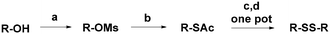Synthesis and preliminary evaluation of the anti-cancer activity on A549 lung cancer cells of a series of unsaturated disulfides†
Fabrizio
Olivito
 *ad,
Nicola
Amodio
b,
Maria Luisa
Di Gioia
c,
Monica
Nardi
de,
Manuela
Oliverio
*ad,
Nicola
Amodio
b,
Maria Luisa
Di Gioia
c,
Monica
Nardi
de,
Manuela
Oliverio
 a,
Giada
Juli
b,
Pierfrancesco
Tassone
b and
Antonio
Procopio
a,
Giada
Juli
b,
Pierfrancesco
Tassone
b and
Antonio
Procopio
 a
a
aDipartimento di Scienze della Salute, Università Magna Græcia, Viale Europa, Germaneto, Catanzaro, 88100, Italy. E-mail: fabrizioolivito@gmail.com
bDipartimento di Medicina Sperimentale e Clinica, Università Magna Græcia, Viale Europa, Germaneto, Catanzaro, 88100, Italy
cDipartimento di Farmacia e Scienze della Salute e della Nutrizione, Università della Calabria, Arcavacata di Rende, Cosenza, 87030, Italy
dDipartimento di Chimica, Università della Calabria, Cubo 12C, Arcavacata di Rende, Cosenza, 87030, Italy
eDipartimento di Agraria, Università Telematica San Raffaele, Via di Val Cannuta, 247, Roma, 00166, Italy
First published on 5th December 2018
Abstract
We synthesized a series of small symmetrical unsaturated disulfides by a multi-step reaction starting from organic alcohols, and we performed a preliminary test to evaluate the effect of these compounds on the viability of A549 lung cancer cells. The garlic-derived natural compound diallyl disulfide, known for its anticancer activity, was used as the lead compound in this study. We synthesized five DADS analogues having different carbon chain lengths and different positions of the double bonds. Two analogues exhibited a promising antitumor activity in vitro, and the allylic double bond did not seem to be the main driving force.
Design, synthesis, and pharmacological assays of new synthetic organic compounds are a common practice within today's scientific community.1,2 Natural compounds present a wide source of structurally and biologically interesting molecules.3,4 In the past few decades, many efforts have been conducted to rationalize the activity of a wide range of natural molecules and to mimic them using synthetic chemistry.5,6 For example, Allium genus plants have been studied for centuries, due to their chemical complexity and their unbelievable pharmacological potential, which still makes this genus fascinating.7,8 Garlic and onion represent its most important species, with tons of them being consumed worldwide mostly for food or related products.9 These and other Allium genus plants are typically characterized by the presence of different organosulfur compounds, many of which still remain unknown. These molecules are only produced when these plants are cut or chopped.10 Organic disulfides constitute one of the most abundant classes of molecules; for this reason, it is likely that they provide an important contribution to their health-benefiting effect.11 Allicin, which belongs to the class of organic thiosulfinates, is one of the most studied out of all kinds of garlic molecules, with proven antimicrobial, antioxidant and anticancer activities.12–14 The first report of the in vivo anticancer activity of this molecule can be dated back to 1958.15 In 2001, a study was conducted about the in vitro anticancer activity of pure allicin on different cancer cell lines.16 In a recent study, allicin was identified as an active anticancer compound present in aqueous garlic extracts; the activity was determined by an MTT cell viability assay on Mus musculus colon carcinoma cells CT26.WT.17 One of the limitations of this molecule is its instability at moderate temperature and under light sources, as well as in biological fluids.18 The main by-product of allicin decomposition is a small molecule called diallyl disulfide (DADS). Hence, it is another molecule that is abundantly detected in garlic extracts. With respect to allicin, DADS has greater stability and preserves many pharmacological activities.19–21 Recent studies were focused on its multi-target action against different types of cancer.22,23 This small molecule produces antiproliferative effects by inducing inhibition of cell proliferation and apoptosis with cell cycle arrest at the G0/G1 or G2/M phase.24 Moreover, DADS induces cell cycle arrest at the G2/M phase and apoptosis in human A549 lung cancer cells in a time- and dose-dependent manner. The increase of intracellular reactive oxygen species (ROS) after treatment was indicated as one of the most important triggering factors.25 Beyond the in vitro tests, DADS reduces carcinogen-induced cancers in experimental animals, as reported by Xia et al. in a recent review.26 Unfortunately, this molecule is sold at just 80% purity due to the presence of other sulfides that can affect its pharmacological activities. In this work, we synthesized the natural molecule DADS as the lead compound and a series of symmetrical unsaturated disulfides with different lengths of the carbon chains and different positions of the double bonds, via a multistep reaction (Scheme 1).
