Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ni nanoparticle supported reduced graphene oxide as a highly active and durable heterogeneous material for coupling reactions†
Surjyakanta
Rana
*,
G. Bishwa Bidita
Varadwaj
and
S. B.
Jonnalagadda
 *
*
School of Chemistry & Physics, College of Agriculture, Engineering & Science, University of KwaZulu-Natal, Durban, South Africa. E-mail: jonnalagaddas@ukzn.ac.za; surjya.nou@gmail.com; Fax: +27 31 260 3091; Tel: +27 31 260 7325 ext. 3090
First published on 29th January 2019
Abstract
We report the loading of highly air stable Ni(0) nanoparticles (average particle size = 11 nm) on the surface of a reduced graphene oxide (RGO) material. The material was characterized using different techniques, including Raman spectroscopy, XRD, TEM, SEM, and HRTEM analysis. The Ni(0)@RGO catalyst showed superb efficiency towards Kumada–Corriu C–C cross-coupling reactions, with 92% yield of 4-methoxybiphenyl at 60 °C. The recycled material can be reused up to the 5th cycle after regeneration by calcination, without loss of activity.
Over the last few years, carbon-based reduced graphene oxide (RGO) has attracted the attention of many researchers, particularly in the field of catalysis.1 Due to its exceptional characteristics, such as its high surface area and electronic properties, reduced graphene oxide has opened up new opportunities for its use as a next generation catalyst and catalyst support material.2–9 The stable dispersion of reduced graphene oxide both in aqueous and organic solvents makes it more ideal as a catalyst support. Generally, in order to enhance the efficiency of conversions in various reactions, different metal nanoparticles loaded on a reduced graphene oxide support have been explored. Graphene oxide can be synthesized by different graphite to sodium nitrate ratio. Out of different ratio of graphite to sodium nitrate materials, a 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio increases the proportion of functional groups on the surface of the graphene oxide material. These functional groups allow for the growth of the metal nanoparticles on the surface.10 Nanoparticles have been explored for various applications due to their high surface to volume ratio.11–16 A survey of the literature shows the use of various metal nanoparticles, such as Au, Pt, and Pd supported RGO, as catalysts for different organic reactions.17–22 Most of these studies used noble metal supported reduced graphene oxide-based materials as catalysts, although only a few have employed low cost metal supported reduced graphene oxide-based materials for selective organic transformations.23–26
1 ratio increases the proportion of functional groups on the surface of the graphene oxide material. These functional groups allow for the growth of the metal nanoparticles on the surface.10 Nanoparticles have been explored for various applications due to their high surface to volume ratio.11–16 A survey of the literature shows the use of various metal nanoparticles, such as Au, Pt, and Pd supported RGO, as catalysts for different organic reactions.17–22 Most of these studies used noble metal supported reduced graphene oxide-based materials as catalysts, although only a few have employed low cost metal supported reduced graphene oxide-based materials for selective organic transformations.23–26
Broadly speaking, C–C cross-coupling reactions have been widely used for the preparation of different pharmaceutical products, molecular organic materials, and bioactive compounds.27–29 Cross-coupling reactions are one of the most significant C–C bond-forming reactions in organic synthesis. This reaction provides impressive results when using Pd complexes with phosphine ligands, or Pd as a ligand-free homogeneous catalyst. Homogeneous phosphine complexes are required to be handled under an inert atmosphere because they are more sensitive to moisture and exposure to air. When a ligand-free homogeneous catalyst is used, its recovery after reaction is very difficult. Therefore, for sustainability purposes, efficient reusable heterogeneous catalysts that can replace homogenous catalysts for cross-coupling reactions are of great significance. Hence, various heterogeneous Pd nanoparticle supported graphene oxide and functional graphene oxide materials have been developed as catalysts for coupling reactions.30–32 Some of these reported Pd-based catalysts are highly efficient and give high yields of the coupling product in the presence of different additives. However, the literature shows very few reports using Ni nanoparticle-based RGO catalysts for C–C coupling reactions.
In this communication, we report the novel synthesis of a Ni(0) modified reduced graphene oxide nanocomposite [Ni(0)RGO] material. During preparation, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of graphite to sodium nitrate was used for the synthesis of the support material. Without the need for any additives, it was capable of catalysing C–C coupling reactions with excellent yields.
