomaterial identification of industrial powders as demonstrated on representative products largely produced at the industrial scale.24 This work under the European project Nanodefine evidenced the reliable nanomaterial identification based on the powder VSSAs determination and comparison with the reference criterion of particle size. Wohlleben et al.24 proposed a screening strategy for nanomaterial and non-nanomaterial identification of powders involving VSSA determination in the first step. They proposed a 6 m2 cm−3 VSSACutoff, corresponding to supposed spherical particles of 1 μm size. If VSSA is found to be lower than 6 m2 cm−3, the powder is classified as a non-nanomaterial. However, EM methods (SEM and TEM) are systematically implemented in the rest of their methodology in case of VSSA upper than the 6 m2 cm−3 threshold to characterize the particles shapes and adjust the VSSA thresholds and/or confirm the classification relying on particle size determination. The first VSSA threshold of this screening strategy seems very strict regarding the materials studied, as all of them have VSSAs greater than 6 m2 cm−3. This involved systematic shape characterization to conduct all the screening strategy, questioning so, the added value of the VSSA criterion if finally, accessing to size and particle shape is mandatory for reliable nanomaterial identification. The approach proposed by the Nanodefine project is cautious and it is currently integrated in the Nanodefiner e-tool,25 a decision support framework for the characterisation of potential nanomaterials. However, it does not seem to allow a wide use of the VSSA criterion in the particular risk assessment context. A simplification of this VSSA criterion would be possible, for instance, reducing the systematic EM characterization, and using the VSSA as the defining criterion for nanomaterial and possibly non-nanomaterial identification. The most recent work on this subject26 demonstrated that it is possible to avoid EM and use the VSSA as an independent parameter of the particle size. It is worth nothing that this latter study was carried out on eight powders only, which limits the generalization of the results.
Therefore, we proposed going further in the investigation of the potential use of the VSSA criterion and presenting a methodology to define a nanomaterial based on it. The simplified methodology we propose puts a stress on VSSA determination after a careful sample preparation implying thermogravimetric analysis before nitrogen adsorption and helium pycnometry, and an accurate data analysis. Representative industrial powders were chosen to classify them in or out of the nanomaterial category based on their VSSAs and the comparison with two thresholds we explained. Our approach is strengthened by an orientating intercomparison of nitrogen adsorption and helium pycnometry (two laboratories). The proposed nanomaterial identification is then compared to the reference size criterion obtained by TEM for validation purposes. The results are finally discussed on the relative merits of this simplified proposition compared to existing literature, and how they implement those coming from previous studies.24,26
2. Operational strategy proposed for nanomaterial identification in the workplace
The operational strategy proposed is illustrated in Fig. 1. To identify a powder as a nanomaterial (or not), one first performs a thermogravimetric analysis (TGA) to select the appropriate outgassing conditions before gas adsorption and helium pycnometry. Then, these methods are implemented to determine the AEx with a convenient analysis of the nitrogen adsorption isotherm and the material skeletal density ρskeletal, respectively. No EM method is involved in these steps.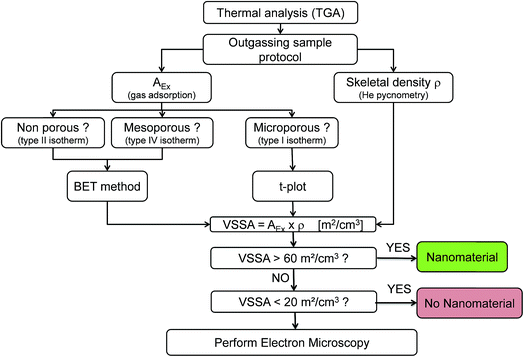 | ||
| Fig. 1 Flow chart of the proposed operational powder characterization strategy for nanomaterial identification in the workplace. | ||
When the VSSA of the powder investigated is determined, the proposed approach compares this latter with the 60 m2 cm−3 threshold, assuming spherical particles. As recommended by the EC definition, our strategy categorizes a powder as nanomaterial as soon as its VSSA is greater than 60 m2 cm−3. If the VSSA is under 20 m2 cm−3, corresponding to the threshold for platelet particles evocated in literature,18 the powder can be classified as non-nanomaterial and no further investigation is proposed. The 40 m2 cm−3 is the VSSACutoff of the fibre like particle corresponding to a fibre diameter of 100 nm and it is necessarily included between the chosen thresholds 20 m2 cm−3 and 60 m2 cm−3. Between 20 and 60 m2 cm−3, complete electron microscopy (TEM or SEM) with particle size distribution determination is suggested to identify the powder as a nanomaterial or not definitely. The chosen thresholds were selected based on the results presented in this study and those available in literature.24,26