Recently, our group developed a new methodology to synthesize organic thioacetates, particularly unsaturated thioacetates, by an environmentally-friendly reaction in aqueous media, starting with alcohols (Table 1).27 Green procedures can be related to the synthesis of pharmacologically active molecules by different strategies.28 In this work, unsaturated thioacetates represent the starting point in the process of obtaining symmetrical unsaturated disulfides in a one-pot procedure, passing from thiols as the intermediate.29 The garlic compound diallyl disulfide (DADS) was freshly prepared with an acceptable level of purity (>95% by NMR) (entry 1). This and other unsaturated compounds play an important role in the pharmacological activity of this type of plant.30,31 Many studies have proved the stronger activity of unsaturated compounds with respect to saturated ones.23 In this direction, we synthesized similar allylic disulfides B, M, and D, only with different carbon chain lengths (entries 3–5). Starting from trans-5-hexen-1-ol, we stereospecifically obtained the newly synthesized trans isomer (E)-1-[[(E)-hex-2-henyl]disulfanyl]hex-2-ene D (entry 5). Starting from cis-5-hexen-1-ol, we obtained the newly synthesized mixture of trans and cis isomers M, in a percentage isomeric cis/trans ratio of 65/35, probably due to a partial rearrangement of the cis isomer to the more stable trans isomer (entry 4). Finally, we synthesized two other symmetrical disulfides A and D with terminal double bonds and different carbon chain lengths (entries 2 and 6). Molecule D was newly synthesized. We proved the acceptable purity of the products by 1H and 13C-NMR spectroscopy. We evaluated the viability of the A549 cell line 24 and 48 hours after the treatment. A comparison between the activity of the lead compound and that of the new analogues was made. We used the human A549 lung adenocarcinoma cell line to assess the effects of the compounds on tumor viability in vitro. The A549 non-small cell lung cancer cell line was purchased from ATCC-LGC Promochem (South West London, UK) as previously reported (Amodio et al., Am. J. Pathol. 2010).32 Cells were maintained in RPMI 1640 medium (Gibco-Invitrogen Cell Culture, Carlsbad, CA), supplemented with 10% fetal bovine serum and 100 units per ml penicillin–streptomycin (Life Technologies, Inc., Carlsbad, CA). Cells were assayed using the Cell Titer-Glo Luminescent Cell Viability assay (Promega), after being treated with increasing concentration of each molecule (50, 100, 250, and 500 μM). Each measurement was repeated three times and the mean value calculated with the relative standard deviation. Cell viability was analyzed by a luminometric assay, and all values were calculated with respect to the control that was labeled C.
| Entry | Reagent | Intermediate 1 | Intermediate 2 | Final producta |
|---|---|---|---|---|
| a All the products were purified using flash column chromatography with alumina and stored at 0 °C. b The final product is a mixture of the two isomers in a cis/trans ratio of 65/35 estimated by 13C-NMR spectroscopy. | ||||
| 1 |

|
 1a
1a
|
 1b
1b
|
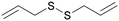 X
X
|
| 2 |

|
 2a
2a
|
 2b
2b
|
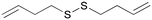 A
A
|
| 3 |
|
 3a
3a
|
 3b
3b
|
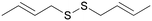 B
B
|
| 4b |

|
 4a
4a
|
 4b
4b
|
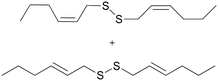 M
M
|
| 5 |
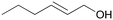
|
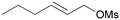 5a
5a
|
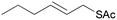 5b
5b
|
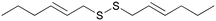 D
D
|
| 6 |

|
 6a
6a
|
 6b
6b
|
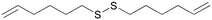 E
E
|
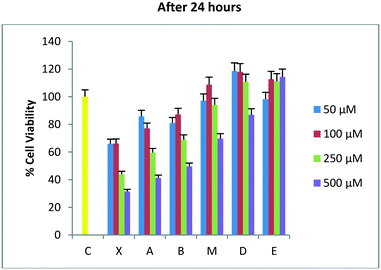
After 24 hours of incubation (Fig. 1), molecules A and B seem to follow the trend of the lead molecule X, with a decrease in cell viability in a concentration-dependent manner. The results relative to the natural molecule X are in accordance with the data reported in the literature.33 The activity of DADS analogues A and B is found to be slightly weaker compared to the reference molecule X, for all the four concentrations used. The presence of a double bond is significant, but it doesn't have to be necessarily allylic, as proved by molecule A that possesses a terminal double bond on its carbon chain. Molecules M and D with allylic double bonds in different isomeric forms triggered a slight decrease in cell viability only at the highest concentration (500 μM). The isomeric mixture M is more active than the trans isomer D at all the four used concentrations, probably due to the higher percentage of the cis isomer. The loss of the anti-tumor activity was also observed for molecule E, which had a terminal double bond on its carbon chain. These results indicate that the carbon chain length is likely to play a more active role in the potential anti-tumor activity of such unsaturated compounds.
