Inherently self-sterilizing charged multiblock polymers that kill drug-resistant microbes in minutes†
Bharadwaja S. T.
Peddinti
a,
Frank
Scholle
b,
Mariana G.
Vargas
a,
Steven D.
Smith
c,
Reza A.
Ghiladi
 *d and
Richard J.
Spontak
*d and
Richard J.
Spontak
 *ae
*ae
aDepartment of Chemical & Biomolecular Engineering, North Carolina State University, Raleigh, NC 27695, USA. E-mail: rich_spontak@ncsu.edu
bDepartment of Biological Sciences, North Carolina State University, Raleigh, NC 27695, USA
cCorporate Research & Development, The Procter & Gamble Company, Cincinnati, OH 45224, USA
dDepartment of Chemistry, North Carolina State University, Raleigh, NC 27695, USA. E-mail: reza_ghiladi@ncsu.edu
eDepartment of Materials Science & Engineering, North Carolina State University, Raleigh, NC 27695, USA
First published on 17th July 2019
Abstract
Drug-resistant microbes loom as a growing threat to global healthcare by greatly increasing the risk of hospital-acquired infections that could ultimately become fatal, especially for elderly, injured and immune-compromised patients. As a consequence, several materials-related antimicrobial strategies have been developed to mitigate this ubiquitous concern, resulting in different levels of success and, in some cases, introducing deleterious complications to environmental safety. Here, we demonstrate that charged multiblock polymers wherein the midblock is selectively sulfonated, and therefore hydrophilic and water-swellable, inherently provide self-sterilizing surfaces that rapidly act (killing more than 99.9999% in just 5 min) against a wide range of Gram-positive and -negative bacteria, three of which are antibiotic-resistant. This surprising response, which depends on the degree of midblock sulfonation, is attributed to a dramatic reduction in surface pH level that is remarkably effective against microbes with a typically anionic outer membrane. These polymers, suitable for use in biomedical applications, smart textiles, separation membranes, commodity fixtures, and food packaging, are equally effective against infectious virus strains. Although the antimicrobial efficacy of these polymers is progressively diminished through complexation of sulfonic acid groups with cationic species during cyclic exposure to electrolyte solutions, these thermoplastic elastomers can be fully rejuvenated to their maximum performance level by relatively short immersion in acidic solutions. As a highly promising addition and alternative design paradigm to the expanding arsenal of antimicrobial materials, these midblock-sulfonated multiblock polymers constitute a facile, inexpensive, comprehensive, and environmentally-benign preventative route by which to combat the worldwide proliferation of drug-resistant microbes.
New conceptsThe increasing threat of drug-resistant pathogens is attracting the attention of the worldwide healthcare community. While interest in self-sterilizing materials is growing, most rely on small improvements in mature methodologies. Here, we investigate the antimicrobial properties of midblock-sulfonated multiblock polymers and demonstrate that these self-organizing amphiphilic (anionic) materials are inherently antibacterial and antiviral without any additives. The major advances reported include (i) inactivation of Gram-positive/negative bacteria, including three drug-resistant strains, to the detection limit of 99.9999% within 5 min, (ii) inactivation of three viruses again to 99.9999% in 5 min, and (iii) mechanistic studies that establish that the low local pH achievable with these materials, which remain mechanically stable as the hydrophilic matrix hydrates while the endblocks serve as physical cross-links, is responsible for the observed antimicrobial efficacy. Discussions with experts regarding anionic polymers capable of inherently killing such a wide variety of pathogens indicate that the combination of broad efficacy and rapid potency exhibited by the present materials is novel. We are excited about this discovery and hope that the biomedical and polymer fields can benefit from our design paradigm to produce stand-alone or coating materials that provide proactive care through prevention for the elderly, immune-compromised or injured, especially in impoverished global communities. |
Drug resistance among infectious pathogens is increasing at an alarming rate around the world and is generating global concern regarding the future of public healthcare, particularly in underdeveloped countries.1 While many medical treatments based on antibiotics and antivirals target specific chemical functionalities to induce microbiocide, several microbes have evolved and developed resistance mechanisms that can compromise such treatment.2 Examples of “nightmare” pathogens, or so-called3 “superbugs,” include methicillin-resistant (MR) Staphylococcus aureus (S. aureus), often referred to as MRSA, vancomycin-resistant (VR) Enterococcus faecium (E. faecium) and carbapenem-resistant Acinetobacter baumannii (A. baumannii).4,5 The worldwide proliferation of drug-resistant infectious microbes has been further exacerbated by medical misdiagnosis, antibiotic overuse, and poor prevention. A symptom of this growing medical crisis is the increasing propensity of healthcare-associated infections (HAIs) that largely threaten elderly, injured and immune-compromised patients in medical and nursing facilities.6 According to the U.S. Centers for Disease Control and Prevention (CDC), ≈1.7 million HAI cases are estimated to occur in the U.S. annually, leading to a 5–6% mortality rate of those affected. At least 23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 37
000 and 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths are attributed to drug-resistant pathogens alone each year in the U.S. and Europe, respectively,7 and these casualty numbers are expected to increase so significantly that, by 2050, deaths due to drug-resistant microbes are predicted to outnumber those caused by cancer.8 In addition to the tragic loss of life, HAIs also introduce an enormous financial burden on healthcare, estimated to vary from $28–45 billion annually.6 In response to public distress associated with infectious diseases, various strategies have been developed to sterilize surfaces, since the transmission of potentially harmful bacteria and viruses lying dormant on surfaces frequently promotes the spread of disease.