3. Materials and methods
3.1 Powders
Fifteen powders produced at the industrial scale were selected for this study. Table 1 describes their manufacturing processes, their main application fields, and available physico-chemical properties, based the safety or commercial product data sheets. These materials were chosen due to their representativeness of the most handled powders in the workplaces, the variety of applications they integrate and their wide range of properties. In particular, the total surface area values At (including AEx and porosity) cover almost 1.4 orders of magnitude. More details about the powder manufacturing processes and applications for TiO2 and SiO2 are presented in ESI part 1.†| Powder | Code | Manufacturing process | Application | A t m2 g−1 | Crystalline phase | Chemical composition weight% |
|---|---|---|---|---|---|---|
| TiO2 | A | Sulfate | Food and paintings | n.a | Anatase | 98 |
| B | Chloride | Buildings | Rutile | 99.5 | ||
| C | Sulfate | 90 | Anatase | 95.2 | ||
| D | 138 | 93.6 | ||||
| E | 350 | 82.7 | ||||
| SiO2 | A1 | Electrometallurgy | 26.3 | Amorphous | 95.58 | |
| A2 | 24.7 | 94.86 | ||||
| B | Pyrogenic | 40 | 99.9 | |||
| C | 150 | 99.9 | ||||
| D | Ink | n.a | 91 | |||
| E | Pharmaceutics | 300 | 99.9 | |||
| 244FP | Precipitated | Food, health and buildings | n.a | 99.6 | ||
| 4850MR | 600 | 99.5 | ||||
| ZnO | ZnO | Wet chemistry | Cosmetics | n.a | n.a | 99.8 |
| CuO | CuO | Sensors and chemical catalysis | 50 | 99 |
3.2 Nitrogen (N2) adsorption
N2 adsorption experiments were carried out in two public research laboratories (Lab A and Lab C) to conduct a comparison of the method and therefore strengthen the data. Lab A performed N2 adsorption with an ASAP 2020 (Micromeritics) whereas Lab C used a 3Flex (Micromeritics) apparatus. All the samples were outgassed under vacuum at 10−2 mmHg at 200 °C and a minimum of 12 h before N2 adsorption. This protocol was chosen based on TGA analysis performed previously with an HR83 (Mettler Toledo) thermobalance. Although TGA does not reproduce exactly the vacuum condition of gas adsorption, it is an interesting method to select a convenient outgassing protocol before N2 adsorption measurements as it is necessary to remove from the powder particle surfaces eventually adsorbed water and pollutants molecules which can entail an underestimation of the surface areas measured. So, when a mass loss stabilization was observed after 30 to 50 minutes under an isothermal segment, one can consider the complete desorption of water and pollutants adsorbed onto particle surfaces. The chosen temperature for outgassing is the temperature where the mass loss stabilization was observed on the thermogram. Generally, adsorbed water and pollutants are removed between 100 °C and 300 °C. In our case, all the powders showed a mass loss plateau after 30 minutes at 200 °C, leading us to generalize the outgassing procedure mentioned above to all the materials studied. All the measurements were triplicated. The external specific surface areas AEx were determined with the BET model (for the case of non-porous or mesoporous materials)27 or with the t-plot model (for the case of microporous material).28 This procedure is in accordance with standards related to gas adsorption and its data analysis.29,303.3 Helium (He) pycnometry
The skeletal densities of the powders were determined by He pycnometry (Accupyc 1340, Micromeritics) in two laboratories (Lab A and Lab B). All the samples were dried in an oven at 105 °C overnight before performing the measurements in Lab A, while this step was done in Lab B using a desorption station Flowprep 060 (Micromeritics), 2 h at 220 °C. Experimental skeletal density measurements were validated by comparison of the results obtained with the theoretical material densities extracted from Handbook of Chemistry (Edition 2017–2018), accounting for the chemical structure of the powders investigated and hypothesis of pure chemical compound. As gas adsorption, the procedure described here is in accordance with ISO12154.313.4 Transmission electron microscopy (TEM)
TEM samples were prepared following a specific method (“grid-on-drop”)32 with optimized colloidal suspensions were formulated based on zeta potential measurements. This step is fully described in ESI part 2.† One TEM grid (Cu 400 Mesh Carbon Film Agar Scientific) per powder sample was analyzed with a TEM CM200 (Philips) at 200 kV and a Cs of 2 mm. Between 40 and 60 micrographs were taken at different magnifications from ×195 to ×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a Gatan Erlangshen (ESW500) 1350 by 1024 and a MSC794 1024 by 1024 CCD cameras. The number size distributions of the constituent particles were determined using ImageJ software (Java version 1.8.0) and by measuring the equivalent projected surface area diameter in case of spherical particle shape; the smallest dimension for fiber-liked or platelets particle shape. Between 200 and 400 particles were counted to establish the number size distributions to have a significant representation of the particle populations considered. The median diameter D50 obtained from the cumulated number size distribution was used to classify the nanomaterial powder either in the nano range or not. This step is also described in ESI part 2.†