1 ratio of graphite to sodium nitrate was used for the synthesis of the support material. Without the need for any additives, it was capable of catalysing C–C coupling reactions with excellent yields.
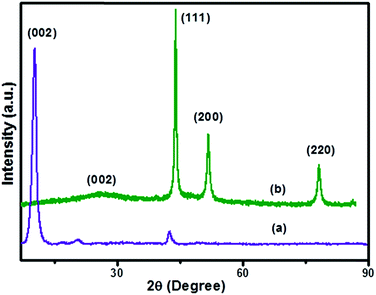
The X-ray diffraction spectra of (a) GO and (b) Ni(0)RGO are illustrated in Fig. 1. In Fig. 1(a), the spectrum shows 2θ ≈ 10.74, corresponding to the (002) plane of GO.4,33,34 In Ni-modified material (Fig. 1(b)), the high intensity peak of the (002) plane of graphene oxide is shifted towards a higher degree, and appears as a broad peak at 2θ ≈ 25, suggesting that the graphene oxide is completely reduced to formed reduced graphene oxide (RGO).
In Fig. 1(b), the peaks at 2θ ≈ 44.79, 52.24, and 78.2 correspond to the (111), (200), and (220) planes of the Ni metal nanoparticles (JCPDS card no. 01-071-4655). The average crystallite size of the synthesized nanomaterial was 11.35 nm, which is calculated from the Scherrer equation. The XRD pattern of Fig. 1(b) gives further confirmation of the absence of Ni(OH)2 and NiO peaks in the Ni(0)RGO sample.
Raman spectra of the (a) GO and (b) Ni(0)RGO catalysts are shown in ESI Fig. S1.† The Raman spectra give information about the disorder in the sp2-hybridized carbon atoms and σ-sp2 bonded carbon atoms, which correspond to the D band and G band, respectively. In case of graphene oxide material, the peaks at 1350 cm−1 and 1590 cm−1 represent the D band and G band, and the intensity of the Id/Ig ratio is 0.63. Following the modification of Ni metal on the graphene oxide surface to form Ni nanoparticles on reduced graphene oxide, the peak positions of the D and G bands remain constant. However, there is an increase in the intensity of the Id/Ig ratio from 0.63 to 0.67.
SEM images of the (a) GO and (b) Ni(0)RGO samples are shown in ESI Fig. S2.† The SEM images give confirmation of the layered structure of graphene oxide. The SEM/EDX and color mapping images of the Ni(0)RGO sample are illustrated in ESI Fig. S3.† This technique gives information about the type of elements present on the material. In this color mapping image, elements like C, Ni, and O are present on the catalyst surface, and are indicated by different colors.
TEM images and electron diffraction patterns of the Ni nanoparticles are shown in Fig. 2. The TEM images provide information about the Ni nanoparticles. The electron diffraction pattern of the three planes, (111), (200), and (220), clearly shows the features of the Ni particles, which resemble the d-spacing of the Ni phases as obtained from the powder X-ray diffraction study. A particle size histogram of the Ni nanoparticles is shown in ESI Fig. S4.† From this histogram, we calculated the average particle size of the Ni nanoparticles as about 11 nm, and these particles were present on the surface of the reduced graphene material. A high resolution image (HRTEM) of the Ni(0)RGO catalyst is shown in ESI Fig. S5.† This HRTEM image (Fig. S5†) provides information about the interplanar spacing of the lattice fringes. The interplanar spacing (d) value was measured to be 0.202 nm, which represents the (111) plane of the Ni crystals.
 | ||
| Fig. 2 TEM image of (a) Ni(0)RGO (scale bar = 100 nm), (b) high magnification image of Ni(0)RGO (scale bar = 20 nm), and (c) electron diffraction pattern image of Ni(0)RGO. | ||
C–C coupling reactions have been reported by many researchers under inappropriate conditions and using highly expensive mono-metals like Pd, Au, and Pt, and bimetal combinations like Pd–Pt, Pd–Au, and Pt–Au as catalysts. Molnár and Papp reported that a C–C cross-coupling reaction over a Pd-modified montmorillonite material at 150 °C gave 92% yield after reacting for 2–3 hours.35 Corral et al. reported that 35% conversion was achieved in the presence of organic solvent after 3 h.36 There are very few examples that use inexpensive metals, like Ni, as a catalyst for this C–C coupling reaction. Bhowmik et al. reported that a C–C coupling reaction with a Ni/RGO catalyst gave 91% yield at 60 °C after reacting for 4 h.37 Sengupta et al. reported that a Ni/RGO catalyst produced a C–S coupling product at 92% yield in 3 h at 100 °C.38 The inbuilt drawback of these studies is the need for either an organic solvent or a high temperature.