000 deaths are attributed to drug-resistant pathogens alone each year in the U.S. and Europe, respectively,7 and these casualty numbers are expected to increase so significantly that, by 2050, deaths due to drug-resistant microbes are predicted to outnumber those caused by cancer.8 In addition to the tragic loss of life, HAIs also introduce an enormous financial burden on healthcare, estimated to vary from $28–45 billion annually.6 In response to public distress associated with infectious diseases, various strategies have been developed to sterilize surfaces, since the transmission of potentially harmful bacteria and viruses lying dormant on surfaces frequently promotes the spread of disease.
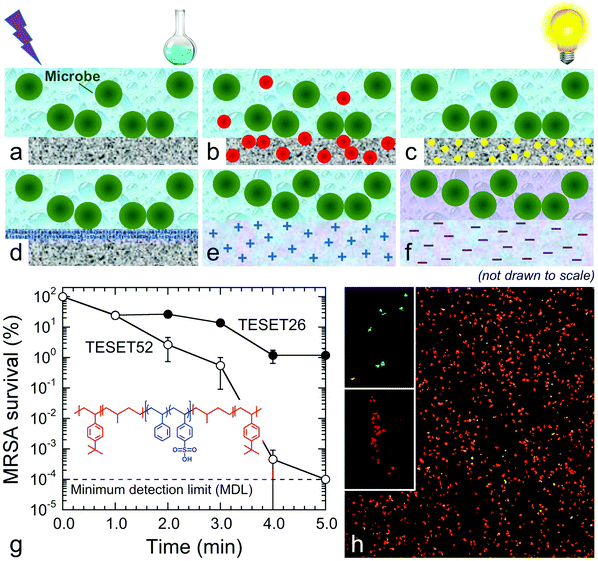
Several of these microbiocidal approaches are illustrated in Fig. 1. In Fig. 1a, for instance, a surface is sterilized by repeated exposure to either radiation9 (e.g., UV light) or a chemical disinfectant10 (e.g., bleach, hydrogen peroxide or a detergent), which can damage the surface, adversely affect the environment or introduce additional health concerns. The approach depicted in Fig. 1b employs metal (e.g., Ag or Cu) or metal oxide (e.g., ZnO or TiO2) nanoparticles or salts11–14 that are embedded inside the matrix, or on the surface, of a polymeric substrate. Although these metals act as toxins to which bacteria can become resistant,15–17 this approach suffers from the likelihood that nanoscale metal can leach into the environment and contaminate the food chain or directly enter into higher-level organisms at the subcellular level.18Fig. 1c displays the principle of photodynamic inactivation, in which photosensitive molecules are introduced into a polymer and are subsequently excited by noncoherent visible light in the presence of molecular oxygen to generate singlet oxygen (1O2), an effective and sustainable microbiocide.19,20 Moreover, since 1O2-induced termination is nonspecific, microbes cannot develop chemical resistance. Some polymers are naturally antibacterial or can be chemically functionalized (or modified with a surface-grown polymer brush21) so that the surface becomes antibacterial (see Fig. 1d). Since the corresponding mechanism of disinfection relies principally on electrostatic interaction between cationic species on the polymer and anionic species on the microbe membrane to induce membrane rupture,22–24 this approach applies mainly to bacteria and can be largely negated by surface abrasion during application. Similarly, as schematically portrayed in Fig. 1e, cationic species can be introduced into soft hydrogels that swell in the presence of the aqueous microbial suspension25–27 or bulk materials.28–30 Unlike conventional plastic surfaces, however, hydrogels are often mechanically weak and prone to cohesive failure.