000 with a Gatan Erlangshen (ESW500) 1350 by 1024 and a MSC794 1024 by 1024 CCD cameras. The number size distributions of the constituent particles were determined using ImageJ software (Java version 1.8.0) and by measuring the equivalent projected surface area diameter in case of spherical particle shape; the smallest dimension for fiber-liked or platelets particle shape. Between 200 and 400 particles were counted to establish the number size distributions to have a significant representation of the particle populations considered. The median diameter D50 obtained from the cumulated number size distribution was used to classify the nanomaterial powder either in the nano range or not. This step is also described in ESI part 2.†
3.5 Equivalent particle size with VSSA
From the VSSA, it can be defined an equivalent diameter dVSSA (eqn (4)):18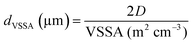 | (4) |
4 Results and discussion
4.1 N2 adsorption, He pycnometry and VSSA
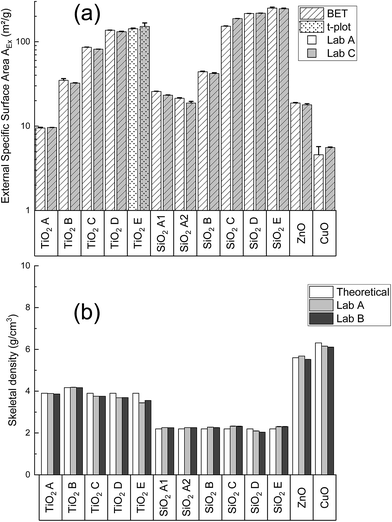
Fig. 2a shows the comparison of AEx obtained in Lab A and Lab C. The AEx of the studied powders cover 1.7 orders of magnitude with the smallest AEx obtained for CuO (4.5 m2 g−1) and the highest AEx obtained for SiO2 E (250 m2 g−1). Both laboratories used BET or t-plot model (TiO2 E and SiO2 4850MR) for AEx determinations. It is worth noting that SiO2 244FP (AEx 142 m2 g−1) and SiO2 4840MR (AEx 92 m2 g−1) were not compared for availability reason of the apparatus in Lab B, that is why these powders are not indicated in the graph. The AEx range covered is not impacted, however. N2 adsorption isotherms of Lab A are available in Fig. S7 ESI part 3.† The comparison shows that, on the one hand, gas adsorption measurements are repeatable since the average coefficient of variation (precision) is 3%. On the other hand, they are reproducible since biases between laboratories are comprised within ±10%. These precision and bias values are slightly higher than those observed in the work undertaken by Hackley and Stefaniak23 on the reference SRM1898 TiO2. Coefficients of variation ranged from 0.10% and 3.96% and bias was generally within ±5% in their work. It is worth mentioning that the so-called intercomparison in our study is clearly reduced compared to the Hackley and Stefaniak work, were much more laboratories were involved. However, the precisions and biases obtained here are comparable to those obtained on more partners. This confirms the reliability of specific surface area determination by gas adsorption method.Fig. 2b shows the comparison of He pycnometry results between Lab A, Lab B and the theoretical skeletal densities. As gas adsorption, repeatability is achieved with He pycnometry since coefficients of variation are comprised within ±5%. A good comparison between the laboratories was observed since biases between Lab A and B are all within ±10% and demonstrate so, a good reproducibility. SiO2 244FP and SiO2 4850MR could not be compared with Lab B, and were only analyzed by Lab A. Lab A found skeletal densities values of 2.07 and 2.17 ± 0.01 g cm−3 for these two powders respectively. One can observe also the skeletal densities measured are very close to the theoretical densities. This means that the studied materials here can be considered as pure metal oxide powders.
These comparison results argue for the use of N2 adsorption and helium pycnometry for VSSA application as they are repeatable and reproducible methods.
Fig. 3 represents the 15 characterized powder ranked by the proposed methodology according to their VSSA. The VSSA values cover 1.3 orders of magnitude (from 28 to 575 m2 cm−3). CuO, TiO2 A, SiO2 A1 and SiO2 A2 are not clearly identified as nanomaterial powders according to our approach since their VSSA is comprised between 20 m2 cm−3 and 60 m2 cm−3. We need in these cases the use of TEM as described above in Section 3.4 to determine their nanomaterial nature, which is presented in the following paragraph. The other powders are defined as nanomaterials according their VSSA higher than the 60 m2 cm−3. At this point, the nanomaterial powders identified with their VSSA according our approach would not require further investigation. However, in order to confirm the reliability of the VSSA, TEM analysis were also performed on these nanomaterials in addition to the one carried out on the four powders requiring nanomaterial classification based on the particle number size distribution.