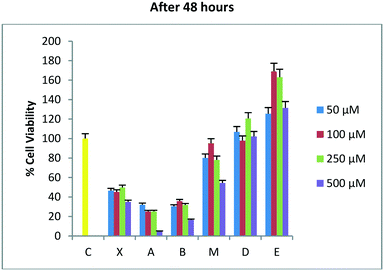
After 48 hours of incubation (Fig. 2), molecules X, A, and B show a significant and time-dependent in vitro anti-tumor activity. These two synthetic derivatives of DADS generate stronger effects at all the four used concentrations. The lead molecule X induces 54% viability inhibition at 50 μM. Molecule A induces a viability inhibition of 68% at the same concentration. Molecule B has a similar effect, with 70% viability inhibition at 50 μM. For molecule X, the percentage inhibition doesn't change significantly from 50 to 250 μM, and reaches almost the same activity as that of the synthetic analogues at 500 μM, with 65% inhibition overall. Molecule A shows a concentration-dependent activity while passing from 50 μM to 100 μM, while no significant changes are observed at 250 μM. Almost complete inhibition was observed for molecule A at 500 μM. Compound B at 50 μM shows effects similar to A, with no improvements at 100 or 250 μM. The inhibition of cell viability by B at 500 μM is weaker than that by molecule A, which shows a value of 84% regarding the same aspect. All other tested compounds do not show significant effects 48 hours after the treatment. The isomeric mixture M is more active than the trans isomer D at all the four used concentrations, even after 48 hours from the treatment.
Finally, we performed a preliminary stability test on more active molecules A and B and on the natural molecule X. These compounds were allowed to stay in their pure form at room temperature (an average of 298.15 K) for one week. After this period the purity was checked and confirmed by 1H-NMR spectroscopy. Small quantities of A, B, and X were dissolved in deuterated chloroform and these samples were left to stand at room temperature (an average of 298.15 K) for one week. After this period, the acceptable purity was checked and confirmed by 1H-NMR spectroscopy for the synthetic compounds A and B, while for the natural molecule X a significant change of purity was evident from the spectrum (molecule X was estimated to be 40% pure by chromatography). This test can be useful for more advanced studies with medicinal interests. Unsaturated organosulfur compounds are widespread in nature, mostly thanks to the plants of genus Allium. These compounds are observed as promising pharmacologically active molecules, but the relationship between the structure and the activity is still not clear. In this work, we synthetized and tested the in vitro anti-tumor potential of different unsaturated disulfides using human adenocarcinoma cells. From the data collected and reported above, we can conclude that unsaturated disulfides hold promise for the inhibition of the cell viability of the A549 cell line. For the first time, the DADS analogues A and B have been proven to be more active on a cancer cell line than the natural molecule diallyl disulfide. In addition, the unsaturation site is not the principal driving force, but other factors, like the size of the molecule and the length of the carbon chain, can be important guidelines for more advanced studies in this field in future.
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. Uramaru, H. Shigematsu, A. Toda, R. Eyanagi, S. Kitamura and S. Ohta, J. Med. Chem., 2010, 53, 8727 CrossRef CAS PubMed.
- A. O. H. El-Nezhawy, A. R. Biuomy, F. S. Hassan, A. K. Ismaiel and H. A. Omar, Bioorg. Med. Chem., 2013, 21, 1661 CrossRef CAS.
- P. F. Koha and T. P. Loh, Green Chem., 2015, 17, 3746 RSC.
- J. Clardy and C. Walsh, Nature, 2004, 432, 829 CrossRef CAS.
- Z. Guo, Acta Pharm. Sin. B, 2017, 7, 119 CrossRef PubMed.