In this work, we report that mechanically robust anionic materials produced by selective hydration of midblock-sulfonated multiblock polymers (see Fig. 1f) provides a surprisingly effective and natural alternative to antimicrobial surfaces. The polymer of primary interest is a thermoplastic elastomeric poly[tert-butylstyrene-b-(ethylene-alt-propylene)-b-(styrenesulfonate)-b-(ethylene-alt-propylene)-b-tert-butylstyrene] (TESET) pentablock polymer that spontaneously self-assembles into a nanostructured substrate due to thermodynamic incompatibility between the contiguous sequences. The morphology of this unique network-forming material can be chemically templated during solvent casting and subsequently annealed in solvent vapor to alter or refine the as-cast morphology.31 Previous studies have established that this amphiphilic polymer can be used for water purification,32 gas separation33,34 and organic photovoltaics.35 Details regarding the molecular characteristics of the polymer are specified elsewhere,31 and the degree of midblock sulfonation examined here ranges from 26 mol% (TESET26) to 52 mol% (TESET52). Due to the presence of sulfonic acid groups along the styrenic midblock, the aqueous medium in microbes and/or their suspensions simultaneously promotes polymer hydration and reduces the pH. The effect of pH on bacteria has been extensively studied, and bacteria are classified according to their preferred pH range: acidophiles (1.0–5.5), neutrophiles (5.5–8.0) and alkalophiles (>8.0). Although bacteria can thrive in environments differing in pH, they tend to maintain a neutral interior irrespective of the external pH. A sudden change in pH, however, promotes stress on the outer membrane and, if sufficiently drastic, destroys the membrane, resulting in enzyme damage, protein denaturation and microbe death.36 We explore this microbiocidal strategy, with experimental details regarding material and microbe preparation/analysis provided in the ESI.†
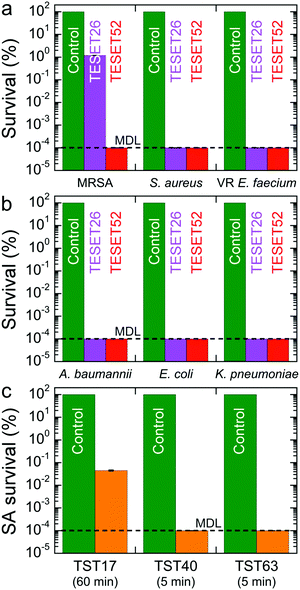
The time-dependent survivability of MRSA suspended in phosphate buffer saline (PBS) solution upon exposure to the TESET polymers is presented in Fig. 1g. Although both polymers display evidence of antibacterial properties from plaque assays, the more highly sulfonated TESET52 polymer is more effective (p < 0.0001) than TESET26 (p = 0.0005) after 3 min, reaching the minimum detection limit (MDL) of 10−4% survival (99.9999% inactivation; i.e., no colony formation observed) in just 5 min. For this reason and unless stated otherwise, the exposure time in the following results is held constant at 5 min. Included in Fig. 1g is the chemical structure of the TESET polymer, wherein the hydrophilic and hydrophobic blocks are color-coded. Evidence of bacterial death is likewise apparent from the confocal laser scanning microscopy (CLSM) images provided in Fig. 1h. In these fluorescent images, methicillin-susceptible S. aureus has been stained to differentiate live (green) and dead (red) controls. The appearance of primarily dead bacteria after exposure to TESET52 for 5 min confirms the antibacterial effectiveness of this material. While commercial SES thermoplastic elastomers (in which E refers to an ethylene-containing polyolefin block) are ubiquitous in a wide range of diverse technologies, their endblock-sulfonated variants do not exhibit antimicrobial properties because, upon self-assembly into nanostructures, the sulfonated endblocks become isolated in a hydrophobic matrix. Fully sulfonated polystyrene, on the other hand, is soluble in aqueous media and dissolves in aqueous solution. The uniquely sulfonated midblocks of the TESET polymer allow considerable swelling in the presence of liquid water—≈170% at 23 °C, but over 1000% at 70 °C, for TESET52 cast from THF37—so that the nanostructured polymer behaves as an anionic, reversible (physical) hydrogel in which swollen midblocks are connected to glassy nanodomains that effectively serve as semi-permanent cross-links.