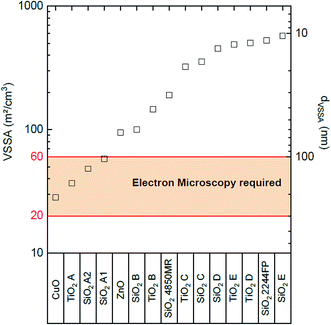 | ||
| Fig. 3 VSSA distribution of the characterized powders and the corresponding dVSSA for a spherical particle shape assumption (shape factor D = 3). | ||
4.2 Comparison of the VSSA-based nanomaterial identification with the particle size criterion
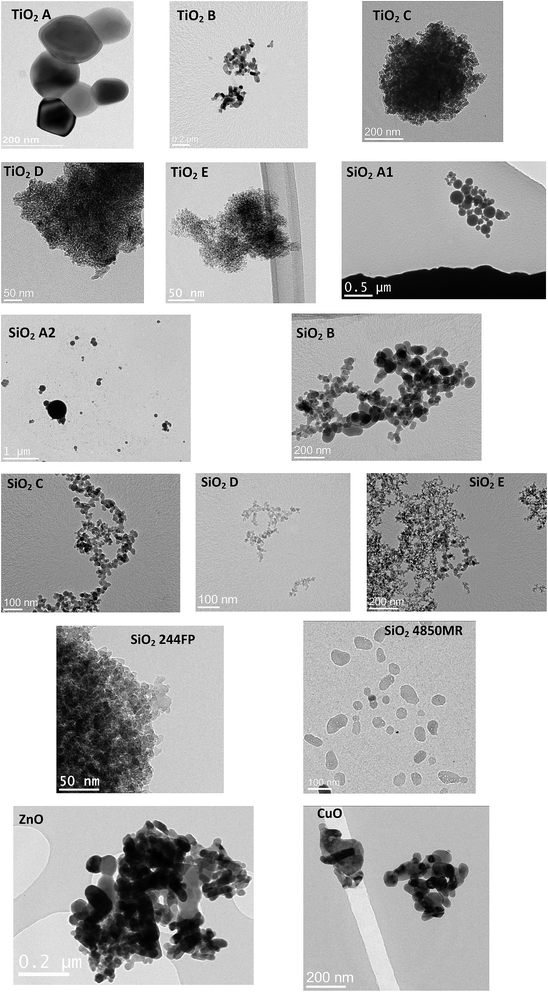
Fig. 4 shows a typical TEM micrograph obtained for the characterized powders. Table 2 summarizes the particle shapes observed and the corresponding D50 median diameters obtained from cumulated number size distributions of the constituent powder particles. The equivalent particle sizes with the VSSA values are indicated for simple comparison purpose.| Powder | Code | Shape | D 50(1) (nm) | D 50(2) (nm) | d VSSA (nm) | TEM classification |
|---|---|---|---|---|---|---|
| a Corresponds to the D50 associated to the first spherical or rod-like population. b Corresponds to the D50 associated to the second spherical or rod-like population. | ||||||
| TiO2 | A | Sphere | 121 ± 10 | — | 163 | Non nanomaterial (borderline) |
| B | 37.5 ± 5.5 | 28.5 ± 5 | 33 | Nanomaterial | ||
| Rod-like | 32 ± 8 | — | ||||
| C | Sphere | 14 ± 4 | 15 | |||
| Rod-like | 11.2 ± 2 | |||||
| D | Sphere | 9.5 ± 4 | 15.2 ± 3 | 11 | ||
| E | 6 ± 3 | — | 12 | |||
| SiO2 | A1 | 94 ± 20 | 103 | Nanomaterial (borderline) | ||
| A2 | 98 ± 25 | 125 | ||||
| B | 54 ± 12 | 17 ± 8 | 60 | Nanomaterial | ||
| C | 15 ± 5 | — | 17 | |||
| D | 11.3 ± 3 | 13 | ||||
| E | 10.8 ± 2 | 10 | ||||
| 244FP | 11.8 ± 5.5 | 11 | ||||
| 4850MR | 32.1 ± 8 | 47.1 ± 5 | 26 | |||
| Rod-like | 37.5 ± 5.5 | — | ||||
| CuO | CuO | Sphere | 48.3 ± 20 | 166 | ||
| Rod-like | 40.5 ± 15 | |||||
| ZnO | ZnO | Sphere | 33 ± 10 | 49 ± 12 | 54 | |
| Rod-like | 31.2 ± 5 | — | ||||
TiO2 powders are composed of spherical particles with one or two populations or rod-like particles with one population. SiO2 powders are composed of spherical particles except SiO2 4850MR containing a rod-like population. ZnO and CuO powders are composed of spherical and rod-like particles.