So, in this communication, we report Ni(0)-modified reduced graphene oxide as a catalyst to improve the yield of this coupling reaction under suitable and appropriate conditions. For the initial study, without the use of the catalyst, the C–C Kumada–Corriu cross-coupling reaction was examined using iodoanisole and phenyl magnesium chloride in the presence of a tetrahdrofuran medium at 60 °C; no methoxybiphenyl product was observed. The same reaction was run under similar conditions using the Ni(0)RGO catalyst, which produced an excellent yield (92%) of methoxybiphenyl (Table 1). Table 1 indicates that the C–C cross-coupling reaction with the RGO catalyst and Ni(II)RGO showed a poorer performance compared to that conducted with the Ni(0)RGO catalyst.
| Catalyst | Time (h) | Temp. (°C) | Yield (%) |
|---|---|---|---|
| a 4-Iodoanisole (1 mmol), catalyst (0.1 mmol), PhMgCl (1.8 mmol), solvent (2 mL), under N2. | |||
| Without catalyst | 5 | 60 | — |
| RGO | 5 | 60 | 9 |
| Ni(II)RGO | 5 | 60 | 62 |
| Ni(0)RGO | 5 | 60 | 92 |
To assess the method, we carried out C–C coupling reactions with different substituents of aryl iodide moieties under similar conditions in the presence of the Ni(0)RGO catalyst, as detailed in Table 2. The product yields indicate that para iodobenzene gives a better yield than ortho iodobenzene. Generally, C–C coupling reactions proceed via three steps: oxidative addition, transmetallation, and reductive elimination.39,40 In the oxidative addition step, the organo halide reacts with Ni(0) to form a Ni(II) complex. In the transmetallation step, the aryl group of the Grignard reagent reacts with the Ni(II) complex to form an Ar–Ni–Ar–(O–CH3) complex and a magnesium iodide salt. In reductive elimination step, the coupling product is formed. Out of the two substituents, the para substituent is more favoured than the ortho substituent, due to steric hindrance.
| Sl no. | Aryl halide (ArX) | Grignard reagent | Product | Yield (%) |
|---|---|---|---|---|
| a Iodoanisole (1 mmol), catalyst (0.1 mmol), PhMgCl (1.8 mmol), solvent (2 mL), under N2, time (5 h), temperature (60 °C). | ||||
| 1 |
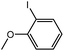
|
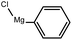
|
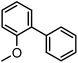
|
71 |
| 2 |
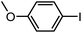
|
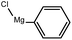
|
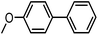
|
92 |
In order to optimize the reaction, we carried out the Kumada cross-coupling reaction at different temperatures under similar conditions (Fig. 3). The yield of the methoxybiphenyl coupling product increases from 78 to 92% with an increase in the temperature from room temperature to 60 °C. The yield of the coupling product did not increase with further increases in the temperature from 60 °C to 70 °C. Thus, the optimum reaction temperature is 60 °C for this coupling reaction using the Ni(0)RGO catalyst.
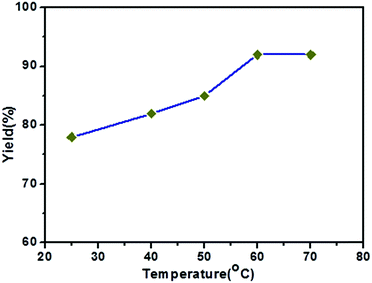 | ||
| Fig. 3 Effect of reaction temperature on the C–C Kumada–Corriu cross-coupling reaction when using the Ni(0)RGO catalyst. | ||
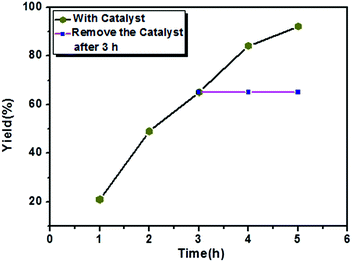
The C–C Kumada–Corriu cross-coupling reaction performance of the Ni(0)@RGO catalyst was evaluated and compared with that of other Ni catalysts in ESI Table S1.† As can be seen from the data, the proposed Ni(0)@RGO catalyst proves to be superior, with a better yield compared to other methods using Ni/RGO-40 and NiCl2(dppf) catalysts.40 The heterogeneity test for the catalyst was carried out under the same reaction conditions. The coupling reaction was stopped after 3 h, the catalyst was filtered, and then the reaction was continued for the remainder of the reaction time (Fig. 4). In Fig. 4, the pink horizontal line shows the constant yield of the coupling product. Hence, no reaction occurred in the absence of the catalyst, and the reaction only happens in presence of heterogeneous catalyst composite.