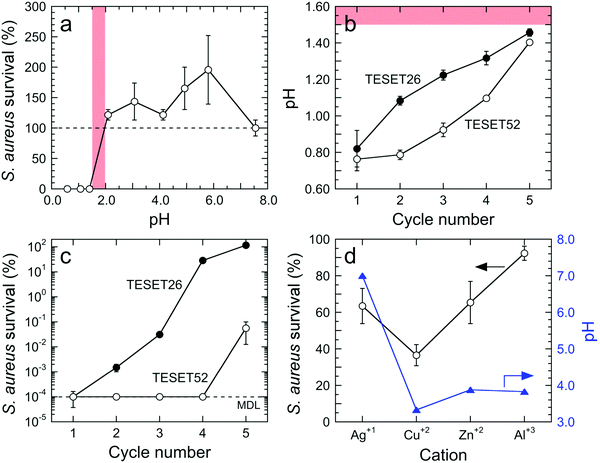
The results displayed for both Gram-positive and -negative bacteria in Fig. 2a and b, respectively, evince the comprehensive antibacterial nature of the TESET polymers. In the case of methicillin-susceptible S. aureus in Fig. 2a (as well as MRSA in Fig. 1g), TESET52 is capable of inactivating 99.9999% of the bacteria within 5 min (p < 0.04), whereas TESET26 with lower midblock sulfonation is significantly more effective at killing methicillin-susceptible S. aureus than MRSA over the same exposure time. Despite their difference in midblock sulfonation, both polymers are equally capable of inactivating VR E. faecium to the MDL after 5 min (see Fig. 2a). Additional results acquired for three Gram-negative bacteria—A. baumannii, Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E. coli)—are included for TESET26 and TESET52 in Fig. 2b and reveal that both polymers are equally effective at inactivating 99.9999% of each bacterial strain. Four strains examined in Fig. 2a and b belong to the ESKAPE classification of common bacteria responsible for nosocomial infections (E. faecium, S. aureus, K. pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Enterobacter species). To confirm the antimicrobial property of the commercial TESET polymers and explore the role of midblock sulfonation, a complementary TST polymer has been synthesized and chemically modified to three different degrees of midblock sulfonation (in mol%): 17 (TST17), 40 (TST40) and 63 (TST63). Details regarding the synthesis and sulfonation of this polymer are available elsewhere.38 The survival of S. aureus on each of these polymers is displayed in Fig. 2c. After 5 min, the TST40 and TST63 polymers reduce the bacterial population to the MDL. In contrast, the inactivation level of S. aureus exposed to TST17 after 60 min is lower, measured as 99.96% (p = 0.0069).
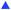
Our findings provided in Fig. 2 indicate that (i) Gram-negative bacteria appear to be more susceptible to inactivation due to midblock sulfonation and (ii) a minimum degree of midblock sulfonation between 26 and 40 mol% is needed to achieve maximum inactivation (to the MDL) of all the bacteria examined. Since these functionalized polymers contain sulfonic acid groups, we posit that an abrupt and drastic change in pH level is responsible for the observed antibacterial inactivation. The pH sensitivity of S. aureus on the control surface is presented in Fig. 3a and establishes that this bacterial strain perishes at pH levels below 1.5, but propagates at pH levels between 2.0 and 8.0. These results therefore identify a clear transition in bacterial response to pH between pH ≈ 1.5 and pH ≈ 2.0. Repeated exposure of both TESET polymers to PBS solution yields the pH levels included in Fig. 3b. Initially, the two polymers promote a highly acidic environment by lowering the solution pH to about 0.8. Upon cycling and presumed complexation of dissociated cations in PBS solution with sulfonic acid groups on the polymers, the solution pH is found to increase monotonically. During the fifth cycle, the solution pH above TESET26 lies near the bacterial transition at pH ≈ 1.5. Corresponding S. aureus assays displayed as a function of TESET cycling in Fig. 3c corroborate that an increase in pH improves bacterial survival after the first cycle for TESET26 and after four cycles for TESET52. In fact, the TESET26 polymer shows no evidence of antibacterial properties during the fifth cycle, whereas the inactivation level induced by TESET52 drops to 99.94%. If the antibacterial effectiveness of these polymers is reduced upon cycling due to progressive complexation of cations from PBS solution,39 it follows that both the pH level and bacterial survival will most likely increase if the sulfonic acid groups are intentionally neutralized by the addition of metal cations.