CuO, SiO2 A1 and SiO2 A2 are identified as nanomaterials with the particle size criterion whereas TiO2 A is a non-nanomaterial. We point out in Table 2 that TiO2 A, SiO2 A1 and SiO2 A2 powders are border-line cases with particle sizes very closed to the 100 nm. The other powders are identified as nanomaterials, which confirmed the previous results. Concerning the obtained dVSSA, they differ from the TEM values for an average bias of 16%. One can note however, some biases are higher than 100% (CuO case). Such discrepancies have already been observed in a previous work. It is important to recall that VSSA and TEM are two different approaches for particle size measurement (indirect for VSSA, direct for TEM) and they rely on different metrics (mass-based metric for VSSA, number-based metric for TEM). The techniques used for their implementation are greatly different and do not rely on the same physical principle (thermodynamic for VSSA, optic for TEM) also. Taking into account these elements and the non-perfect nature of the constituent particles, the hypothesis made for the dVSSA cannot be systematically convenient for a good comparison with TEM, it is not so surprising to obtain important deviations between dVSSA and D50. Moreover, VSSA cannot distinguish neither the different particle size populations as it is an integral analysis, nor the particle shape. However, our approach and the suggested thresholds do not seem to be impacted by the particle shape or the size composition for nanomaterial classification. We did not obtain a false negative or a false positive when comparing the nanomaterial categorization by VSSA and constituent particle size distribution criteria with the proposed limits. Four materials would require TEM analysis to be sure about the classification (no false negative or false positive expected so) whereas all the others are correctly classified. A false positive detection with VSSA implies that a powder is identified as nanomaterial whereas the particle size criterion demonstrates the opposite. A false negative detection implies VSSA identifies a powder as non-nanomaterial whereas the size criterion certifies it is a nanomaterial. In a risk assessment context, a false negative is the worst case that can occur since the preventative measures may be reduced contrary to a false positive case. Here, even if dVSSA greatly differ from the constituent particle size obtained through TEM, it is worth recalling that the final objective is the correct identification of nanomaterial and also non-nanomaterial powders with the VSSA criterion, no matter what is the equivalent particle size obtained. The following paragraph justifies the 20 m2 cm−3 and 60 m2 cm−3 thresholds choice based on these results and those available in literature.
4.3 Discussion about the proposed strategy
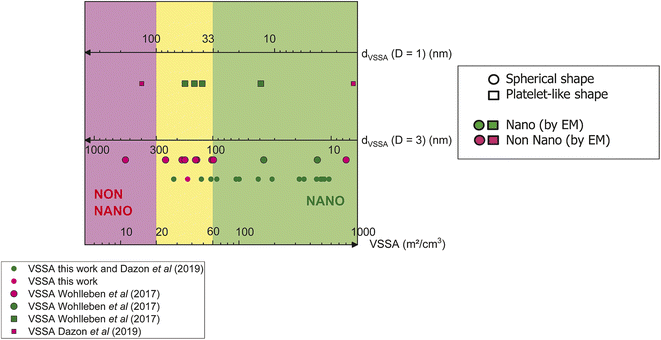
Fig. 5 shows the VSSA distributions obtained in this work and previous studies.24,26The thresholds 20 m2 cm−3 and 60 m2 cm−3 of our methodology are indicated through 3 colour zones. Nanomaterials identified by VSSA (top axis, above 60 m2 cm−3) are in the green zone, non-nanomaterials (below 20 m2 cm−3) are in the pink zone and those requiring EM (between 20 m2 cm−3 and 60 m2 cm−3) are in the yellow zone.
The plots forms correspond to the particle shape observed with EM methods in the different studies (a circle for spheres and squares for platelets). The corresponding dVSSA are also indicated in Fig. 5 for the different shape factor D (D = 1 for platelet particle, middle axis, and D = 3 for spherical particle, bottom axis).
The plot colours correspond to the nanomaterial or non-nanomaterial identification by EM (green for nanomaterial and pink for non-nanomaterial). So, if the colour of the plot and the colour of the zone are the same, VSSA and EM are in accordance for nanomaterial or non-nanomaterial identification. A green plot in the pink zone indicates a false negative and a pink plot in the green zone indicates a false positive.
Based on the VSSA values and the thresholds, it can be seen that 14 powders from a set of 33 materials (40%) considered can be reliably classified as nanomaterial and 2 powders as non-nanomaterial (6%) without additional EM characterization based on the proposed methodology. The non-nanomaterials in question here are a CaCO3 (ref. 26) of 15 m2 cm−3 VSSA value and a BaSO4 (ref. 24) powder with an 11 m2 cm−3 VSSA value. Without knowing particle shape, the comparison with the 60 m2 cm−3 (suggesting so a spherical shape in all cases), leads to a non-nanomaterial classification. If we compare now the VSSA values of these two materials with adjusted VSSACutoff in line with the particle shape (20 m2 cm−3 for the CaCO3 (D = 1) and 60 m2 cm−3 for BaSO4 (D = 3)), we also obtain their identification as non-nanomaterial. The latter fact suggests so that knowing the particle shape is not really necessary for the solely nanomaterial identification when using the VSSA criterion.