For commercialization purposes, the recovered catalyst was regenerated, followed by washing with solvent several times, and drying at the same temperature. The used catalyst was tested under the same reaction conditions several times (ESI Fig. S6†). The catalyst was active up to the 5th cycle. In the 6th cycle, the yield decreased by up to 13% due to leaching of the metal particles from the support surface.
In conclusion, Ni nanoparticles, with an average particle size of 12 nm, supported on reduced graphene oxide were successfully prepared by a simple procedure. The material demonstrated excellent catalytic activity (92% yield), good stability, and reusability for the Kumada–Corriu cross-coupling reaction. The absence of Ni(OH)2 and NiO particles and the presence of Ni(0) particles was confirmed by XRD analysis. TEM analysis confirmed that the Ni nanoparticles, with an average particle size of 11 nm, were uniformly distributed over the reduced graphene oxide surface. The catalyst also displayed good stability during recycling tests.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support received from the School of Chemistry & Physics and the College of Agriculture, Engineering & Science, University of KwaZulu-Natal, Durban, South Africa in the form of research facilities and financial support.Notes and references
- B. F. Machado and P. Serp, Catal. Sci. Technol., 2012, 2, 54–75 RSC.
- L. T. Qu, Y. Liu, J. B. Baek and L. Dai, ACS Nano, 2010, 4, 1321–1326 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- S. Rana, S. Maddila and S. B. Jonnalagadda, Catal. Sci. Technol., 2015, 5, 3235–3241 RSC.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- G. B. B. Varadwaj and V. O. Nyamori, Nano Res., 2016, 9, 3598–3621 CrossRef CAS.
- S. Rana, S. Maddila, K. Yalagala and S. B. Jonnalagadda, Appl. Catal., A, 2015, 505, 539–547 CrossRef CAS.
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. R. Peres and A. K. Geim, Science, 2008, 320, 1308 CrossRef CAS PubMed.
- L. T. Qu, Y. Liu, J. B. Baek and L. Dai, ACS Nano, 2010, 4, 1321–1326 CrossRef CAS PubMed.
- P. T. Yin, S. Shah, M. Chhowalla and K. Le, Chem. Rev., 2015, 115(7), 2483–2531 CrossRef CAS PubMed.
- R. Saravanan, E. Sacari, F. Gracia, M. M. Khan, E. Mosquera and V. K. Gupta, J. Mol. Liq., 2016, 221, 1029–1033 CrossRef CAS.
- S. Rajendran, M. M. Khan, F. Gracia, J. Qin, V. K. Gupta and S. Arumainathan, Sci. Rep., 2016, 6, 31641 CrossRef CAS PubMed.
- R. Saravanan, S. Karthikeyan, V. K. Gupta, G. Sekaran, V. Narayanan and A. Stephen, Mater. Sci. Eng. C, 2013, 33, 91–98 CrossRef CAS PubMed.
- R. Saravanan, M. M. Khan, V. K. Gupta, E. Mosquera, F. Gracia, V. Narayanan and A. Stephen, RSC Adv., 2015, 5, 34645–34651 RSC.
- M. Ghaedi, S. Hajjati, Z. Mahmudi, I. Tyagi, S. Agarwa, A. Maity and V. K. Gupta, Chem. Eng. J., 2015, 268, 28–37 CrossRef CAS.
- A. Asfaram, M. Ghaedi, S. Agarwal, I. Tyagi and V. K. Gupta, RSC Adv., 2015, 5, 18438–18450 RSC.