The results provided in Fig. 3d for S. aureus confirm this expectation. In the case of Ag1+ from AgNO3, the PBS solution in contact with TESET52 becomes fully neutralized (to pH ≈ 7), but little antibacterial performance (≈36.5% inactivation compared to 99.9999% in Fig. 2) is observed, even though Ag is a well-documented antibacterial metal.11 This substantial reduction in effectiveness is attributed to nearly complete complexation of Ag1+ with the sulfonic acid groups on the polymer. In contrast, the pH levels measured for two divalent cations (Cu2+ from CuSO4 and Zn2+ from ZnCl2) and one trivalent cation (Al3+ from AlCl3) all lie between 3.0 and 4.0, indicating that complexation is incomplete. Copper, another recognized antibacterial metal,12 exhibits the highest level of inactivation (≈63.5%) of the series, whereas the efficacy of Zn is comparable to that of Ag. Aluminum, on the other hand, does not possess antibacterial properties and, upon complexation, induces negligibly little bacterial inactivation (≈7.7%). These results verify that the ability of the sulfonic acid groups on the TESET (and, by inference, TST) polymers to lower the pH of bacterial suspensions is largely, if not exclusively, responsible for rapid and dramatic bacterial inactivation. Two other considerations that must be examined relate to the possibility that polymer swelling upon exposure to PBS solution either mechanically compromises the outer bacterial membrane or draws bacteria to the low-pH polymer surface. To discern if swelling influences our findings, we have investigated the survival of MRSA after 5 min in the presence of both TESET polymers previously swollen in deionized water to their equilibrium level. While the effectiveness of TESET26 is lower (75% compared to ≈99% in Fig. 1g), the bacterial inactivation level attained by TESET52 remains unaltered, signifying that the degree of sulfonation should exceed 26 mol% to achieve reliable antibacterial properties with negligible flow-induced complications during polymer swelling.
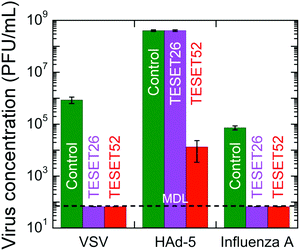
Although materials containing biocidal additives tend to be pathogen-specific, some materials40–44 exhibit broad-spectrum antimicrobial efficacy, making them suitable for use against bacteria and viruses. In this vein, Fig. 4 displays the ability of the TESET polymers to inactivate three virus species: vesicular stomatitis virus (VSV), human adenovirus-5 (HAd-5) and influenza A virus. Of these, only HAd-5 is non-enveloped (i.e., protected by a protein shell), which hinders inactivation. The results presented in Fig. 4 indicate that, after an exposure time of 5 min, the TESET polymers eradicated both enveloped viruses below the MDL of 66.7 PFU mL−1. In the case of HAd-5, however, TESET26 is altogether ineffective, whereas TESET52 successfully inactivates 99.997% of the virus population, which is comparable to that achieved by photodynamic inactivation after an exposure time of 60 min.20 Insofar as we are aware, no other polymer is inherently antiviral (we recognize that polymers can be made antiviral by the incorporation of additives). We have demonstrated that two series of midblock-sulfonated block polymers exhibit remarkably high antimicrobial efficacy due to a unique combination of (i) molecular architecture that permits water-induced swelling and physical hydrogel formation while retaining robust mechanical properties, and (ii) the ability of the hydrated sulfonic acid groups to drastically lower the pH of the bacterial suspension. The results reported herein confirm that these polymers afford a largely unexplored route to accelerated, effective and comprehensive antimicrobial materials that are activated by the simple presence of water. Although their antimicrobial effectiveness is progressively reduced upon repeated exposure to microbial suspensions, we have further observed that these materials can be fully rejuvenated by exposure to acid (with the time required depending on concentration; see Fig. S1 in the ESI†) to remove cations that have complexed with the sulfonic acid groups. Limited toxicity tests (see Fig. S2 in the ESI†) further indicate that these materials can also kill mammalian cells over the course of minutes and must be carefully regulated if they come into direct contact with live cells during use. Since these midblock-sulfonated polymers can be cast into different shapes, coated onto various surfaces and recycled, the antimicrobial strategy introduced here nonetheless provides an alternative and comprehensive approach to combat the global threat of drug-resistant infectious microbes without compromising environmental welfare.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by The Nonwovens Institute at North Carolina State University. The CLSM images were acquired at the NC State Cellular and Molecular Imaging Facility, supported by the National Science Foundation (Grant DBI-1624613). We thank Dr J. E. Flood (Kraton Polymers) for valuable discussions.References
- R. Laxminarayan and D. L. Heymann, Challenges of drug resistance in the developing world, BMJ [Br. Med. J.], 2012, 344, 25–27 CrossRef.