We observed also on Fig. 5 that only two false positive cases appear (6%) and no false negative. If we focus on the green circle plots in the yellow zone, corresponding to the CuO, SiO2 A1 and SiO2 A2 of this work, the VSSA comparison with the 60 m2 cm−3 leads to non-nanomaterial identification. EM evidenced that these powders are nanomaterials, which entails a false negative detection with VSSA. If we decide to limit the nanomaterial identification with VSSA and do not follow EC recommendation (to confirm non-nanomaterial classification with VSSA by size measurement), these three powders constitute false negatives, which is a worst case for risk assessment. That is why we suggest largest thresholds than those proposed by previous work24 since we observed that half of the cases are reliably identified as nanomaterial (or not) with the solely VSSA criterion whereas the second part is classified by EM (size measurement). No experimental errors can be associated with these two limits since there is no sufficient published data today allowing a statistical relevancy for any safety margin propositions.
Concerning the particle shape, our proposition deliberately does not integrate it, contrary to the screening strategy proposed by the European project Nanodefine24 where simple EM is indicated to adjust the VSSACutoff in case of values above the first limit of 6 m2 cm−3. In our proposition, we considered that, when EM is required, VSSA no longer appears necessary since a direct implementation of the reference size criterion for nanomaterial identification is possible. The particle shape is a property to investigate, for instance, in the REACH regulation (annexes for nanoforms documentation were recently amended and are now available)33 if appropriate. In this context, the Nanodefine project proposes a more conservative approach and allows the particle shape characterization in most of the cases, which is in accordance with REACH if regulation is aimed. However, for the simple purpose of identification of nanomaterial in a strategy with an initial step of risk assessment, this particle shape determination could be optional, since it is not a critical property to mention at this level, and we demonstrate it does not seem to have an influence on the final results. The data obtained in this work, and the comparison with previous studies24,26 suggests that the 20 m2 cm−3 and the 60 m2 cm−3 thresholds are reasonable and conservative to reduce as much as possible false negative cases based on the whole available data in the open literature. The 20 m2 cm−3 appears optimistic threshold in the case of platelet-like particles, but further investigation on this type of powders is necessary to make evolve this limit.
In all cases, Nanodefine results and ours suggest that it is important to well define the context surrounding the material characterization in order to select the appropriate strategy to implement, given also the area concerned which may also depend on technical possibilities (deadlines, cost, procedures etc.).
However, because of the lack of data concerning the critical false negative case, VSSA seems a tenuous alternative criterion to consider without further particle size and shape determination in a regulatory context or for risk assessment, which impacts a confident use of this parameter according to our proposition. In top of that, the powders studied in this work, but also in the literature, do not display constituent particle size distributions composed of a nanomaterial and non-nanomaterial distinct populations for a same substance. Therefore, the proposed strategy is restricted for industrial monomodal or slightly polydispersed materials to date. Only one specifically prepared mixed powder has ever been studied until now24 in the Nanodefine project to evaluate the limits of the VSSA for nanomaterial identification. The results obtained encourage one to continue the investigation of such multimodal materials to enhance the relative merits of using VSSA for nanomaterial and non-nanomaterial identification, and consider in the future as a defining criterion. Awaiting new feedback, one can implement this proposed simplified methodology, and go further in the characterization in cases where the particle shape is required and/or any doubt about a possible false negatives obtained, depending on the results (VSSA very close to 20 m2 cm−3 for instance).
5 Conclusions
In the context of risk assessment to nanomaterial powders, a first essential step is the nanomaterial identification. Indeed, if a powder is not categorized as a nanomaterial, a conventional risk assessment strategy related to chemicals is applied. To date, this first step lacks of an operational methodology to characterize the nanoparticulate nature of the powders. Besides, risk prevention professionals do not necessarily have skills in the domain of material characterization, nor the equipment to implement in a reliable way the techniques suggested in the literature to apply the recommended nanomaterial definition of European Commission relying on reference particle size criterion and complementary volume specific surface area (VSSA) of powders.In this work, we proposed a simplified and operational powder characterization methodology based on the previous European Nanodefine project results and relying on the VSSA determination involving nitrogen adsorption and helium pycnometry with a previous selection of outgassing conditions with thermogravimetric analysis. The proposed methodology distinguishes nanomaterials (VSSA above 60 m2 cm−3) and non-nanomaterials (VSSA below 20 m2 cm−3) zones involving neither particle shape determination nor size measurement to confirm the results. For VSSA included between 20 m2 cm−3 and 60 m2 cm−3, electron microscopy is recommended to clearly identify the “nano” nature (or not) of the powders. The procedure was tested on fifteen representative industrial powders (TiO2, SiO2, ZnO and CuO) and applied also on previous published data in literature. Moreover, an orientating intercomparison of characterization methods implemented for VSSA determination was carried out to give more robustness to the results.