- M. W. P. Bebbington, Chem. Soc. Rev., 2017, 46, 5059 RSC.
- E. Block, Angew. Chem., Int. Ed. Engl., 1992, 31, 1135 CrossRef.
- J. M. Kim, H. J. Chang, W. K. Kim, N. Chang and H. S. Chun, J. Agric. Food Chem., 2006, 54, 6547 CrossRef CAS PubMed.
- L. C. Armando, European Pat. 1721534A, 2006 Search PubMed.
- M. G. Jones, J. Hughes, A. Tregova, J. Milne, A. B. Tomsett and H. A. Collin, J. Exp. Bot., 2004, 55, 1903 CrossRef CAS PubMed.
- V. Lanzotti, J. Chromatogr. A, 2006, 1112, 3 CrossRef CAS.
- S. Ankri and D. Mirelman, Microbes Infect., 1999, 2, 125 CrossRef.
- J. Y. Chan, A. C. Yuen, R. Y. Chan and S. W. Chan, Phytother. Res., 2013, 27, 637 CrossRef CAS.
- S. Oommen, R. J. Anto, G. Srinivas and D. Karunagaran, Eur. J. Pharmacol., 2004, 485, 97 CrossRef CAS.
- A. S. Weisberger and J. Pensky, Cancer Res., 1958, 18, 1301 CAS.
- K. Hirsch, M. Danilenko and J. Giat, Nutr. Cancer, 2000, 38, 245 CrossRef CAS PubMed.
- L. Jenny, S. Gupta, J. S. Huang, L. P. Jayathilaka and B. S. Lee, Anal. Biochem., 2013, 436, 187 CrossRef PubMed.
- H. Fujisawa, K. Suma, K. Origuchi, H. Kumagai, T. Seki and T. Ariga, J. Agric. Food Chem., 2008, 56, 4229 CrossRef CAS PubMed.
- C. Gao, X. Jiang, H. Wang, Z. Zhao and W. Wang, J. Drug Metab. Toxicol., 2013, 4, 1 Search PubMed.
- V. Robert, B. Mouillé, C. Mayeur, M. Michaud and F. Blachier, Carcinogenesis, 2001, 22, 1155 CrossRef CAS.
- G. J. Heo, S. H. Hwang, S. C. Park, M. De Zoysa and G. W. Shin, J. Anim. Vet. Adv., 2012, 11, 2280 CrossRef.
- R. Liu, Y. Yang, L. Yi, J. Qing, Q. Yely, W. Wang, J. Wang, Y. Tang and H. Tan, Oncol. Lett., 2018, 15, 6377 Search PubMed.
- T. Seki, T. Hosono, S. Suda, K. Kimura and T. Ariga, J. Food Drug Anal., 2012, 20, 309 CAS.
- L. Yi and Q. Su, Food Chem. Toxicol., 2013, 57, 362 CrossRef CAS PubMed.
- X. J. Wu, F. Kassie and V. Mersch-Sundermann, Mutat. Res., 2005, 579, 115 CrossRef CAS PubMed.
- L. Z. Xia, et al. , Chemother.: Open Access, 2017, 6, 1–8 Search PubMed.
- F. Olivito, P. Costanzo, M. L. Di Gioia, M. Nardi, M. Oliverio and A. Procopio, Org. Biomol. Chem., 2018, 16, 7753 RSC.
- (a) M. Nardi, P. Costanzo, A. De Nino, M. L. Di Gioia, F. Olivito, G. Sindona and A. Procopio, Green Chem., 2017, 19, 5403 RSC; (b) R. Paonessa, M. Nardi, M. L. Di Gioia, F. Olivito, M. Oliverio and A. Procopio, Nat. Prod. Commun., 2018, 13, 1117 Search PubMed.
- F. Zheng and D. A. Pratt, Chem. Commun., 2013, 49, 8181 RSC.
- S. Tsao and M. Yin, J. Antimicrob. Chemother., 2001, 47, 665 CrossRef CAS.
- J. W. Kim, J. E. Huh, S. H. Kyung and K. H. Kyung, Food Sci. Biotechnol., 2004, 13, 235 CAS.
- N. Amodio, et al. , Am. J. Pathol., 2010, 177, 2622 CrossRef CAS PubMed.
- X. Wu, F. Kassie and V. Mersch-Sundermann, Mutat. Res., 2005, 579, 115 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and compound characterization. See DOI: 10.1039/c8md00503f |
| This journal is © The Royal Society of Chemistry 2019 |