- Z. Tang, S. Shen, J. Zhuang and X. Wang, Angew. Chem., Int. Ed., 2010, 49, 4603–4607 CrossRef CAS PubMed.
- E. Yoo, T. Okada, T. Akita, M. Kohyama, I. Honma and J. Nakamura, J. Power Sources, 2011, 196, 110–115 CrossRef CAS.
- S. Sharma, A. Ganguly, P. Papakonstantinou, X. Miao, M. Li, J. L. Hutchison, M. Delichatsios and S. Ukleja, J. Phys. Chem. C, 2010, 114, 19459–19466 CrossRef CAS.
- A. R. Siamaki, A. E. R. S. Khder, V. Abdelsayed, M. S. El-Shall and B. F. Gupton, J. Catal., 2011, 279, 1–11 CrossRef CAS.
- M. Bayati, J. M. Abad, R. J. Nichols and D. J. Schiffrin, J. Phys. Chem. C, 2010, 114, 18439–18448 CrossRef CAS.
- Y. Li, X. B. Fan, J. J. Qi, J. Y. Ji, S. L. Wang, G. L. Zhang and F. B. Zhang, Mater. Res. Bull., 2010, 45, 1413–1418 CrossRef CAS.
- C. Sarkara and S. K. Dolui, RSC Adv., 2015, 5, 60763–60769 RSC.
- H. Zhao, G. Mao, H. Han, J. Song, Y. Liu, W. Chu and Z. Sun, RSC Adv., 2016, 6, 41108–41113 RSC.
- N. Hussain, P. Gogoi, P. Khared and M. R. Das, RSC Adv., 2015, 5, 103105–103115 RSC.
- A. Kamal, V. Srinivasulu, J. N. S. R. C. Murty, N. Shankaraiah, N. Nagesh, T. Srinivasa Reddy and A. V. Subba Rao, Adv. Synth. Catal., 2013, 355, 2297–2307 CrossRef CAS.
- F. Diederich, P. J. Stang and R. R. Tykwinski, Acetylene Chemistry: Chemistry, Biology, and Material Science, Wiley-VCH, Weinheim, 2005 Search PubMed.
- S. Atobe, M. Sonoda, Y. Suzuki, H. Shinohara, T. Yamamoto and A. Ogawa, Chem. Lett., 2011, 40, 925–927 CrossRef CAS.
- K. Sonogashira, J. Organomet. Chem., 2002, 653, 46–49 CrossRef CAS.
- S. Mouss, A. R. Siamak, B. Frank Gupton and M. Samy El-Shall, ACS Catal., 2012, 2(1), 145–154 CrossRef.
- J. Durand, E. Teuma and M. Gómez, Eur. J. Inorg. Chem., 2008, 23, 3577–3586 CrossRef.
- T. P. N. Tran, A. Thakur, D. X. Trinh, A. T. N. Dao and T. Taniike, Appl. Catal., A, 2017, 549, 60–67 CrossRef.
- Y. J. Li, W. Gao, L. J. Ci, C. M. Wang and P. M. Ajayan, Carbon, 2010, 48, 1124–1130 CrossRef CAS.
- H.-K. Jeong, Y. P. Lee, R. J. W. E. Lahaye, M.-H. Park, K. H. An, I. J. Kim, C.-W. Yang, C. Y. Park, R. S. Ruoff and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 1362–1366 CrossRef CAS PubMed.
- Á. Molnár and A. Papp, Synlett, 2006, 18, 3130–3134 CrossRef.
- J. A. Corral, M. I. López, D. Esquivel, M. Mora, S. Jiménez-Sanchidrián and F. J. Romero-Salguero, Materials, 2013, 6, 1554–1565 CrossRef CAS PubMed.
- K. Bhowmik, D. Sengupta, B. Basu and G. De, RSC Adv., 2014, 4, 35442–35448 RSC.
- D. Sengupta, K. Bhowmik, G. De and B. Basu, Beilstein J. Org. Chem., 2017, 13, 1796–1806 CrossRef CAS PubMed.
- A. C. Frisch and M. s. Beller, Angew. Chem., Int. Ed., 2005, 44, 674–688 CrossRef CAS PubMed.
- B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346–1416 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8na00245b |
| This journal is © The Royal Society of Chemistry 2019 |