It was seen that the VSSA criterion alone allows a classification of 46% of the studied powders in the nanomaterial category and 6% in the non-nanomaterial category without systematic confirmation by particle size measurement. Comparison with literature results suggests the chosen thresholds in our strategy limits the false negative cases, considered as the worst situation in the risk assessment implementation in workplaces.
This operational strategy appears generic and accessible for non-specialists in materials characterization. Deeper investigations are still necessary however to confirm this, since this work was carried out on a restricted number of material powders with simple compositions in terms of chemistry as well as particle sizes and shapes. Powder mixings characterized by two or more particle size modes, different shapes (platelet-like, needle-like, fiber…) should be investigated through an experimental program such as that presented here to further validate or propose new evolutions on the VSSA criterion. Awaiting new data, the presented methodology can be reasonably applied for the same type of powders studied here or in the Nanodefine project.
Conflicts of interest
The authors confirm that there are no conflicts to declare.Acknowledgements
The authors would like to thank Emily Bloch from Lab C for performing N2 adsorption measurements.References
- R. J. B. Peters, H. Bouwmeester, S. Gottardo, V. Amenta, M. Arena, P. Brandhoff, H. J. P. Marvin, A. Mech, F. B. Moniz, L. Q. Pesudo, H. Rauscher, R. Schoonjans, A. K. Undas, M. V. Vettori, S. Weigel and K. Aschberger, Nanomaterials for products and application in agriculture, feed and food, Trends Food Sci. Technol., 2016, 54, 155–164 CrossRef CAS.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world, J. Nanopart. Res., 2012, 14, 1109 CrossRef.
- S. Foss Hansen, L. R. Heggelund, P. Revilla Besora, A. Mackevica, A. Boldrin and A. Baun, Nanoproducts - what is actually available to European consumers?, Environ. Sci.: Nano, 2016, 3, 169–180 RSC.
- French Ministry of Ecology Sustainable Development and Energy, Report on the declaration of substances imported, manufactured or distributed in France in 2016 2017, available at https://www.r-nano.fr.
- D. Brouwer, Exposure to manufactured nanoparticles in different workplaces, Potential Hazard of Nanoparticles: From Properties to Biological & Environmental Effects, Toxicology, 2010, 269, 120–127 CrossRef CAS.
- T. Kuhlbusch, C. Asbach, H. Fissan, D. Gohler and M. Stintz, Nanoparticle exposure at nanotechnology workplaces: A review, Part. Fibre Toxicol., 2011, 8, 22 CrossRef.
- K. H. Dunn, A. C. Eastlake, M. Story and E. D. Kuempel, Control Banding Tools for Engineered Nanoparticles: What the Practitioner Needs to Know, Ann. Work Exposures Health, 2018, 62, 362–388 CrossRef CAS PubMed.
- E. Kuijpers, C. Bekker, D. Brouwer, M. le Feber and W. Fransman, Understanding workers' exposure: Systematic review and data-analysis of emission potential for NOAA, J. Occup. Environ. Hyg., 2017, 14, 349–359 CrossRef CAS.
- K. Donaldson, P. J. A. Borm, G. Oberdorster, K. E. Pinkerton, V. Stone and C. L. Tran, Concordance Between In Vitro and In Vivo Dosimetry in the Proinflammatory Effects of Low-Toxicity, Low-Solubility Particles: The Key Role of the Proximal Alveolar Region, Inhalation Toxicol., 2008, 20, 53–62 CrossRef CAS.
- C. Buzea, I. I. Pacheco and K. Robbie, Nanomaterials and nanoparticles: Sources and toxicity, Biointerphases, 2007, 2, MR17–MR71 CrossRef.
- S. Murugadoss, D. Lison, L. Godderis, S. Van Den Brule, J. Mast, F. Brassinne, N. Sebaihi and P. H. Hoet, Toxicology of silica nanoparticles: an update, Arch. Toxicol., 2017, 91(9), 2967–3010 CrossRef CAS.
- J. Wu, C. Wang, J. Sun and Y. Xue, Neurotoxicity of Silica Nanoparticles: Brain Localization and Dopaminergic Neurons Damage Pathways, ACS Nano, 2011, 5, 4476–4489 CrossRef CAS.
- P. T. O'Shaughnessy, Occupational health risk to nanoparticulate exposure, Environ. Sci.: Processes Impacts, 2013, 15, 49–62 RSC.