- R. Laxminarayan, A. Duse, C. Wattal, A. K. M. Zaidi, H. F. L. Wertheim, N. Sumpradit, E. Vlieghe, G. L. Hara, I. M. Gould, H. Goossens, C. Greko, A. D. So, M. Bigdeli, G. Tomson, W. Woodhouse, E. Ombaka, A. Q. Peralta, F. N. Qamar, F. Mir, S. Kariuki, Z. A. Bhutta, A. Coates, R. Bergstrom, G. D. Wright, E. D. Brown and O. Cars, Antibiotic resistance-the need for global solutions, Lancet Infect. Dis., 2013, 13, 1057–1098 CrossRef.
- M. Smith, How ninja polymers are fighting killer superbugs. BBC News, April 17, 2018, http://www.bbc.com/news/business-43781851.
- N. Cimolai, MRSA and the environment: implications for comprehensive control measures, Eur. J. Clin. Microbiol. Infect. Dis., 2008, 27, 481–493 CrossRef CAS.
- A. N. Neely and M. P. Maley, Survival of enterococci and staphylococci on hospital fabrics and plastic, J. Clin. Microbiol., 2000, 38, 724–726 CAS.
- R. D. Scott II, The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention, Centers for Disease Control and Prevention, Atlanta, 2009, http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf.
- B. Allegranzi, Report on the burden of endemic health care-associated infection worldwide. World Health Organization, 2011 http://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf;jsessionid=272099680942145FE65A51BE09AA426A?sequence=1.
- J. O’Neill, Tackling drug-resistant infections globally: final report and recommendation. Review on antimicrobial resistance. London, 2016, amr-review.org.
- W. A. M. Hijnen, E. F. Beerendonk and G. J. Medema, Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review, Water Res., 2006, 40, 3–22 CrossRef CAS.
- J. A. Otter and G. L. French, Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor, J. Clin. Microbiol., 2009, 47, 205–207 CrossRef CAS.
- A. P. Richter, J. S. Brown, B. Bharti, A. Wang, S. Gangwal, K. Houck, E. A. C. Hubal, V. N. Paunov, S. D. Stoyanov and O. D. Velev, An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core, Nat. Nanotechnol., 2015, 10, 815–823 CrossRef PubMed.
- N. Cioffi, L. Torsi, N. Ditaranto, G. Tantillo, L. Ghibelli, L. Sabbatini, T. Bleve-Zacheo, M. D’Alessio, P. G. Zambonin and E. Traversa, Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties, Chem. Mater., 2005, 17, 5255–5262 CrossRef CAS.
- V. B. Schwartz, F. Thétiot, S. Ritz, S. Pütz, L. Choritz, A. Lappas, R. Förch, K. Landfester and U. Jonas, Antibacterial surface coatings from zinc oxide nanoparticles embedded in poly(n-isopropylacrylamide) hydrogel surface layers, Adv. Funct. Mater., 2012, 22, 2376–2386 CrossRef CAS.
- A. Kubacka, M. S. Diez, D. Rojo, R. Bargiela, S. Ciordia, I. Zapico, J. P. Albar, C. Barbas, V. A. P. Martins dos Santos, M. Fernández-García and M. Ferrer, Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium, Sci. Rep., 2015, 4, 4134 CrossRef PubMed.
- J. J. Harrison, H. Ceri and R. J. Turner, Multimetal resistance and tolerance in microbial biofilms, Nat. Rev. Microbiol., 2007, 5, 928–938 CrossRef CAS PubMed.
- Z. Ma, F. E. Jacobsen and D. P. Giedroc, Coordination chemistry of bacterial metal transport and sensing, Chem. Rev., 2009, 109, 4644–4681 CrossRef CAS.
- K. S. Chaturvedi, C. S. Hung, J. R. Crowley, A. E. Stapleton and J. P. Henderson, The siderophore yersiniabactin binds copper to protect pathogens during infection, Nat. Chem. Biol., 2012, 8, 731–736 CrossRef CAS.
- M. C. Stensberg, Q. Wei, E. S. McLamore, D. M. Porterfield, A. Wei and M. S. Sepúlveda, Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging, Nanomedicine, 2011, 6, 879–898 CrossRef CAS.
- E. Feese, H. Sadeghifar, H. S. Gracz, D. S. Argyropoulos and R. A. Ghiladi, Photobactericidal porphyrin-cellulose nanocrystals: synthesis, characterization, and antimicrobial properties, Biomacromolecules, 2011, 12, 3528–3539 CrossRef CAS.
- B. S. T. Peddinti, F. Scholle, R. A. Ghiladi and R. J. Spontak, Photodynamic polymers as comprehensive anti-infective materials: Staying ahead of a growing global threat, ACS Appl. Mater. Interfaces, 2018, 10, 25955–25959 CrossRef CAS.
- A. E. Ozcam, K. E. Roskov, R. J. Spontak and J. Genzer, Generation of functional PET microfibers through surface-initiated polymerization, J. Mater. Chem., 2012, 22, 5855–5864 RSC.
- L. B. Rawlinson, S. M. Ryan, G. Mantovani, J. A. Syrett, D. M. Haddleton and D. J. Brayden, Antibacterial effects of poly(2-(dimethylamino ethyl)methacrylate) against selected Gram-positive and Gram-negative bacteria, Biomacromolecules, 2010, 11, 443–453 CrossRef CAS PubMed.
- G. Cheng, H. Xue, Z. Zhang, S. Chen and S. Jiang, A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities, Angew. Chem., Int. Ed., 2008, 47, 8831–8834 CrossRef CAS.
- G. Gao, K. Yu, J. Kindrachuk, D. E. Brooks, R. E. W. Hancock and J. N. Kizhakkedathu, Antibacterial surfaces based on polymer brushes: investigation on the influence of brush properties on antimicrobial peptide immobilization and antimicrobial activity, Biomacromolecules, 2011, 12, 3715–3727 CrossRef CAS.
- P. Li, Y. F. Poon, W. Li, H. Y. Zhu, S. H. Yeap, Y. Cao, X. Qi, C. Zhou, M. Lamrani, R. W. Beuerman, E. T. Kang, Y. Mu, C. M. Li, M. W. Chang, S. S. J. Leong and M. B. C. Park, A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability, Nat. Mater., 2011, 10, 149–156 CrossRef CAS.
- M. A. Aziz, J. D. Cabral, H. J. L. Brooks, S. C. Moratti and L. R. Hantona, Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use, Antimicrob. Agents Chemother., 2012, 56, 280–287 CrossRef CAS.
- L. Mi and S. Jiang, Synchronizing nonfouling and antimicrobial properties in a zwitterionic hydrogel, Biomaterials, 2012, 33, 8928–8933 CrossRef CAS PubMed.
- Z. Zhou, D. Wei, Y. Guan, A. Zheng and J. Zhong, Extensive in vitro activity of guanidine hydrochloride polymer analogs against antibiotics-resistant clinically isolated strains, Mater. Sci. Eng., C, 2011, 31, 1836–1843 CrossRef CAS.
- A. Song, S. G. Walker, K. A. Parker and N. S. Sampson, Antibacterial studies of cationic polymers with alternating, random, and uniform backbones, ACS Chem. Biol., 2011, 6, 590–599 CrossRef CAS.
- J. Hoque, P. Akkapeddi, V. Yadav, G. B. Manjunath, D. S. S. M. Uppu, M. M. Konai, V. Yarlagadda, K. Sanyal and J. Haldar, Broad spectrum antibacterial and antifungal polymeric paint materials: synthesis, structure-activity relationship, and membrane-active mode of action, ACS Appl. Mater. Interfaces, 2015, 7, 1804–1815 CrossRef CAS.