- D. R. Boverhof, C. M. Bramante, J. H. Butala, S. F. Clancy, M. Lafranconi, J. West and S. C. Gordon, Comparative assessment of nanomaterial definitions and safety evaluation considerations, Regul. Toxicol. Pharmacol., 2015, 73, 137–150 CrossRef CAS.
- EC, Commission recommendation of 18 October 2011 on the definition of nanomaterial, Official Journal of the European Union, L: Legislation, 2011, L275, 49–62 Search PubMed.
- X. Gao and G. V. Lowry, Progress towards standardized and validated characterizations for measuring physicochemical properties of manufactured nanomaterials relevant to nano health and safety risks, NanoImpact, 2018, 9, 14–30 CrossRef.
- F. Babick, J. Mielke, W. Wohlleben, S. Weigel and V.-D. Hodoroaba, How reliably can a material be classified as a nanomaterial? Available particle-sizing techniques at work, J. Nanopart. Res., 2016, 18, 158 CrossRef.
- G. Roebben, H. Rauscher, V. Amenta, K. Aschberger, A. B. Sanfeliu, L. Calzolai, H. Emons, C. Gaillard, N. Gibson, U. Holzwarth, R. Koeber, T. Linsinger, K. Rasmussen, B. Sokull-Klüttgen and H. Stamm, Towards a Review of the EC Recommendation for a Definition of the Term "Nanomaterial" Part 2: Assessment of Collected Information Concerning the Experience with the Definition, European Commission, Joint Research Centre, Institute for Reference Materials and Measurements, Luxembourg, 2014 Search PubMed.
- S. Lowell, J. E. Shields, M. A. Thomas and a. M. Thommes, in Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, ed. S. S. B. Media, Kluwer Academic Publisher, 2004, ch. 19 Search PubMed.
- J. Rouquerol and F. Rouquerol, in Adsorption by Powders and Porous SolidsAcademic Press, Oxford, 2nd edn, 2014, pp. 57–104 Search PubMed.
- E. Olson, The importance of sample preparation when measuring specific surface area, Journal of GXP Compliance, 2012, 16, 52–62 Search PubMed.
- J. Rouquerol and F. Rouquerol, in Adsorption by Powders and Porous Solids, Academic Press, Oxford, 2nd edn, 2014, pp. 57–104 Search PubMed.
- V. A. Hackley and A. B. Stefaniak, “Real-world” precision, bias, and between-laboratory variation for surface area measurement of a titanium dioxide nanomaterial in powder form, J. Nanopart. Res., 2013, 15, 1742 CrossRef.
- W. Wohlleben, J. Mielke, A. Bianchin, A. Ghanem, H. Freiberger, H. Rauscher, M. Gemeinert and V.-D. Hodoroaba, Reliable nanomaterial classification of powders using the volume-specific surface area method, J. Nanopart. Res., 2017, 19, 61 CrossRef PubMed.
- R. Brüngel, J. Rückert, W. Wohlleben, F. Babick, A. Ghanem, C. Gaillard, A. Mech, H. Rauscher, S. Weigel & C. M. Friedrich. The NanoDefiner e-tool — A decision support framework for recommendation of suitable measurement techniques for the assessment of potential nanomaterials. In 2017 IEEE 12th Nanotechnology Materials and Devices Conference, NMDC, 2017, 2018, pp. 71–72 Search PubMed.
- C. Dazon, O. Witschger, S. Bau, V. Fierro and P. L. Llewellyn, Nanomaterial identification of powders: comparing volume specific surface area, X-ray diffraction and scanning electron microscopy methods, Environ. Sci.: Nano, 2019, 6, 152–162 RSC.
- S. Brunauer, P. H. Emmet and E. Teller, Adsorption of gases in multimolecular layers, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- B. C. Lippens and J. H. de Boer, Studies on pore systems in catalysts: V. The t method, J. Catal., 1965, 4, 319–323 CrossRef CAS.
- ISO 9277, Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method, 2010, p. 24 Search PubMed.
- ISO 15901-3, Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption—Part 3: Analysis of Micropores by Gas Adsorption, 2007, p. 27 Search PubMed.
- ISO 12154, Determination of Density by Volumetric Displacement—Skeleton Density by Gas Pycnometry, 2014, p. 20 Search PubMed.
- J. Mast and L. Demeestere, Electron tomography of negatively stained complex viruses: application in their diagnosis, Diagn. Pathol., 2009, 4, 5 CrossRef.
- Official Journal of the European Union, COMMISSION REGULATION (EU) 2018/1881 of 3 December 2018 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annexes I, III,VI, VII, VIII, IX, X, XI, and XII to address nanoforms of substances, L308, 61.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00010k |
| This journal is © The Royal Society of Chemistry 2019 |