- K. P. Mineart, B. Lee and R. J. Spontak, A solvent-vapor approach towards the control of block ionomer morphologies, Macromolecules, 2016, 49, 3126–3137 CrossRef CAS.
- G. M. Geise, B. D. Freeman and D. R. Paul, Characterization of a sulfonated pentablock copolymer for desalination applications, Polymer, 2010, 51, 5815–5822 CrossRef CAS.
- L. Ansaloni, Z. Dai, J. J. Ryan, K. P. Mineart, Q. Yu, K. T. Saud, M. B. Hägg, R. J. Spontak and L. Deng, Solvent-templated block ionomers for base- and acid-gas separations: effect of humidity on ammonia and carbon dioxide permeation, Adv. Mater. Interfaces, 2017, 4, 1700854 CrossRef.
- Z. Dai, L. Ansaloni, J. J. Ryan, R. J. Spontak and L. Deng, Incorporation of an ionic liquid into a midblock-sulfonated multiblock polymer for CO2 capture, J. Membr. Sci., 2019, 588, 117193 CrossRef CAS.
- H. A. Al-Mohsin, K. P. Mineart and R. J. Spontak, Highly flexible aqueous photovoltaic elastomer gels derived from sulfonated block ionomers, Adv. Energy Mater., 2015, 5, 1401941 CrossRef.
- P. Lund, A. Tramonti and D. D. Biase, Coping with low pH: molecular strategies in neutralophilic bacteria, FEMS Microbiol. Rev., 2014, 38, 1091–1125 CrossRef CAS.
- K. P. Mineart, J. D. Dickerson, D. M. Love, B. Lee, X. Zuo and R. J. Spontak, Hydrothermal conditioning of physical hydrogels prepared from a midblock-sulfonated multiblock copolymer, Macromol. Rapid Commun., 2017, 38, 1600666 CrossRef.
- K. P. Mineart, J. J. Ryan, B. Lee, S. D. Smith and R. J. Spontak, Molecular and morphological characterization of midblock-sulfonated styrenic triblock copolymers, J. Polym. Sci., Part B: Polym. Phys., 2017, 55, 490–497 CrossRef CAS.
- While the PBS solution used to perform the inactivation experiments in this study contains numerous cations that can complex with the sulfonic acid groups on the polymer, naturally hydrated live pathogens (alone or suspended in water) activate the polymer surface without adversely affecting it due to the low concentration of cations available. The surfaces of these polymers remain fully active insofar as ion complexation is minimized or altogether avoided. Even if complexation does occur, however, it can be reversed to recover full antimicrobial efficacy (see the ESI†).
- D. A. Salick, D. J. Pochan and J. P. Schneider, Design of an injectable β-hairpin peptide hydrogel that kills methicillin-resistant Staphylococcus aureus, Adv. Mater., 2009, 21, 4120–4123 CrossRef CAS.
- Y. He, E. Heine, N. Keusgen, H. Keul and M. Möller, Synthesis and characterization of amphiphilic monodisperse compounds and poly(ethylene imine)s: influence of their microstructures on the antimicrobial properties, Biomacromolecules, 2012, 13, 612–623 CrossRef CAS.
- K. E. S. Locock, T. D. Michl, J. D. P. Valentin, K. Vasilev, J. D. Hayball, Y. Qu, A. Traven, H. J. Griesser, L. Meagher and M. Haeussler, Guanylated polymethacrylates: a class of potent antimicrobial polymers with low hemolytic activity, Biomacromolecules, 2013, 14, 4021–4031 CrossRef CAS.
- L. Schnaider, S. Brahmachari, N. W. Schmidt, B. Mensa, S. Shaham-Niv, D. Bychenko, L. Adler-Abramovich, L. J. W. Shimon, S. Kolusheva, W. F. DeGrado and E. Gazit, Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity, Nat. Commun., 2017, 8, 1365 CrossRef PubMed.
- H. Meng, P. K. Forooshani, P. U. Joshi, J. Osborne, X. Mi, C. Meingast, R. Pinnaratip, J. Kelley, A. Narkar, W. He, M. C. Frost, C. L. Heldt and B. P. Lee, Biomimetic recyclable microgels for on-demand generation of hydrogen peroxide and antipathogenic application, Acta Biomater., 2019, 83, 109–118 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mh00726a |
| This journal is © The Royal Society of Chemistry 2019 |